INTRODUCTION
Pulmonary tuberculosis (TB) is a widespread debilitating disease that threatens public health throughout the world. It is estimated that one third of the world's population live with a latent Mycobacterium tuberculosis infection, which problematically presents a potential source of active TB in the future [1]. About 5–10% of individuals infected by M. tuberculosis will progress to active TB during their lifetime [Reference Horsburgh2, Reference Walzl3]. In 2012, 8·6 million people were diagnosed with active TB worldwide, 1·3 million of whom died from the disease [4]. Of the 8·6 million TB cases, 450 000 were multidrug-resistant TB (MDR-TB) and 150 000 patients died from MDR-TB. China carries the second largest TB burden in the world. According to the 5th Nationwide TB Epidemiological Sampling Survey in 2010 [5], the prevalence of active and smear-positive TB were 459/100 000 and 66/100 000, respectively, in people aged ⩾15 years.
After implementation of the directly observed treatment strategy (DOTS), identification, diagnosis and treatment of TB have been increasingly standardized. To reduce the occurrence of MDR-TB, the World Health Organization (WHO) adjusted the pulmonary TB treatment plan in 2010. In the adjusted plan, it is recommended that new patients with pulmonary TB receive a treatment regimen with 6 months of rifampicin: 2HRZE/4HR [6]. During the long process of TB treatment, no efficient method is available to evaluate the curative effects with precision, especially for smear-negative TB, which accounts for a majority (85·6% in China) of all TB cases. Therefore, it is crucial to find a method to more precisely evaluate the curative effects of TB treatment.
Chitotriosidase, secreted by activated macrophages [Reference Di Rosa7–Reference Kologlu10], is involved in the mechanism of innate host defence against fungal infection [Reference Debono and Gordee11]. Tuberculosis bacillus is a type of intracellular bacterium mainly parasitized in macrophages. Alveolar macrophages secrete chitotriosidase after being activated by the M. tuberculosis residing in alveolar macrophages. The chitotriosidase activity of plasma in TB patients was first investigated in 2007 [Reference Bargagli12]. However, the result of the study was not significant, which could be attributed to the small sample size. As reported, chitotriosidase activity of smear-negative culture-positive TB cases is higher compared to non-active TB patients and healthy controls [Reference Tasci13]. The chitotriosidase level in patients with 6 months' anti-TB treatment significantly decreased to a level that was close to healthy controls [Reference Cakir14]. These studies suggest that chitotriosidase may serve as a biomarker in evaluating the curative effects of TB treatment. Therefore, the aim of our study was to explore the difference in chitotriosidase activity in TB patients at different treatment stages and to investigate the relationship between chitotriosidase and radiological parameters.
METHODS
Selection of cases
Sources of cases: Cases were randomly selected with a two-step stratified sampling method. First, five counties out of 122 counties/cities/districts in Hunan Province were randomly selected using a random number table (e.g. Qidong county, Yueyanglou district, Yueyang county, Zixing city, Hongjiang city). According to the TB management regulation, all suspected TB patients would be transferred to local Centers for Disease Control (CDCs) for confirmation based on WHO diagnostic criteria [15]. Once confirmed, the patients would receive treatment at the CDCs free of charge. Therefore, we identified cases from local CDCs. All patients who were newly diagnosed or had been receiving TB treatment at the CDCs of these five counties between April and August 2009 were selected. Patients with HIV/TB co-infection or other co-infections including fungi and nematodes were excluded because these co-infections could enhance serum chitotriosidase activity. Patients who stopped receiving medications or were previously treated were also excluded.
Selection of healthy controls
Controls were also selected using the stratified sampling strategy. First, one from the 14 community health service centres (e.g. that in Xingang community) in Kaifu district, Changsha city was selected using the random number table. Next, one from the six community health service stations (e.g. Xin'ansi) managed by Xingang Community Health Service Centre was randomly selected. Next, controls were matched with cases using a frequency-matching method. Because the ratio of male to female TB patients was about 2·5:1 in Hunan [Reference Chen16], the healthy controls were selected from permanent residents in Xin'ansi community by a gender-age frequency-matching method. All controls were confirmed free from active TB.
We also excluded participants (both cases and controls) who were not Han nationality.
Estimation of sample size
Sample size was calculated with:
 ${n_{ij}} = \displaystyle{{{{({Z_{a/2}} + {Z_{1 - \beta}} )}^2} \times (\sigma _2^2 + \sigma _2^2 )} \over {\delta _{ij}^2}}. $
${n_{ij}} = \displaystyle{{{{({Z_{a/2}} + {Z_{1 - \beta}} )}^2} \times (\sigma _2^2 + \sigma _2^2 )} \over {\delta _{ij}^2}}. $
The standard deviation (s.d.) σ in controls and the >6-month treatment group was estimated to be 4·2 [Reference Cakir14]; assuming a difference between these two groups of δ = 2, α = 0·05 (two-sided), and β = 0·10, the estimated sample size was 93, i.e. at least 93 participants of each group eligible for inclusion.
The protocol of our study was approved by the Ethics Review Committee of Central South University. A written informed consent form was signed by all participants. After that, 5 ml of blood was collected through venepuncture using an EDTA-containing tube. Plasma and packed cells were separated by centrifugation at 2500 g for 10 min and stored at −80 °C until used. In addition, all TB patients underwent a chest X-ray examination to determine the degree of radiological extent (DRE). The healthy controls received free physical examinations, including chest X-ray.
Determination of DRE
DRE was determined through chest X-rays by two chest physicians independently. In case of inconsistent interpretations, a final decision was made by consensus after the physicians evaluated the chest X-ray together. A previously described scoring system with modification was used [Reference Cakir14, Reference Deniz17]. Briefly, in each chest X-ray radiograph, each lung was divided into three zones: the middle zone was set between the levels of the second and fourth ribs at the costosternal junction, the upper zone was above the middle zone, and the lower zone was below the middle zone. A chest X-ray score was determined by visually estimating the extent of the opacities attributable to TB (e.g. nodular infiltration, consolidation, atelectasis, or linear density) and separately evaluating the existence of cavitation. The scoring was based on the percentage of opacities in each lung zone (PER) and the total diameter of the cavities (D). The scoring system was set as follows:
-
Score 0: no abnormality;
-
Score 1: PER of <25% without obvious cavitation;
-
Score 2: PER of 25–50% without obvious cavitation, or PER <25% and D < 2 cm;
-
Score 3: PER of 50–75% without obvious cavitation, or PER <50% and D = 2–4 cm;
-
Score 4: PER >75%, or any cavitary disease with D > 4 cm.
Treatment regimen
The medications used for TB treatment included isoniazid (H), rifampicin (R), pyrazinamide (Z), ethambutol (E), thioacetazone (T), and streptomycin (S). The treatment regimens used in Hunan in 2009 were based on the WHO-recommended regimen [15]. Specifically, the smear-positive patients were treated with 3 times/week HRZE for the first 2 months and 3 times/week HR for 4 months (2H3R3Z3E3/4H3R3). The smear-negative patients were treated similarly except that E was not used (2H3R3Z3/4H3R3).
Detection of chitotriosidase activity
We adopted a published method for measurement of chitotriosidase activity [Reference Hollak18]. Specifically, 5 μl of EDTA plasma was incubated with 100 μl of 0·022 mm 4-methylumbelliferyl-fl-d-NN,N'-triacetylchitotriose (4 MU-chitotrioside; Sigma Chemical Co., USA) as substrate in a citrate/phosphate buffer (0·1/0·2 m), pH 5·2, at 37 °C. After 60 min, the reaction was stopped with addition of 0·3 m glycine/NaOH buffer (2 ml, pH 10·6). The fluorescence intensity was tested at an excitation wavelength of 360 nm and emission wavelength of 450 nm. The unit of chitotriosidase activity is ‘nmol/h.ml’.
Statistical analysis
Data were analysed with SPSS v. 19.0 (IBM Corporation, USA). Categorical variables were analysed by χ 2 test. Measurement data in multiple groups were tested with analysis of variance (ANOVA) and least significant difference (LSD). Correlation between chitotriosidase activity and DRE was examined by Spearman's rank correlation test, with the correlation coefficient expressed as r. All tests of hypothesis were two-tailed with a type-1 error at 5%.
Ethical standards
The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national and institutional committees on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008.
RESULTS
Basic information of the study population
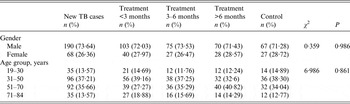
A total of 695 participants were involved in our study, including 601 cases and 94 healthy controls (control group). The cases were at different treatment stages: new (n = 258, case group 1), <3 months (143, case group 2), 3–6 months (102, case group 3), and >6 months (98, case group 4). The age of the study population ranged from 19 to 84 years. There was no significant difference in gender (P = 0·986) or age (P = 0·861) between cases and controls (Table 1).
Table 1. Distribution of gender and age of the study population
Chitotriosidase activity
Comparison between case groups and control group
The average levels of plasma chitotriosidase activity in the four case groups and the control group were 54·47 ± 19·27, 34·77 ± 8·67, 21·54 ± 7·56, 12·73 ± 3·99 and 10·53 ± 3·47 nmol/h.ml, respectively. The ANOVA revealed significant difference in chitotriosidase activity (P < 0·001). Chitotriosidase activity in the pulmonary TB patients declined with continuity of treatment. After 6 months' anti-TB treatment, chitotriosidase activity in both smear-positive and smear-negative patients significantly decreased to levels which were close to the healthy controls (Table 2).
Table 2. Chitotriosidase activity of the study groups
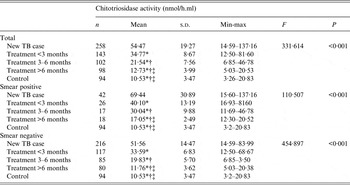
* The least significant difference (LSD) pairwise comparison showed statistical significance compared with the new case group.
† The LSD pairwise comparison showed statistical significance compared with the treatment <3 months group.
‡ The LSD pairwise comparison showed statistical significance compared with the treatment 3–6 months group.
Correlation between chitotriosidase activity and DRE
The consistency of DRE interpretations by the two chest physicians was satisfactory (kappa = 0·908, Table 3). Serum chitotriosidase activity is moderately and significantly correlated with DRE (r = 0·607, P < 0·001; Fig. 1, Table 4).
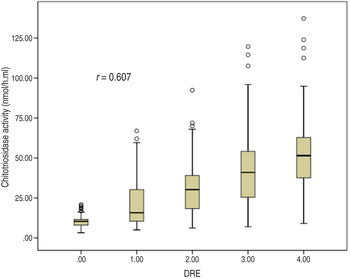
Fig. 1. Correlation between chitotriosidase activity and the degree of radiological extent (DRE).
Table 3. The inter-rater correlation of the degree of radiological extent (DRE) interpretation
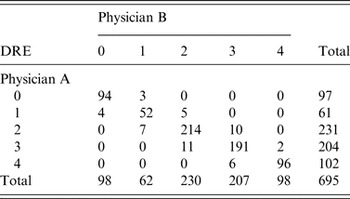
Kappa = 0·908.
Table 4. Chitotriosidase activity in groups with different degree of radiological extent (DRE)

* All healthy controls with DER = 0.
† The least significant difference (LSD) pairwise comparison showed statistical significance compared with the score 0 group.
‡ The LSD pairwise comparison showed statistical significance compared with the score 1 group.
§ The LSD pairwise comparison showed statistical significance compared with the score 2 group.
∥ The LSD pairwise comparison showed statistical significance compared with the score 3 group.
DISCUSSION
Plasma chitotriosidase activity levels in the four case groups and the control group were 54·47, 34·77, 21·54, 12·73 and 10·53 nmol/h.ml, respectively. As the treatment continued, the chitotriosidase activity of TB patients gradually declined. Chitotriosidase activity levels of both smear-positive and smear-negative patients decreased after 6 months' treatment and approached normal levels. Moreover, chitotriosidase activity is positively correlated with DRE (r = 0·607, P < 0·001), suggesting that worse DREs correspond to higher chitotriosidase activity. Our results, as well as a previous study [Reference Cakir14], suggest that chitotriosidase is capable of reflecting the curative effects of TB treatment.
In humans, alveolar macrophages can be activated by M. tuberculosis parasitizing inside and this activation can trigger macrophages to secrete chitotriosidase [Reference van Eijk9, Reference Korolenko19]. Moreover, activated macrophages play an important role in the formation of tuberculous granulomas [Reference Guirado, Schlesinger and Kaplan20]. Meanwhile, the activated macrophages can promote the secretion of interferon gamma as well as tumuor necrosis factor alpha, which will indirectly enhance the secretion of chitotriosidase [Reference Di Rosa7, Reference Malaguarnera8]. However, it is still unclear how chitotriosidase functions physiologically in human tissue. Nevertheless, it has been widely accepted that chitotriosidase is a biomarker of macrophages, it is also a biomarker of resistance of the host against the infection of chitin-related pathogen [Reference Guan21–Reference Ramanathan23]. The improvement of chitotriosidase activity in TB patients and its correlation with DRE suggest that chitotriosidase is possibly involved in the immunopathogenesis of TB [Reference Cakir14]. As reported, chitotriosidase activity of sarcoidosis patients was weakened with the remission of clinical symptoms, and was enhanced as the chest X-ray worsened [Reference Harlander24]. This evidence indicates that chitotriosidase activity is very likely to be a biomarker in evaluating the curative effects of anti-TB treatment.
In 2007, Bargagli et al. [Reference Bargagli12] first analysed plasma chitotriosidase activity in TB patients. The difference between TB patients and controls was not significant because of the small sample size. However, chitotriosidase activity of TB pleural effusions was significantly higher than non-TB lymphocytic pleural effusions [Reference Bouzas, San Jose and Tutor25]. Serum chitotriosidase levels in 17 smear-negative, culture-positive TB cases were significantly higher than in 38 smear-negative, culture-negative TB cases and 20 healthy controls (68·05, 29·73 and 28·4 nmol/h.ml, respectively) [Reference Tasci13]. A follow-up study [Reference Cakir14] shows that the average chitotriosidase activity of 42 TB patients is significantly higher compared to 30 controls (39·73 ± 24·97 vs. 9·63 ± 4·55 nmol/h.ml, P < 0·001). After 6 months' anti-TB treatment, chitotriosidase levels significantly decreased and approached the levels of healthy controls. At present, TB treatment effectiveness is evaluated by clinical symptoms and chest X-rays, which are strongly subjective and therefore unsatisfactory. The aforementioned evidence and our results imply that chitotriosidase might be a promising marker to evaluate the curative effects of TB treatment.
There are some limitations to our study. First, a cross-sectional study was designed to enrol cases at different treatment stages. Our study result just modestly suggests that chitotriosidase activity would be weakened as the treatment continued. However, whether chitotriosidase could serve as a marker to evaluate the effects of TB treatment should be further confirmed by follow-up studies, which are limited by potential difficulties, especially loss to follow-up. Second, the sample size calculation was performed after the sample collection. However, according to the method proposed by Dupont & Plummer [Reference Dupont and Plummer26], the power, calculated using our sample size, was 0·964, which suggests that our sample size had adequate power. Third, we did not consider the possible effects of sarcoidosis, fungi and nematode infections on enzyme activity in the controls. Nevertheless, these diseases are not prevalent in the general population, and are therefore unlikely to impact the results observed in our study.
In conclusion, our results suggest that chitotriosidase is a potential marker of the curative effects of TB treatment. Follow-up studies in various populations are needed in the future.
ACKNOWLEDGEMENTS
We thank our partners Dr Liqiong Bai (Hunan Institute of Tuberculosis Prevention and Treatment), and Ying Liang (School of Public Health, Central South University) for their input into this work.
This research received no specific grant from any funding agency, commercial or not-for-profit sectors.
DECLARATION OF INTEREST
None.







