At over 200 million years, the ginkgo tree is the oldest tree on earth (Reference GabyGaby, 1996). Traditionally ginkgo has been used to treat so-called ‘cerebral insufficiency’ (Reference Kleijnen and KnipschildKleijnen & Knipschild, 1992a ). Cerebral insufficiency is a very vague and inclusive diagnosis, covering symptoms such as depression, tiredness, poor memory and concentration, absent-mindedness and confusion (Reference Oken, Storbach and KayeOken et al, 1998). Occasionally no clinical explanation is found for cerebral insufficiency (Reference Kleijnen and KnipschildKleijnen & Knipschild, 1992b ). This review, therefore, only includes studies where ginkgo has been used to treat patients with an established diagnosis of dementia.
Clinical studies
Two reviews of the clinical studies have been published (Reference Oken, Storbach and KayeOken et al, 1998; Reference Kleijnen and KnipschildKleijnen & Knipschild, 1992b ). The second included 40 trials that mainly used ginkgo to treat cerebral insufficiency. The majority of patients did not have an established diagnosis of dementia. Most trials used 120-160 mg daily of ginkgo and 4 to 6 weeks' treatment was required before effectiveness was noted. The authors concluded that ginkgo was effective in cerebral insufficiency. Limitations of the published trials included low patient numbers and incomplete reporting of patient characteristics. Ginkgo has a characteristic bitter taste, which could lead to unblinding. Very few negative reports had been published, and the review noted a possible publication bias.
Oken et al (Reference Oken, Storbach and Kaye1998) identified 57 studies and reviews; again, ginkgo was mainly used to treat cerebral insufficiency. The authors conducted a meta-analysis on four trials that met strict inclusion criteria. These criteria were a confirmed diagnosis of Alzheimer's disease; the use of a standardised ginkgo extract; a randomised double-blind and placebo-controlled study; stated exclusion criteria; and cognitive function being one outcome measure. The paper concluded that 3 to 6 months of therapy with 120-240 mg of ginkgo daily had a small but significant effect on cognitive function in Alzheimer's disease. The review calculated the mean size effect of 0.40 (P < 0.0001) and concluded that this was comparable to the effect of donepezil in the Rogers et al (Reference Rogers, Farlow and Doody1998) study.
The Medline search identified five randomised controlled clinical trials that used ginkgo to treat Alzheimer's disease and vascular dementia (see Table 1 for a summary of the results from these five trials). One recently published paper investigated the efficacy of ginkgo and appeared to show that ginkgo was no more effective than placebo (Reference Van Dongen, Van Rossum and KesselsVan Dongen et al, 2000). This paper has been excluded from the review article because only approximately 30% of subjects in the study had dementia (either vascular or Alzheimer's). The remaining 70% of patients had non-dementia age-associated memory impairment.
Table 1. Double-blind placebo-controlled studies involving ginkgo for the treatment of dementia
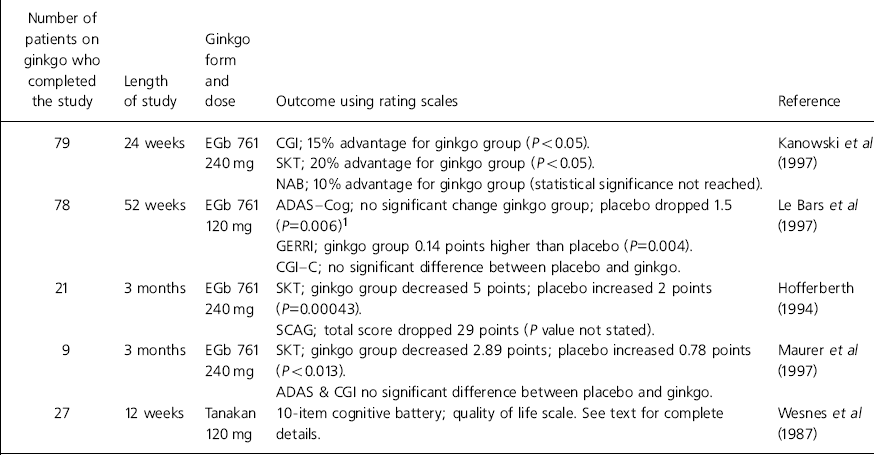
| Number of patients on ginkgo who completed the study | Length of study | Ginkgo form and dose | Outcome using rating scales | Reference |
|---|---|---|---|---|
| 79 | 24 weeks | EGb 761 | CGI; 15% advantage for ginkgo group (P<0.05). | Kanowski et al (Reference Kanowski, Herrmann and Stephen1997) |
| 240 mg | SKT; 20% advantage for ginkgo group (P<0.05). | |||
| NAB; 10% advantage for ginkgo group (statistical significance not reached). | ||||
| 78 | 52 weeks | EGb 761 | ADAS—Cog; no significant change ginkgo group; placebo dropped 1.5 (P=0.006)1 | Le Bars et al (Reference Le Bars, Katz and Berman1997) |
| 120 mg | ||||
| GERRI; ginkgo group 0.14 points higher than placebo (P=0.004). | ||||
| CGI—C; no significant difference between placebo and ginkgo. | ||||
| 21 | 3 months | EGb 761 | SKT; ginkgo group decreased 5 points; placebo increased 2 points (P=0.00043). | Hofferberth (Reference Hofferberth1994) |
| 240 mg | ||||
| SCAG; total score dropped 29 points (P value not stated). | ||||
| 9 | 3 months | EGb 761 | SKT; ginkgo group decreased 2.89 points; placebo increased 0.78 points (P<0.013). | Maurer et al (Reference Maurer, Ihl and Dierks1997) |
| 240 mg | ||||
| ADAS & CGI no significant difference between placebo and ginkgo. | ||||
| 27 | 12 weeks | Tanakan | 10-item cognitive battery; quality of life scale. See text for complete details. | Wesnes et al (Reference Wesnes, Simmons and Rook1987) |
| 120 mg |
Most of the studies used ginkgo extract EGb 761, which contains 22-27% flavone glycosides (including quercetin, kaempferol and their glycosides) and 5-7% terpene lactones (consisting of 2.6-3.3% bilobalide and 2.8-3.4% ginkgolides A, B, and C) (Reference Fugh-Berman and CottFugh-Berman & Cott, 1999).
A common drawback of most of the trials was the tendency to use non-standard rating scales as the primary outcome measure. Only two trials used the Alzheimer's Disease Assessment Scale — Cognitive subscale (ADAS—Cog) (Reference Le Bars, Katz and BermanLe Bars et al, 1997; Reference Maurer, Ihl and DierksMaurer et al, 1997). One of these trials used the ADAS scale as a secondary outcome measure and failed to show that ginkgo had any significant effect compared to placebo (Reference Maurer, Ihl and DierksMaurer et al, 1997). Other limitations of the results include the short duration of most of the studies. Only one study reached over 24 weeks and this had a high drop-out rate (Reference Le Bars, Katz and BermanLe Bars et al, 1997). Furthermore, the number of patients on ginkgo completing the studies was relatively low; in total only 214 patients received ginkgo in double-blind, placebo-controlled conditions. The most effective dose of ginkgo also requires clarification.
Kanowski et al (Reference Kanowski, Herrmann and Stephen1997) conducted a trial involving 216 patients with mild to moderate primary degenerative dementia (either Alzheimer type or multi-infarct) according to DSM—III—R criteria (American Psychiatric Association, 1987). After a 4-week placebo run-in period patients randomly received either ginkgo or placebo.
An overall response was defined as a positive response in 2 of 3 outcome measures. A change in item 2 of the Clinical Global Impression (CGI) (Reference GuyGuy, 1970; Collegium Internationale Psychiatriae Scalarum, 1986) to ‘much’ or ‘very much’ improved was defined as a positive response. A decrease of at least 4 points in the Syndrom-Kurztest (SKT) (Reference ErzigkeitErzigkeit, 1986), which assesses attention and memory, and a decrease in the Nurnberger Alters-Beobachtungsskala (NAB) daily living skills scale (Reference Oswald and FleischmannOswald & Fleischmann, 1986) of at least 2 points, were also positive responses.
Data were obtained from 156 patients who completed the 24-week study. There was an overall response to therapy in 28% of patients on ginkgo compared to 10% of patients on placebo. This difference in response rate was significant (P < 0.005, Fisher's exact test). This significance was confirmed by intention-to-treat analysis.
Le Bars et al (Reference Le Bars, Katz and Berman1997) conducted a multi-centre double-blind placebo-controlled trial involving 309 patients with mild to severe Alzheimer or multi-infarct dementia according to DSM—III—R (American Psychiatric Association, 1987) and ICD-10 criteria (World Health Organization, 1992). Patients were assessed with the ADAS—Cog (Reference Rosen, Mohs and DavisRosen et al, 1984), the Geriatric Evaluation by Relative's Rating Instrument (GERRI) (Reference SchwartzSchwartz, 1983) and the CGI — Change scale (Reference GuyGuy, 1976).
In the ginkgo group 50% of patients completed the study, compared with 38% of patients on placebo. At the end of the trial the ginkgo group had a GERRI reading 0.14 points better than the placebo group (P=0.005). The number of patients with a positive or negative response to placebo and ginkgo at 52 weeks was evaluated with a cumulative logit analysis. A 4-point improvement in the ADAS—Cog (equivalent to a 6 month delay in disease progression) occurred in 27% of ginkgo patients, but only 14% of patients on placebo. On the GERRI scale 37% of patients on ginkgo improved compared with 23% of patients on placebo (P=0.003).
While acknowledging the very high drop-out rates, the paper concluded that ginkgo stabilises the dementia process, and in 20% of cases improves cognitive and social functioning for 6 to 12 months.
One study involving 40 patients investigated the efficacy of ginkgo in Alzheimer's disease at 1, 2 and 3 months (Reference HofferberthHofferberth, 1994). Table 1 illustrates the results from the primary outcome measure, the SKT and the Sandoz Clinical Assessment Geriatric Scale (SCAG), which rated psychopathological changes (Reference Shader, Harmatz and SalzmannShader et al, 1974).
A 5-point improvement in the SKT value occurred in 52.4% of patients on ginkgo. In 13 of the 18 items on the SCAG there was a statistically significant improvement. The improvement in the items of rating confusion, mental clarity, indifference to surroundings, recent memory loss, animosity and appetite loss was significantly greater in the ginkgo group compared with placebo (P < 0.01). The investigator's global impression was that ginkgo was more effective than placebo. In 16 cases its efficacy was rated as ‘good’, whereas in no cases was placebo rated as ‘good’.
Maurer et al (Reference Maurer, Ihl and Dierks1997) investigated the effect of ginkgo on 20 patients with mild to moderate Alzheimer's disease. The main outcome measure in the double-blind randomised placebo-controlled parallel group trial was the SKT score; secondary measures were the CGI and the ADAS.
Table 1 illustrates the results obtained from the nine patients in each group who completed the trial. While acknowledging the low number of subjects involved, the authors concluded that the 19% improvement in the SKT score produced by ginkgo was comparable to other studies.
Wesnes et al (Reference Wesnes, Simmons and Rook1987) used ginkgo to treat cognitive impairment in 54 elderly patients with dementia. Patients were included in the randomised double-blind parallel-group placebo-controlled trial if they scored 14 or more on the Crichton Geriatric Behavioural Scale (Reference Robinson and AndersonRobinson, 1964). At weeks 0 and 12 a quality of life questionnaire assessed the interest and frequency of new activities, and whether old activities had been abandoned. Cognition tests were conducted at weeks 0, 4, 8 and 12. At week 12 ginkgo did not significantly increase the frequency of new activities or engagement in old activities. Patients on ginkgo did, however, show a significantly increased interest in new activities (P=0.015), whereas placebo had no significant effect (P=0.43).
The data from several cognitive tests had to be combined to form scores for accuracy and speed for differences between placebo and ginkgo to be detected. The accuracy score for both the placebo and the ginkgo group improved during the study; significance was reached at week 8. This improvement could be owing to a training effect associated with repeated testing. At week 12 the improvement in accuracy was significantly greater in the ginkgo group than the placebo group. In patients taking ginkgo, speed of mental processing improved significantly throughout the study, which could again be due to a training effect. At weeks 4 and 8, but not week 12, mental processing was significantly faster in the ginkgo group than the placebo group. The study concluded that the improvements in mental efficiency and living skills shown suggest that ginkgo may be an effective therapy in the early stages of primary degenerative dementia.
Owing to the absence of comparative double-blind trials involving both ginkgo and a cholinesterase inhibitor it is difficult to make conclusions regarding the relative effectiveness of ginkgo. Furthermore, the lack of uniformity makes it very difficult to compare the studies involving ginkgo with those involving cholinesterase inhibitors. As already mentioned, unlike much of the data on cholinesterase inhibitors, the studies using ginkgo tended to use a wide variety of non-standard rating scales. When a ginkgo and cholinesterase inhibitor trial used the same scale other conditions often varied. For example, while both Rogers et al (Reference Rogers, Farlow and Doody1998) and Le Bars et al (Reference Le Bars, Katz and Berman1997) used ADAS—Cog, the subject groups were not identical. The donepezil trial (Reference Rogers, Farlow and DoodyRogers et al, 1998) only involved patients with mild to moderate Alzheimer's disease, whereas the ginkgo trial (Reference Le Bars, Katz and BermanLe Bars et al, 1997) included patients with severe disease.
Mechanism of action
Ginkgo may improve cognitive function in patients with dementia in a number of ways. Suggested mechanisms include improving cerebral blood-flow, inhibiting platelet aggregation and increasing tolerance to hypoxia (Reference Kleijnen and KnipschildKleijnen & Knipschild, 1992a ). Ginkgo may also have a neuroprotective effect, stabilise membranes and possess anti-oxidant activity (Reference Fugh-Berman and CottFugh-Berman & Cott, 1999).
Side-effects and drug interactions
Ginkgo appears to be well-tolerated. A German post-marketing study only identified 183 out of 10 815 patients who reported side-effects when treated with ginkgo (Reference Fugh-Berman and CottFugh-Berman & Cott, 1999). The most commonly reported side-effects were nausea (27), headache (24), stomach problems (15) and diarrhoea (15).
It has been suggested that ginkgo may increase bleeding times and potentiate the activity of anticoagulants (Reference Wong, Smith and BoonWong et al, 1998). There are at least four reports in the literature of haemorrhage or prolonged bleeding in patients taking ginkgo (Reference Oken, Storbach and KayeOken et al, 1998). These four cases included one case each of ginkgo potentiating the anti-coagulant effect of aspirin and warfarin. The use of ginkgo should, therefore, be avoided in patients with clotting disorders, those taking anticoagulants or those awaiting surgery (Reference Fugh-BermanFugh-Berman, 2000).
Summary
The Medline search identified two review articles and five clinical trials. From the limited evidence available it appears that ginkgo has a small but significant effect on the symptoms of dementia. Limitations of the published data include the use of vague diagnostic criteria, non-standard rating scales and the fact that only a few patients with a confirmed diagnosis of dementia have received ginkgo in controlled trials.
The correct dose of ginkgo needs clarification. Ginkgo appears relatively free from side-effects, although its potential anti-coagulant effect is of concern. Ginkgo's mode of action is unclear.
Although there are no direct trials, one review concluded that the effect of ginkgo appears comparable to that of donepezil. This requires confirmation in a comparative double-blind trial. A very useful study would be four-armed comparing the effect of placebo, done-pezil, ginkgo and combined donepezil/ginkgo therapy.




eLetters
No eLetters have been published for this article.