Free radicals and/or oxygen derivatives are continuously generated during regular cellular metabolism. At low concentrations, these reactive oxygen species (ROS) may be beneficial or even indispensable in processes such as defence against micro-organisms, contributing to phagocytic bactericidal activity. However, when an imbalance between ROS generation and ROS removal occurs, oxidative stress arises( Reference Rando 1 ). The detrimental effects include oxidative damage to molecules of great biological importance, including lipids, proteins and DNA, causing alterations that produce a range of cellular damage which can ultimately lead to cell death( Reference Halliwell and Gutteridge 2 ).
Several effective antioxidant systems prevent oxidative damage in fish. Among them, various antioxidant enzymes (AOE) prevent the cascade of oxidation reactions, intercepting and inactivating the reactive intermediates and closing the lipid-peroxidation catalytic cycle. This defence system includes enzymes such as catalase (CAT), superoxide dismutase (SOD) and glutathione peroxidase (GPX)( Reference Halliwell and Gutteridge 2 , Reference Bell, Cowey and Adron 3 ). In addition to these enzymes, dietary micronutrients such as vitamins E and C as well as carotenoids have also been regarded as antioxidant defences in fish( Reference Wilhelm-Filho 4 – Reference Hamre 8 ). During larval stages, oxidation risks are particularly high, due to the increased metabolic rate, oxygen demand, water and long-chain PUFA (LC-PUFA) tissue contents found in fish larvae. Therefore, adequate supplementation of marine fish larvae with antioxidant elements is important when formulating diets in order to avoid in vivo lipid peroxidation. However, few studies have dealt with the effects of antioxidants in early fish feeding( Reference Mourente, Tocher and Díaz 9 – Reference Betancor, Atalah and Caballero 12 ). Among the other antioxidants, Se is an essential trace mineral in animal nutrition obtained partly from the surrounding water( Reference Lall and Bishop 13 ), but mostly from the diet( Reference Halver 14 ). The importance of Se to oxidative stress involves its presence at the active site of the antioxidant enzyme GPX( Reference Felton, Landolt and Grace 15 ), which reduces H2O2 at the expense of reduced glutathione( Reference Arteel and Sies 16 ). In fish, studies have shown a synergistic action between vitamin E and Se( Reference Poston, Combs and Leibovitz 17 , Reference Bell and Cowey 18 ). Moreover, a Se deficiency may lead to reduced levels of tissue α-tocopherol in several fish species( Reference Bell, Cowey and Adron 3 , Reference Poston, Combs and Leibovitz 17 – Reference Gatlin, Poe and Wilson 20 ). Additionally, a recent study in grouper (Epinephelus malabaricus) juveniles suggested a sparing effect between Se and vitamin E( Reference Lin and Shiau 21 ).
One of the most important factors that can lead to oxidative stress in fish is their high requirement for LC-PUFA, which are high in diets and, subsequently, in fish tissue( Reference McEvoy, Naess and Bell 22 , Reference Evjemo, Reitan and Olsen 23 ). For instance, severe dystrophic lesions in the epaxial musculature of sea bass larvae have been related to the deleterious effect of oxidative stress due to the high ingestion of LC-PUFA, particularly DHA( Reference Betancor, Atalah and Caballero 12 , Reference Betancor, Caballero and Benítez-Santana 24 ). Muscle growth is an essential process during larval stages, as a massive increment of muscle fibres takes place from hatching to maturity( Reference Johnston, Abercromby and Vieira 25 ) with damage to the musculature appearing to compromise larval growth( Reference Betancor, Atalah and Caballero 12 , Reference Betancor, Caballero and Benítez-Santana 24 ).
Muscle formation processes require the influence of growth factors and a sequence of cellular events that result in the regulation of myoblasts (myosatellite cells)( Reference Allen and Boxhorn 26 , Reference Johnston 27 ). Insulin-like growth factors I and II (IGF-I and IGF-II) are two myogenic regulatory factors which increase satellite cell proliferation and differentiation( Reference Goldspink, Wilkes and Ennion 28 , Reference Bower, Li and Taylor 29 ). In various species of fish, it has been shown that hepatic( Reference Duan and Plisetskaya 30 – Reference Hevrøy, Azpeleta and Shimizu 33 ) and muscular( Reference Terova, Rimoldi and Chini 32 – Reference Chauvigné, Gabillard and Weil 34 ) IGF-I and IGF-II mRNA levels depend on feeding status. Differences in the regulation of myogenesis such as myosin isoform expression have also been observed during the earliest stages of development as well as during temperature acclimatisation( Reference Watabe 35 , Reference Silva, Rowlerson and Valente 36 ). However, nutritional regulation of the various components of the IGF signalling pathways in muscle growth in fish as well as myosin expression is not well studied.
The purpose of the present study was to investigate whether diets supplemented with Se (5 mg/kg dry weight (DW)) and vitamin E can protect sea bass larvae muscle from oxidative stress when high DHA levels are included in diets. To reach this objective, growth, survival, thiobarbituric acid-reactive substances (TBARS), fatty acid profile, α-tocopherol and Se contents, and mRNA expression levels of CAT, SOD, GPX, IGF-I, IGF-II and myosin heavy chain (MyHC) genes were determined in sea bass larvae fed diets with different LC-PUFA, Se and vitamin E contents.
Methods
Fish
All experiments were designed according to the Animal Welfare Ethics Committee guidelines of Las Palmas University. The experiment was carried out at the Grupo de Investigación en Acuicultura facilities (Telde, Canary Islands, Spain). Sea bass (Dicentrarchus labrax) larvae were obtained from natural spawnings from the Instituto de Acuicultura de Torre de la Sal (Castellón, Spain). Before the start of the feeding experiment, larvae were fed enriched (DHA Protein Selco®; INVE) yeast-fed rotifers until they reached 14 d post-hatching (dph). Larvae (total length 8·58 (sd 0·64) mm and dry body weight 0·36 (sd 0·0) mg) were randomly distributed into the experimental tanks at a density of 1000 larvae/tank and fed one of the experimental diets for 21 d. All tanks (170 litres light grey colour cylinder fibreglass tanks) were supplied with filtered sea water (34 g/l salinity) at an increasing rate of 1·0–1·5 litres/min during the feeding trials. Water entered the tank from bottom to top; water quality was tested daily and no deterioration was observed. Water was continuously aerated (125 ml/min), attaining 5–8 g/l dissolved oxygen and saturation ranged between 60 and 80 %. Water temperature ranged from 19·5 to 21·0°C.
Diets
For the feeding experiment, three isonitrogenous and isolipidic experimental microdiets (pellet size < 250 μm) similar in their EPA content and different in DHA, Se and vitamin E content were formulated (Table 1) using concentrated fish oils INCROMEGA™ EPA500 and DHA500 (CRODA) as sources of EPA and DHA in TAG form and dl-α-tocopheryl acetate (Sigma-Aldrich) as a source of vitamin E. The diets were chosen based on previous trials( Reference Betancor, Atalah and Caballero 12 , Reference Betancor, Caballero and Benítez-Santana 24 ) and their names elected according to the level of dietary DHA and vitamin E content. A positive control diet was formulated to include 1 g DHA/100 g DW and 150 mg vitamin E/100 g DW (diet 1/150). The negative control diet consisted of 5 g DHA/100 g DW and 300 mg vitamin E/100 g DW (diet 5/300). The third diet had identical DHA and vitamin E content to the 5/300 diet, but was supplemented with Se in organic form (diet 5/300+Se, Sel-Plex® 2000, 2000 mg/kg; Alltech). The protein source was derived from squid meal, naturally containing 14 % lipid, defatted three consecutive times to allow complete control of the microdiet fatty acid profile. No other ingredients were defatted due to their poor lipid content. The squid meal was defatted with a chloroform:squid meal ratio of 3:1, rinsed and dried in an oven at 37°C until complete solvent evaporation. EPA500 and DHA500 were added in different quantities to the defatted meal (2·4 % lipid content) to obtain the desired ratios. Oleic acid (Merck) was added to equalise the lipid content in each diet (Table 1) and soyabean lecithin (Acrofarma) was included as a source of phospholipids (Table 1). The microdiets were processed as described previously( Reference Liu, Caballero and Izquierdo 37 ). Briefly, the squid powder and water-soluble components were mixed, followed by the lipid and fat-soluble vitamins and, before adding gelatine (Panreac), dissolved in warm water, as a binder. The paste was pelleted and oven dried at 38°C for 24 h. The pellets were ground and sieved to obtain particle size below 250 μm. To avoid peroxidation, the diets were stored under N2 at − 20°C until use. The diets were analysed for proximate and fatty acid composition on a dry basis and manually supplied; fourteen times/d at 45 min intervals from 09.00 to 19.00 hours. Daily feed supplied was 2, 2·5 and 3 g/tank during the first, second and third week of feeding, respectively.
Table 1 Formulation of the experimental diets
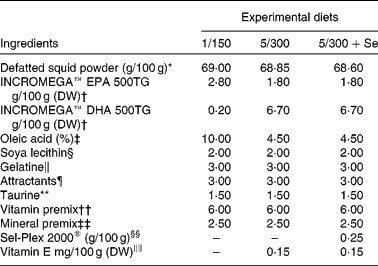
DW, dry weight; 1/150, 1 g DHA/100 g DW and 150 mg vitamin E/100 g DW diet; 5/300, 5 g DHA/100 g DW and 300 mg vitamin E/100 g DW diet; 5/300+Se, 5 g DHA/100 g DW and 300 mg vitamin E/100 g DW diet supplemented with Se.
* Riber and Son.
† Croda Chemicals Europe.
‡ Merck.
§ Acrofarma.
∥ Panreac.
¶ Attractant premix supplied per 100 g diet: inosine-5-monophosphate, 500·0 mg; betaine, 660·0 mg; l-serine, 170·0 mg; l-phenylalanine, 250·0 mg; dl-alanine, 500·0 mg; l-sodium aspartate, 330·0 mg; l-valine, 250·0 mg; glycine, 170·0 mg.
** Sigma-Aldrich.
†† Vitamin premix supplied per 100 g diet: cyanocobalamin, 0·03 mg; astaxanthin, 5·0 mg; folic acid, 5·4 mg; pyridoxine-HCI, 17·2 mg; thiamin, 21·7 mg; riboflavin, 72·5 mg; calcium-pantothenate, 101·5 mg; p-aminobenzoic acid, 145·0 mg; nicotinic acid, 290·1 mg; myo-inositol, 1450·9 mg; retinol acetate, 0·2 mg; ergocalcipherol, 3·6 mg; menadione, 17·3 mg; α-tocopheryl acetate, 150·0 mg.
‡‡ Mineral premix supplied per 100 g diet: NaCl, 215·133 mg; MgSO4.7H2O, 677·545 mg; NaH2PO4.H2O, 381·453 mg; K2HPO4, 758·949 mg; Ca(H2PO4).2H2O, 671·610 mg; FeC6H5O7, 146·884 mg; C3H5O3.0·5Ca, 1617·210 mg; Al2(SO4)3.6H2O, 0·693 mg; ZnSO4.7H2O, 14·837 mg; CuSO4.5H2O, 1·247 mg; MnSO4.H2O, 2·998 mg; KI, 0·742 mg; CoSO4.7H2O, 10·706 mg.
§§ Sel-Plex 2000, 2000 mg Se/kg; Alltech.
∥∥ dl-α-Tocopheryl acetate; Sigma-Aldrich.
Growth and survival
Final survival was determined by counting live larvae at the beginning and end of the experiment. Growth was determined by measuring dry body weight (105°C for 24 h) and total length (Profile Projector V-12A Tokyo; Nikon) of thirty fish/tank at the beginning, middle and end of the trial.
Biochemical analysis
All the remaining larvae in each tank were washed with distilled water, sampled and kept at − 80°C for biochemical composition, TBARS, Se and vitamin E analysis after 12 h of starvation at the end of the trial. Moisture( 38 ), protein( 38 ) and lipid( Reference Folch, Lees and Sloane Stanley 39 ) contents of larvae and the diets were analysed.
Total lipid fatty acid analysis
Fatty acid methyl esters were obtained by transmethylation of total lipids as described by Christie( Reference Christie 40 ). Fatty acid methyl esters were separated by GLC, quantified by flame ionisation detection (GC-14A; Shimadzu) under the conditions described previously( Reference Izquierdo, Arakawa and Takeuchi 41 ) and identified by comparison with previously characterised standards and GLC–MS.
Determination of vitamin E content
Vitamin E concentrations were determined in the diets and total larvae using HPLC at the University of Stirling (Scotland, UK). Samples were weighed, homogenised in pyrogallol and saponified as described by McMurray et al. ( Reference McMurray, Blanchflower and Rice 42 ) for the diets or according to Cowey et al. ( Reference Cowey, Adron and Walton 43 ) for larval tissues. HPLC analysis was performed using a 150 × 4·60 mm reversed-phase Luna 5 μm C18 column (Phenomenox). The mobile phase was 98 % methanol supplied at a flow rate of 1·0 ml/min. The effluent from the column was monitored at a wavelength of 293 nm and quantification achieved by comparison with (+)-α-tocopherol (Poole) as the external standard.
Selenium determination
Total Se concentration was measured in total larvae and the diets, and analyses were carried out at the University of Stirling (Scotland, UK). Samples were acidified in a microwave digestor (MarsXpress; CEM) with 5 ml of 69 % pure HNO3, then poured after digestion into a 10 ml volumetric flask and made up to volume with distilled water. A total of 0·4 ml of this solution were then added to a 10 ml sample tube, 10 μl of the internal standard (Ga and Sc, 10 ppm) included and 0·3 ml of methanol added. The tubes were made up to volume with distilled water and total Se measured by collision/reaction by inductively coupled plasma MS (Thermo Scientific) using argon and hydrogen as the carrier gas.
Measurement of thiobarbituric acid-reactive substances
TBARS from triplicate samples were determined using a method adapted from that used by Burk et al. ( Reference Burk, Trumble and Lawrence 44 ). Approximately 20–30 mg of larval tissue per sample were homogenised in 1·5 ml of 20 % TCA (w/v) containing 0·05 ml of 1 % butylated hydroxytoluene in methanol. To this 2·95 ml of freshly prepared 50 mm-thiobarbituric acid solution were added before mixing and heating for 10 min at 100°C. After cooling, protein precipitates were removed by centrifugation (Sigma 4K15) at 2000 g and the supernatant was read in a spectrophotometer (Evolution 300; Thermo Scientific) at 532 nm. Absorbance was recorded against a blank at the same wavelength. The concentration of thiobarbituric acid-malonaldehyde (MDA), expressed as μmol MDA/g tissue was calculated using an extinction coefficient of 0·156 cm/μm.
Histopathological sampling
From each tank, thirty larvae were collected every 7 d from the beginning of the feeding trial, fixed in 10 % buffered formalin for 1–2 d, dehydrated through graded alcohols, then xylene and finally embedded in paraffin wax. Then, six paraffin blocks containing five larvae/tank were sectioned at 3 μm, and stained with haematoxylin and eosin for histopathological evaluation( Reference Martoja and Martoja Pearson 45 ).
From each tank, ten larvae were fixed for 24 h at 4°C in 2·5 % glutaraldehyde in 0·2 m-phosphate buffer (pH 7·2). Samples were then rinsed in phosphate buffer and post-fixed for 1 h in 2 % osmium tetroxide in 0·2 m-potassium ferrocyanide. Each larva was then embedded in an Eppon/Araldite resin block. Thick serial transverse and longitudinal sections of the larvae were cut at 2 μm, stained with toluidine blue and examined by light microscopy( Reference Hoffman, Flores and Coover 46 ). Thin sections were cut at 50 nm and stained with lead citrate before observing with a ZEISS EM 910 transmission electron microscope (Zeiss) at the Electron Microscope Service of the University of Las Palmas de Gran Canaria.
RNA extraction and quantitative RT-PCR
Molecular biology analysis was carried out at the University of Insubria (Varese, Italy). Total RNA was extracted from sea bass larvae (approximately 200 mg; pool per tank), using the PureYield RNA Midiprep System (Promega). The quantity and purity of RNA was assessed by a spectrophotometer. Visualisation on 1 % agarose gel stained with ethidium bromide showed that RNA was not degraded. After DNase treatment (Invitrogen), 3 μg of total RNA were reverse transcribed into complementary DNA in a volume of 12 μl, including 1 μl of oligo-dT16 primer (50 pmol) and 1 μl of 10 mm-deoxynucleotide triphosphates. This mix was heated at 65°C for 5 min and chilled on ice before 4 μl of 5 × reverse transcription buffer, 2 μl of 0·1 m-dithiothreitol, 1 μl RNase out and 1 μl of Moloney murine leukaemia virus was added. After incubation at 37°C for 50 min, the reaction was stopped by heating at 75°C for 15 min.
PCR primer sequences used for the PCR amplification of the complementary DNA of target genes were CAT, SOD, GPX, IGF-I, IGF-II and MyHC. To perform PCR, a 4 μl aliquot of complementary DNA was amplified using 25 μl GoTaq Green Master Mix (Promega) in 50 μl of final volume and 50 pmol of each designed primer.
A total of thirty-one PCR amplification cycles (eight touchdown) were performed for all primer sets, using an automated Thermal Cycler (MyCycler; BioRad). An aliquot of each sample was then subjected to electrophoresis on a 1 % agarose gel in 1 × Tris-acetate–EDTA buffer (Bio-Rad) and bands were detected by ethidium bromide staining. Samples were run with a 100 bp–1·5 kb DNA ladder to control the molecular weight of DNA. The negative control (a reaction mixture without complementary DNA) confirmed the absence of genomic contamination. The PCR products from each primer set amplification were cloned using the pGEM®-T easy vector (Promega) and subsequently sequenced in both directions (T7 and SP6).
TaqMan® real-time RT-PCR was performed on a StepOne Real Time PCR System (Applied Biosystems) using Assays-by-DesignSM PCR primers (Applied Biosystems) and gene-specific fluorogenic probes. Primer sequences and Taqman® probes of the target genes were as follows:
Target gene: sea bass CAT
Forward primer: 5′-ATGGTGTGGGACTTCTGGAG-3′
Reverse primer: 5′-GCTGAACAAGAAAGACACCTGATG-3′
Taqman® probe: 5′-CAGACACTCAGGCCTCA-3′
Target gene: sea bass SOD
Forward primer: 5′-TGGAGACCTGGGAGATGTAACTG-3′
Reverse primer: 5′-TCTTGTCCGTGATGTCGATCTTG-3′
Taqman® probe: 5′-CAGGAGGAGATAACATTG-3′
Target gene: Sea bass GPX
Forward primer: 5′-AGTTAATCCGGAATTCGTGAG-3′
Reverse primer: 5′-AGCTTAGCTGTCAGGTCGTAAAAC-3′
Taqman® probe: 5′-AATGGCTGGAAACGTG-3′
Target gene: Sea bass IGF-I
Forward primer: 5′-GCAGTTTGTGTGTGGAGAGAGA-3′
Reverse primer: 5′-GACCGCCGTGCATTGG-3′
Taqman® probe: 5′-CTGTAGGTTTACTGAAATAAAA-3′
Target gene: Sea bass IGF-II
Forward primer: 5′-TGCAGAGACGCTGTGTGG-3′
Reverse primer: 5′-GCCTACTGAAATAGAAGCCTCTGT-3′
Taqman® probe: 5′-CAAACTGCAGCGCATCC-3′
Target gene: Sea bass MyHC
Forward primer: 5′-TGGAGAAGATGTGCCGTACTCT-3′
Reverse primer: 5′-CGTGTCATTGATTTGACGGACATTT-3′
Taqman® probe: 5′-AACTGAGTGAACTGAAGACC-3′
Data from TaqMan® PCR runs were collected using ABI's Sequence Detector Program. Cycle threshold values corresponded to the number of cycles at which fluorescence emission monitored in real time exceeded the threshold limit. The cycle threshold values were used to create standard curves to serve as a basis for calculating the absolute amounts of mRNA in total RNA. To reduce pipetting errors, master mixes were prepared to set up duplicate reactions (2 × 30 μl) for each sample.
Calculations
Larval survival was determined by comparing the number of larvae at the beginning of the trial with the larvae number measured in individual tanks at 35 dph to which the average number of larvae sampled from the tanks during the trial was added. Percentage survival could then be calculated for each tank to get a mean and standard deviation per treatment. The incidence of muscular lesions was calculated as the percentage of injured larvae per tank compared with the total larvae observed, with standard deviation referring to deviation among the tanks. Specific growth rate (SGR) was calculated as
where W 0 and W 1 are the initial and final DW (tank means), respectively, and t 2 − t 1 is the time interval in days between the beginning and end of the experimental trial (21 d).
Statistical analysis
Survival, growth and molecular biology data were tested for normality and homogeneity of variances with Levene's test. Where necessary, data were log transformed before further statistical analysis. The χ2 test was employed for incidence of muscular lesions and TBARS content. Survival, growth and biochemical analysis data were treated using one-way ANOVA and molecular biology results were treated using a general linear model (GLM). Means were compared by Duncan's test. Results are presented as means and standard deviations. The tank was considered as the experimental unit, except for the estimation of the incidence of muscular lesions, where each individual larva was considered as a unit. For percentage data (final survival), arcsine transformation was performed before the analysis. For the analysis of one-way ANOVA, the following GLM was used:
where Y ij is the mean value of the tank, μ is the mean population, α i is the fixed effect of the diet and ε ij is the residual error. For the analysis of molecular biology data, a two-variable GLM was employed to analyse possible interactions between treatment and time:
where Y ijk is the mean value of the tank, μ is the mean population, α i is the fixed effect of the diet, δ j is the fixed effect of the time, (αδ) ij is the interaction between diet and time and ε ijk is the residual error. Significance was accepted at P ≤ 0·05. Statistical analysis was performed using SPSS software (SPSS for Windows 14.0; SPSS, Inc., 2005).
Results
The diet containing about 1 % DHA (1/150) showed a higher monoenoic fatty acid level than the diets containing 5 % DHA (5/300 and 5/300+Se) due to a higher oleic acid content in the former diet (Table 2). Elevation of dietary DHA (5/300 diet) increased n-3 and n-3 LC-PUFA fatty acid contents, as well as the n-3:n-6 ratio. Vitamin E levels were more than two times higher in the diets containing 300 mg/100 g compared with the control diet (1/150) (Table 3). Se contents differed among the dietary treatments, with a higher level of this mineral found in the diet supplemented with Se compared with the others (Table 3).
Table 2 Main fatty acids (% total fatty acids) of the experimental diets fed to sea bass larvae

1/150, 1 g DHA/100 g dry weight and 150 mg vitamin E/100 g dry weight diet; 5/300, 5 g DHA/100 g dry weight and 300 mg vitamin E/100 g dry weight diet; 5/300+Se, 5 g DHA/100 g dry weight and 300 mg vitamin E/100 g dry weight diet supplemented with Se; ND, not determined; LC-PUFA, long-chain PUFA; ARA, arachidonic acid.
Table 3 Gross composition, α-tocopherol and selenium content in the experimental diets fed to sea bass larvae (Mean values and standard deviations)
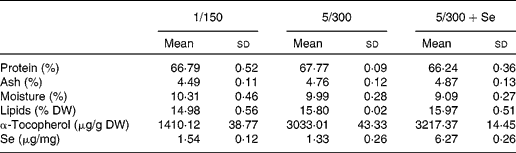
DW, dry weight; 1/150, 1 g DHA/100 g DW and 150 mg vitamin E/100 g DW diet; 5/300, 5 g DHA/100 g DW and 300 mg vitamin E/100 g DW diet; 5/300+Se, 5 g DHA/100 g DW and 300 mg vitamin E/100 g DW diet supplemented with Se.
All the experimental diets were well accepted by the larvae. Dietary increase of vitamin E or Se did not significantly affect larval survival (P = 0·158; Table 5). The highest larval growth, in terms of total length, was found in the larvae fed the positive control diet containing the lowest vitamin E and DHA contents (diet 1/150). However, increases in vitamin E and DHA (diet 5/300) significantly reduced the larval growth (P = 0·001), whereas Se inclusion (diet 5/300+Se) significantly increased this parameter (P = 0·02; Table 5). The average SGR was higher (P = 0·024) in the larvae fed the 1/150 diet, although no differences were found in the larvae fed the 5/300+Se diet.
In terms of fatty acid composition, larvae fed the 5/300 diet resulted in a higher concentration of n-3 and n-3 LC-PUFA, reflecting the higher content of these components in the diets (Table 4). However, larvae fed the 1/150 diet showed a higher retention rate of DHA (279·26 %) and EPA (68·24 %) compared with larvae fed the 5/300 (73·48 and 43·93 %, respectively) and 5/300+Se diets (77·00 and 44·75 %, respectively) and also a higher content of 18 : 1n-9, displaying levels similar to those found in the former microdiet. In contrast, the 5/300 larvae showed a higher retention rate of arachidonic acid (about 50 %) compared with the 1/150 larvae (29·83 %).
Table 4 Main fatty acid compositions of total lipids from sea bass larvae fed the experimental diets for 21 d (% total fatty acids) (Mean values and standard deviations)

1/150, 1 g DHA/100 g dry weight and 150 mg vitamin E/100 g dry weight diet; 5/300, 5 g DHA/100 g dry weight and 300 mg vitamin E/100 g dry weight diet; 5/300+Se, 5 g DHA/100 g dry weight and 300 mg vitamin E/100 g dry weight diet supplemented with Se; LC-PUFA, long-chain PUFA.
a,b,c Mean values within a row with unlike superscript letters were significantly different (P < 0·05).
The level of lipid oxidation, as indicated by the MDA content (μmol/g larval tissues), was significantly higher (P = 0·001) in the larvae fed the diets with the highest DHA content. Nevertheless, the inclusion of Se showed a beneficial effect preventing the formation of H2O2, as denoted by the decrease in MDA levels (Table 5). The lowest peroxidation level was observed in the larvae fed the 1/150 diet. Despite the increase in dietary α-tocopherol in the 5/300 and 5/300+Se diets, the content of this nutrient in larvae did not significantly increase in comparison with larvae fed the 1/150 diet (P = 0·601; Table 5). Therefore, the retention rate of dietary vitamin E in the larvae fed the 5/300 (17·87 %) and 5/300+Se (17·38 %) diets was lower than the 1/150 larvae (44·68 %). Sea bass larvae fed the diets supplemented with Se showed a significantly higher (P = 0·001) content of this mineral compared with the other larvae, this level being 1·7 times higher than the larvae fed the 1/150 diet and 2·4 times higher than the 5/300 larvae (Table 5). Thus, although Se contents in the 1/150 and 5/300 diets were similar, the larvae fed the latter diet showed lower Se levels together with the highest TBARS value.
Table 5 Sea bass larvae performance and levels of lipid peroxidation products (thiobarbituric acid-reactive substances (TBARS)), vitamin E (α-tocopherol) and selenium content of sea bass larvae at the beginning and after eating the experimental diets for 21 d (Mean values and standard deviations)

1/150, 1 g DHA/100 g dry weight and 150 mg vitamin E/100 g dry weight diet; 5/300, 5 g DHA/100 g dry weight and 300 mg vitamin E/100 g dry weight diet; 5/300+Se, 5 g DHA/100 g dry weight and 300 mg vitamin E/100 g dry weight diet supplemented with Se; SGR, specific growth rate.
a,b,c Mean values within a row with unlike superscript letters were significantly different (P < 0·05).
Histopathological examinations revealed the presence of lesions affecting the larval axial musculature. These lesions showed the typical features of necrotic degeneration of muscle, characterised by marked eosinophilia, loss of striations and adjacent nucleus. The incidence of muscular lesions increased with an increase in DHA dietary content (Table 5). However, inclusion of Se was shown to reduce this incidence to almost half.
In semithin sections, more detailed features of these muscular lesions could be observed. In the severely damaged fibres, a coagulation of the muscular proteins could be observed as a darkening of the surrounding fibres due to hypercontraction (Fig. 1(a)). In initial, mild stages of the condition, an increase in the presence of vacuoles within the fibres was observed, together with the loss of the shape of muscular fibres, alteration of sarcoplasmic membranes and variation in the diameter of fibres (Fig. 1(b)).

Fig. 1 Semithin micrographs of (a) longitudinal and (b) transversal sections (400 × ) from larvae fed the diet 5/300 (5 g DHA/100 g dry weight and 300 mg vitamin E/100 g dry weight) showing (a) coagulation of muscular proteins in the affected fibre ( → ) and hypercontraction of the surrounding muscular fibres (*). (b) Mild affected fibres showed loss of the polyhedral structure, abundant vacuoles (*) and dilatation of sarcoplasmic membranes ( → ).
Transmission electron microscopy showed muscle degeneration with the presence of hydropic and autophagic vacuoles, considered secondary lysosomes, within some affected fibres in the larvae fed the 5/300 diet (Fig. 2(a)). Altered mitochondria, observed as swollen double membrane organelles, were seen in the affected fibres and presented loss of the cristae (Fig. 2(a)) in contrast to the normal ones (Fig. 2(b)). Additionally, satellite cells were observed to be attached to the existing damaged muscle fibres under the basal lamina (Fig. 2(c)).

Fig. 2 Electromicrographs of transversal sections of sea bass larvae fed the diet 5/300 (5 g DHA/100 g dry weight and 300 mg vitamin E/100 g dry weight). (a) Damaged muscle fibre showing autophagic (AV) and hydropic vacuoles (HV) and swollen mitochondria (arrow; 8000 × ). (b) Unaffected fibre where normal mitochondria can be observed ( → ; 8000 × ). (c) Presence of a satellite cell (SC) with a mitochondrion (*) between two damaged muscle fibres, with the presence of vacuoles and degenerated mitochondria ( → ; 5000 × ). MF, normal myofilaments; disMF, disarrayed myofilaments.
The general pattern of antioxidant enzyme gene expression in all groups of sea bass larvae was characterised by a rapid increase between 14 and 26 dph, followed by a decrease back to the levels slightly higher than those observed at 14 dph, by 29 dph (Fig. 3(a)–(c)). The only exception to this trend was observed in GPX gene expression, where sea bass larvae fed the 1/150 diet had a lower mRNA level of this enzyme at 35 dph in comparison with that at 14 dph (Fig. 3(c)). CAT gene expression was higher in the larvae fed the diets containing a high content of DHA at all sampling points, although no statistical differences were observed (Fig. 3(a)). However, GLM analysis showed differences between all the three treatments (P = 0·001; Fig. 3(a); Table 6). The SOD mRNA copy number was significantly higher (P = 0·004) at 35 dph in the 5/300 larvae (Fig. 3(b)). GPX expression level was highest in the larvae fed the diets containing a high level of DHA compared with larvae fed low DHA levels. Nevertheless, the larvae fed the diets supplemented with Se showed a lower number of GPX mRNA copies, comparable with the larvae fed the 1/150 diet (Fig. 3(c)).

Fig. 3 (a) Catalase (CAT), (b) superoxide dismutase (SOD), (c) glutathione peroxidase (GPX), (d) insulin-like growth factor I (IGF-I), (e) insulin-like growth factor II (IGF-II) and (f) myosin heavy chain (MyHC) expression levels measured by real-time PCR in Dicentrarchus labrax larvae when fed the diets 1/150 (♦; 1 g DHA/100 g dry weight and 150 mg vitamin E/100 g dry weight), 5/300 (■; 5 g DHA/100 g dry weight and 300 mg vitamin E/100 g dry weight) or 5/300+Se (●; 5 g DHA/100 g dry weight and 300 mg vitamin E/100 g dry weight supplemented with Se). mRNA copy number of each gene was normalised as a ratio to 100 ng total RNA. Values are means, with standard deviations represented by vertical bars. a,b Mean values with unlike letters were significantly different in gene expression among the treatments at given sampling points.
Table 6 Effects of the dietary treatment, time and their interaction on the global gene expression

D, diet; T, time; CAT, catalase; SOD, superoxide dismutase; GPX, glutathione peroxidase; IGF-I, insulin-like growth factor I; IGF-II, insulin-like growth factor II; MyHC, myosin heavy chain.
*P ≤ 0·05, **P ≤ 0·01.
Regarding the IGF genes, IGF-I mRNA copy number increased from 14 to 26 dph in all treatments, showing a decrease at day 35 in the 1/150 and 5/300+Se larvae. In contrast, the 5/300 larvae showed an increasing IGF-I expression levels throughout the experimental trial, with significantly higher levels at 35 dph (P = 0·006; Fig. 3(d)). No significant differences were observed among the treatments taking into account the whole experimental period (Fig. 3(d); Table 6). The mRNA levels of IGF-II followed a similar increasing pattern in the 5/300 and 5/300+Se larvae from 14 to 26 dph, in contrast to the 1/150 larvae. At 26 dph, the 5/300+Se larvae showed a marked decrease in IGF-II expression, whereas in the 5/300 larvae, a steady increase could be observed. In the larvae fed the 1/150 diet, a decrease in IGF-II expression could be observed at all sampling points (Fig. 3(e)).
MyHC expression levels were elevated in the larvae fed the 5/300 diet, with a lower expression of this gene mRNA copy number was found at 26 dph, when Se was included in the diet (P = 0·007). At 35 dph, no statistical differences were observed when Se was added to the 5/300 diet, with the lowest values in the 1/150 larvae (Fig. 3(f)). GLM analysis showed significant differences between the 5/300 larvae and the larvae fed the other dietary treatments (P = 0·001; Fig. 3(f); Table 6).
Interactions were found in the gene expression between dietary treatment and time within the experimental trial for SOD and IGF-I (Table 6), indicating that the increase in the expression of these genes may be induced for the larval stage and the effect of the diet. However, IGF-I showed no differences in the expression among the treatments or during the whole experimental trial (Table 6).
Discussion
The aim of the present study was to evaluate the oxidative status of sea bass larvae when Se and vitamin E were included in the diet, at high DHA level inclusion. Previously, Betancor et al. ( Reference Betancor, Atalah and Caballero 12 , Reference Betancor, Caballero and Benítez-Santana 24 ) provided evidence of the appearance of muscular dystrophy in sea bass larvae when fed high DHA, implying that an excessive production of free radicals was present. Furthermore, the same authors showed that an increase in vitamin E alone could not prevent its adverse effects. Se and vitamin E have different but complementary biochemical functions which may allow these nutrients to interact physiologically( Reference Combs and Scott 47 ).
Se contents in the 5/300+Se diet were adjusted according to the levels found in copepods( Reference Hamre, Srivastava and Rønnestad 48 , Reference Van der Meeren, Olsen and Hamre 49 ), natural live prey of marine fish larvae, although they were higher than those recommended by the National Research Council( 50 ) for juveniles of other fish species. Since marine fish larvae have a rapid growth rate, it is possible that they may well have a higher requirement than the juveniles which have been used in most of the requirement studies quoted by the National Research Council. In addition, the Se level did not seem to be excessive, as the larvae fed this diet did not show reduced growth in comparison with the larvae fed the same vitamin E and DHA contents. Reduced growth is one of the first symptoms occurring when excessive levels of Se are fed to fish( Reference Penglase, Nordgreen and Van der Meeren 11 , Reference Lin and Shiau 21 , Reference Hilton, Hodson and Slinger 51 – Reference Jaramillo, Peng and Gatlin 53 ). Moreover, the Se source used in the present study was derived from yeast, making it less likely to be toxic as Se toxicity is highly dependent on its speciation( Reference Tinggi 54 ), with mineral Se being more toxic than organic Se( Reference Hilton, Hodson and Slinger 51 , Reference Rider, Davies and Jha 55 ).
A dose-dependent effect of dietary vitamin E on larval tissue concentration was not observed in the present study, with the highest vitamin E content found in the larvae fed the lowest level of vitamin E (150 mg/100 g DW). These results are in contrast with previous reports where vitamin E concentrations in fish were linked to dietary input( Reference Kiron, Puangkaew and Ishizaka 56 , Reference Puangkaew, Kiron and Satoh 57 ). It is also noteworthy that vitamin E levels were influenced by the dietary DHA ratio, being lower in the larvae fed the diets containing the higher amount of DHA (5/300 diet). This indicates that more vitamin E was being utilised as an antioxidant in the larvae fed higher DHA levels to protect tissue lipids from an increased oxidation risk. Consequently, α-tocopherol was accumulated at lower amounts in larval tissues. These results match those of previous reports where vitamin E concentration in juvenile or adult fish was lower when high contents of n-3 LC-PUFA were included in the diets( Reference Puangkaew, Kiron and Satoh 57 ). In addition, the low DHA retention rate observed in the 5/300 larvae could probably be due to the high in vivo lipid oxidation induced by this fatty acid.
In agreement with this, a dietary DHA increase resulted in higher levels of MDA, whereas Se supplementation improved protection against peroxidation by decreasing TBARS values. Moreover, Se incorporation rate was very low in the 5/300+Se larvae (42·26 %) in contrast to the 1/150 (100 %) and 5/300 (83·46 %) larvae, suggesting that this mineral was being used at the active sites of the antioxidant enzyme GPX( Reference Felton, Landolt and Grace 15 ). The synergism between tocopherol and Se has previously been observed in rainbow trout (Oncorhynchus mykiss) and salmon (Salmo salar) using diets deficient in vitamin E, Se or both( Reference Poston, Combs and Leibovitz 17 , Reference Bell and Cowey 18 ). In the present study, tocopherol levels were high enough to avoid a deficiency in this nutrient, suggesting that vitamin E addition as the sole antioxidant is not sufficient enough to control lipid peroxidation when high levels of DHA are included in fish larval diets. Therefore, the role of tocopherol as an effective antioxidant depends on the extent of oxidative stress in fish and is thus related to the degree of unsaturation of dietary fatty acids.
The availability of the literature on the activities of AOE in fish is mainly focused on pollutant detoxification( Reference Ji, Li and Liu 58 , Reference Kim, Ji and Lee 59 ) or developmental aspects( Reference Mourente, Tocher and Díaz 9 , Reference Peters and Livingstone 60 ). A few reports exist concerning the effect of dietary components on their activity and gene expression during early developmental stages of marine fish larvae( Reference Tovar-Ramírez, Mazurais and Gatesoupe 61 ). The results from the present study demonstrate that there is an increase in the expression of specific antioxidant genes in sea bass larvae exposed to oxidative stress in order to neutralise the generated ROS. Moreover, when sea bass larvae were exposed to high dietary DHA contents (5 %), the induction of antioxidant enzyme genes coincided with increases in MDA levels. Accordingly, studies in Manchurian trout (Brachymystax lenok) larvae( Reference Zhang, Mu and X 62 ) revealed that high dietary lipid levels produced elevated MDA levels, inducing an antioxidant response noticeable by an increase in the activity of AOE.
In the present study, an initial increase in the expression of each AOE was observed in all treatments at 26 dph, including the control group. Fernández-Díaz et al. ( Reference Fernández-Díaz, Kopecka and Canavete 63 ) found that the administration of inert diets to Solea senegalensis larvae produced increased CAT and SOD activity compared with larvae fed with Artemia. Therefore, the observed initial increase in expression could be due to the use of inert food.
In an attempt to dismutate superoxide anions and to decompose H2O2, increases in SOD, CAT and GPX expression were detected in fish larvae fed a high-DHA and Se-free diet. Similarly, exposure to high-DHA diets caused a significant increase in CAT and GPX in the larvae fed the Se-supplemented diets. Given that increases in SOD activity were less significant in Se-supplemented larvae, it can be concluded that H2O2 formation declined or that CAT activity was sufficient to remove H2O2. These results agree with Monteiro et al. ( Reference Monteiro, Rantin and Kalinin 64 ) who observed that Se supplementation had a protective effect against oxidative stress caused by methyl parathion in Brycon cephalus, as denoted by a decrease in CAT and SOD activity. In contrast with these authors, Se supplementation did not increase the GPX level, or decreased GPX caused by methyl parathion. In mammals, it is likely that maintaining the activity of known selenoproteins, including GPX, is not the mechanism by which Se acts since it appears to be saturated at normal nutritional intakes. Thus, supranutritional levels of Se are required to reduce the incidence of human and animal diseases( Reference Brown and Arthur 65 ). Therefore, it appears that other selenoproteins could be implicated in tissue antioxidant defence mechanisms. Among all selenoproteins, selenoprotein P seems to play an important role as an antioxidant defence in mammals( Reference Burk, Hill and Boeglin 66 ) by associating with endothelial cells. Previous studies in zebrafish (Danio rerio) indicate that selenoprotein P is utilised to a larger extent than in human subjects, as it is encoded by two genes and has seventeen selenocysteine residues, the largest number of selenocysteine residues found in any known protein( Reference Tubajeva, Ransom and Harney 67 , Reference Kryukov and Gladyshev 68 ). In consequence, the action of selenoprotein P or any other selenoproteins could be critical as an antioxidant defence against lipid H2O2 in sea bass larvae when Se requirements are covered. Thus, further studies are required to clarify the antioxidant mode of Se action in marine fish larvae.
As observed in our previous studies( Reference Betancor, Atalah and Caballero 12 , Reference Betancor, Caballero and Benítez-Santana 24 ), high dietary DHA levels caused pathological changes in sea bass larvae muscle. However, in the present study, inclusion of Se proved to be efficient in controlling the damage caused by ROS, reducing the incidence of muscle injury to almost half as compared with the 5/300 diet. Moreover, certain properties of muscle may render it especially susceptible to ROS injury( Reference Rando 1 ). For instance, muscle is prone to oxidative injury as a result of increased electron flux due to its requirement and ability to undertake rapid and coordinated changes in energy supply and oxygen flux during contraction( Reference Haycock, Jones and Harris 69 ). There is also a very high concentration of myoglobin in the muscle, and it is known that such a haem-containing protein may confer greater sensitivity to free radical-induced damage by the conversion of H2O2 to a more reactive species( Reference Ostdal, Skibsted and Andersen 70 ). Furthermore, the requirement of skeletal muscle membranes for phospholipids containing large proportions of PUFA may render those membranes particularly susceptible to oxidative stress( Reference Murphy and Kehrer 71 ). Finally, low Se accumulation in muscular tissues will make this tissue more susceptible to oxidative damage( Reference Monteiro, Rantin and Kalinin 64 , Reference Elia, Prearo and Pacini 72 ). However, interaction of LC-PUFA with other cellular components should be taken into account to complete the scenario. In this sense, studies using juvenile salmon fed with high-EPA and DHA diets showed loss of mitochondrial β-oxidation, reduced lipid deposition and apoptosis in white adipose tissue, indicating that high supplementation rates of these LC-PUFA may lead to oxidative stress( Reference Todorčević, Kjær and Djaković 73 ).
IGF-I and IGF-II are polypeptides well known for promoting proliferation and differentiation in many vertebrates with nutritional status having a profound effect on the IGF system in fish( Reference Duan 74 ). However, most nutritional studies have focused on the effects of food restriction( Reference Terova, Rimoldi and Chini 32 , Reference Hevrøy, Azpeleta and Shimizu 33 , Reference Moriyama, Swanson and Nishi 75 ), dietary protein or carbohydrate content( Reference Enes, Sánchez-Gurmaches and Navarro 76 , Reference Pérez-Sánchez, Martipalanca and Kaushik 77 ) and probiotics( Reference Carnevali, de Vivo and Sulpizio 78 ), whereas little information is known about the effect of lipids on this system. Moreover, no information exists about the effect of oxidative stress on IGF in fish larvae. In the present study, an increase in IGF-I and IGF-II in the larvae fed the 5/300 diet was observed, especially when no Se was added, suggesting oxidative stress may play a role in the expression of these growth factors. Accordingly, the larvae fed the highest content of DHA showed a higher incidence of muscular lesions and the presence of abundant satellite cells. Satellite cells are able to regenerate damaged muscle by forming new myofibres by fusing to existing muscle fibres or fusing together( Reference Schultz and McCormick 79 ). It is known that to control the satellite cell population, growth factors are required( Reference Seale and Rudnicki 80 ). In mammals, IGF-I appears to utilise multiple signalling pathways in the regulation of the satellite cell pool such as the mitogen-activated protein or phosphatidylinositol-3-OH kinase( Reference Coolican, Samuel and Ewton 81 , Reference Semsarian, Wu and Ju 82 ). In agreement with this, Pozios et al. ( Reference Pozios, Ding and Degger 83 ) showed that IGF-II and IGF-I potently activate cell proliferation and DNA synthesis in embryonic zebrafish cells via mitogen-activated protein and phosphatidylinositol-3-OH kinase, suggesting that the increase in IGF-I mRNA copies observed in the present study in the larvae with the highest incidence of muscular lesions could be due to the regeneration process carried out by satellite cells. Similarly, the mitogenic effect of IGF in fish has also been described in cultured muscle cells from rainbow trout( Reference Castillo, Codina and Martínez 84 ).
Late markers of myogenesis include the myofibrillar protein MyHC. By monitoring the expression patterns of this marker gene, the effect that nutritional status has on muscle growth can be determined( Reference Bower, Li and Taylor 29 ). Regeneration of fish muscle has only rarely been described. Rowlerson et al. ( Reference Rowlerson, Radaelli and Mascarello 85 ) demonstrated a vigorous regeneration in juvenile sea bream (Sparus aurata) after mechanical injury, with myosin expression in regenerating fibres resembling that seen in newly produced fibres in post-larval white muscle. In the present study, a higher expression of myosin was observed in the 5/300 larvae, especially when Se was not added to the diets with a positive correlation observed between the incidence of muscular lesions and myosin mRNA copies at 35 dph (y = 2 × 10− 6 x − 29·567; R 2 0·9133).
In the present study, the high levels of AOE and MDA content observed in the 5/300 larvae demonstrate an adaptive response in attempting to neutralise the generated ROS. Moreover, a reactive response was observed by the increase in IGF and MyHC expression in larval tissues, suggesting regenerative processes in the injured muscle. Organic Se proved to enhance the cell antioxidant capacity, protecting muscle, as shown by the decrease in the incidence of muscular lesions, MDA content and AOE expression. Therefore, when high levels of LC-PUFA are included in sea bass larvae microdiets, an adequate combination of dietary α-tocopherol and Se must be included to avoid the appearance of oxidative stress in larval tissues and favour culture performance.
Acknowledgements
M. B. B. was supported by a grant given by the Cabildo Insular de Gran Canaria. The present study was funded by the Spanish Ministry of Sciences and Education (AGL2009-14661). The authors are thankful to Dr Matthew Sprague for his assistance in proof reading the manuscript. Acknowledgments are due to Instituto de Acuicultura de Torre la Sal that kindly provided the sea bass eggs, Croda Company and Alltech that provided DHA and EPA 50 and the Se yeast, respectively. The authors disclose no conflicts of interest. M. B. B. carried out the experiment, performed all analyses, participated in the interpretation of the results and drafted the manuscript. M. J. C. and M. I. participated in the animal experimental design, interpretation of the results and the preparation of the manuscript. R. S. and T. B.-S. participated in the animal feeding and sampling. E. A. collaborated to formulate and prepare the experimental microdiets. G. T. supervised the molecular biology analysis and J. G. B. supervised the vitamin E and Se analysis.











