Highlights of this study
We provided a systematic and updated evaluation of n-3 PUFA supplementation on LV remodelling in patients with CHF.
The benefits of n-3 PUFA mediated LVEF improvement became more prominent when the accumulated dosage reached 600 g.
n-3 PUFA supplementation reduced the levels of pro-inflammatory mediators including TNF-α, IL-6 and Hs-CRP.
Chronic heart failure (CHF) is a clinical syndrome characterised by insufficient cardiac function, representing one of the largest contributors to mortality worldwide. The development of CHF arises from a molecular and cellular transformation termed ‘ventricular remodelling’, which is featured by geometrical changes in the overall left ventricular (LV) shape and depression of LV ejection fraction (LVEF)(Reference Bhatt, Ambrosy and Velazquez1). Ventricular remodelling is the main pathological basis of the occurrence and development of CHF and is a decisive factor affecting the morbidity and mortality of CHF. Heart failure (HF) patients can be categorised by LVEF (including HF with reduced ejection fraction (EF) (HFrEF), HF with preserved EF (HFpEF), HF with borderline EF), and approximately 50 % of cases are HFrEF(Reference Yancy, Jessup and Bozkurt2). Chronic inflammation is a key process in the pathophysiology of CHF. A number of pro-inflammatory cytokines have been implicated in the pathogenesis of HF including TNF-α, interferon-γ, IL-1β, IL-6, IL-17 and IL-18(Reference Shirazi, Bissett and Romeo3). For example, increasing circulating levels of TNF-α and IL-6 can weaken LV function and promote LV remodelling in a multicentre clinical trial of HF patients(Reference Moertl, Hammer and Steiner4). A research has showed the negative inotropic effect of IL-1β, and currently IL-1β blocking agents are applied to treat HF states(Reference Van Tassell, Seropian and Toldo5). Although current treatments can improve the syndrome to some extent, CHF remains a worsening global problem especially in ageing populations(Reference Ziaeian and Fonarow6). Therefore, it is vital to explore effective prevention to block or slow down the progression of CHF.
Recommendations for the use of n-3 PUFA supplementation are included in several guidelines for the prevention of CHD(Reference Kris-Etherton, Harris and Appel7–Reference Krauss, Eckel and Howard9). Previous epidemiological researches found that supplementation of n-3 PUFA may prevent the development and progression of CHF(Reference Mozaffarian, Bryson and Lemaitre10,Reference Yamagishi, Iso and Date11) . A study showed that inflammatory cytokines (TNF-α, IL-1β) can reduce cardiac function and increase cardiac fibrosis to advance cardiac remodelling of HF patients(Reference Van Linthout and Tschöpe12), and n-3 PUFA reduced the levels of inflammation factors to prevent abnormal LV remodelling. In the GISSI-HF trial, 1 g daily n-3 PUFA therapy provided a small but statistically significant improvement in LVEF and further reduced the mortality of HF by 9 % after 3·9 years follow-up(Reference Tavazzi, Maggioni and Marchioli13,Reference Ghio, Scelsi and Latini14) . A meta-analysis performed in 2012 demonstrated that fish oil increased the LV systolic function rather than diastolic function in non-ischaemic HF patients(Reference Xin, Wei and Li15). The OMEGA-REMODEL trial suggested that dietary supplementation of high-dose n-3 PUFA reduced LV remodelling and inflammatory biomarkers(Reference Heydari, Abdullah and Pottala16). However, other clinical trials reported that n-3 PUFA supplementation provides less beneficial cardiovascular outcomes on patients(Reference Roncaglioni and Massimo17–Reference Kromhout, Giltay and Geleijnse19). For example, researchers failed to find a protective effect for fish intake in the prevention of HF in the population-based Rotterdam Study(Reference Dijkstra, Brouwer and van Rooij20). Thus, the current meta-analysis aimed to provide a systematic and updated evaluation of n-3 PUFA supplementation on LV remodelling in patients with CHF.
Methods
We implemented a systematic review and meta-analysis according to the Quality of Reporting of Meta-analyses (QUOROM) guidelines in all stages(Reference Moher, Cook and Eastwood21). The protocol of our study was registered in the PROSPERO database: CRD42020154553.
Literature search strategy and selection criteria
We performed a search through Pubmed, Clinical key and Web of Science up to January 1 in 2021 and the search terms were (n-3 OR n-3 fatty acids OR n-3 Fatty Acids OR n-3 Polyunsaturated Fatty Acid OR n-3 PUFA OR n-3 Oils) AND (cardiac function or heart function) AND (clinical trials). J.L. and Q.S.M independently screened all eligible citations including titles, abstracts, and full texts and references when necessary.
Eligible studies were included as the following criteria: (a) non-repetitive clinical trials of n-3 PUFA supplementation (both dietary supplements and capsules of n-3); (b) participants were patients who were diagnosed as CHF; (c) provided information about cardiac function and (d) English language publications.
Date extraction
We extracted the following data from each of the included studies: the first author, the journal, publication year, country, age, male number of participants, aetiology of patients (dilated cardiomyopathy (DCM), ischaemic cardiomyopathy (ICM), ICM/DCM), BMI, daily dosage of n-3 PUFA, duration, total dosages of n-3 PUFA, the original values at baseline and the end of the trials including LVEF, LV end systolic volume/diameter (LVESV/LVESD), LV end diastolic volume/diameter (LVEDV/LVEDD) and circulating inflammatory mediators including TNF-α, IL-6 and hypersensitive c-reactive protein (Hs-CRP) (mean values and standard deviations). Total dosages were calculated as (daily dosage) × (total number of days at the time of examination) (one month was equivalent to 30 d). The outcomes were assessed by the changes in LVEF, LVESV, LVEDV, LVESD, LVEDD, TNF-α, IL-6 and Hs-CRP, from the baseline to the end of intervention. The sd changes of outcomes were calculated by averaging the placebo sd and n-3 PUFA intervention sd. For the LV remodelling indices, including LVEF, LVESV, LVEDV, LVESD, LVEDD, TNF-α, IL-6 and Hs-CRP, most of the literature provided the mean values and standard deviations at baseline and the end of the intervention. However, some studies only reported variables in the form of median and interquartile range. In such case, mean values and standard deviations were converted with the information of median, interquartile range and sample size using an estimation method published by Hozo and colleagues(Reference Hozo, Djulbegovic and Hozo22). In Hozo’s paper, the median itself is the best estimator for mean when the sample size exceeds 25, which is the case for the included studies in our paper. And the sd was estimated using the formula: (1) 15 < sample size ≤ 70, sd = (Max–Min)/4; (2) sample size > 70, sd = (Max–Min)/6(Reference Hozo, Djulbegovic and Hozo22).
Quality assessment of included studies
The quality of all included randomised controlled trials (ten trials) was assessed by authors using the Cochrane Collaboration’s Tool and the Revised JADAD’s Scale. On the Cochrane Collaboration’s Tool, seven specific aspects were addressed and the judgement was expressed by low, unclear and high. The Revised JADAD’s Scale assessed the risk of bias and scored by 0, 1 and 2. Additionally, the Newcastle–Ottawa Scale analysed and scored the prospective studies.
Statistical analyses
The primary study outcomes were assessed by the changes of LV remodelling indices including LVEF, LVESV, LVEDV, LVESD and LVEDD, and circulating inflammatory mediators including TNF-α, IL-6 and Hs-CRP in both n-3 PUFA treated and placebo groups, from the baseline to the end of intervention. All statistical analyses were conducted using the statistical software STATA software, version 12.0 (StataCorp LP). For the continuous variables, the pooled effects were presented as weighted mean difference (WMD) with 95 % CI. I 2 test was used to evaluate the clinical heterogeneity, and I 2 ≥ 50 % indicated obvious heterogeneity(Reference Higgins, Thompson and Deeks23). The random-effects model was used to assess the pooled data considering both intra and interstudy variations. A forest plot was conducted to show the relationship between n-3 PUFA and LV remodelling indices. Sensitivity analysis was performed to determine the reliability of the data by sequentially eliminating each of the included studies. Publication bias was measured using a Begg and Egger regression asymmetry test.
Results
Selection of studies
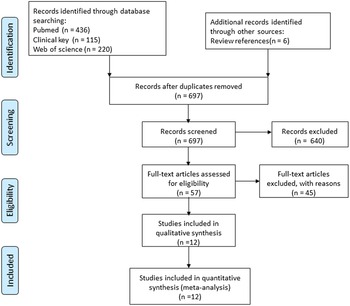
The search strategy resulted in 777 articles for consideration in the present meta-analysis. After removing duplicated eighty records, the titles and abstracts of the remaining 697 records were further examined, and 640 records were excluded based on the inclusion criteria. A full-text examination was performed in the remaining fifty-seven studies, and twelve studies were eligible for the current analysis based on the selection criteria, of which ten studies were randomised controlled trials(Reference Moertl, Hammer and Steiner4,Reference Ghio, Scelsi and Latini14,Reference Heydari, Abdullah and Pottala16,Reference Chrysohoou, Metallinos and Georgiopoulos24–Reference Kojuri, Ostovan and Rezaian30) , and two studies were prospective studies(Reference Kohashi, Nakagomi and Saiki31,Reference Campos-Staffico, Costa and Carvalho32) . The flow diagram of the initial literature search and trial selection is shown in Fig. 1.

Fig. 1. Flow diagram of the systematic review and meta-analysis.
Baseline characteristics
Baseline characteristics of included trials are summarised in Table 1. A total of 2162 participants were included, with age (years) range from 55·1 to 74·0. The twelve trials were variously performed in the eastern (China(Reference Zhao, Shao and Teng27), Iran(Reference Kojuri, Ostovan and Rezaian30), Japan(Reference Kohashi, Nakagomi and Saiki31)) and the western (Denmark(Reference Skou, Toft and Christensen25), Italy(Reference Ghio, Scelsi and Latini14,Reference Radaelli, Cazzaniga and Viola26,Reference Nodari, Metra and Milesi28,Reference Nodari, Triggiani and Campia29) , Austria(Reference Moertl, Hammer and Steiner4), USA(Reference Heydari, Abdullah and Pottala16), Brazil(Reference Campos-Staffico, Costa and Carvalho32), Greece(Reference Chrysohoou, Metallinos and Georgiopoulos24)) countries. Baseline and follow-up LVEF scores were available in eleven studies. In the eleven clinical trials, nine studies were diagnosed as HFrEF (LVEF ≤ 40 %)(Reference Moertl, Hammer and Steiner4,Reference Ghio, Scelsi and Latini14,Reference Chrysohoou, Metallinos and Georgiopoulos24,Reference Radaelli, Cazzaniga and Viola26–Reference Kohashi, Nakagomi and Saiki31) , whereas two studies were diagnosed as HFpEF (LVEF ≥ 50 %)(Reference Heydari, Abdullah and Pottala16,Reference Campos-Staffico, Costa and Carvalho32) . Changes in LVESV(Reference Ghio, Scelsi and Latini14,Reference Skou, Toft and Christensen25,Reference Nodari, Metra and Milesi28–Reference Kojuri, Ostovan and Rezaian30) and LVEDD(Reference Ghio, Scelsi and Latini14,Reference Chrysohoou, Metallinos and Georgiopoulos24,Reference Zhao, Shao and Teng27,Reference Nodari, Triggiani and Campia29,Reference Kojuri, Ostovan and Rezaian30) were available in five trials. LVEDV(Reference Ghio, Scelsi and Latini14,Reference Skou, Toft and Christensen25,Reference Nodari, Triggiani and Campia29,Reference Campos-Staffico, Costa and Carvalho32) and LVESD(Reference Higgins, Thompson and Deeks23,Reference Zhao, Shao and Teng27,Reference Nodari, Triggiani and Campia29,Reference Kojuri, Ostovan and Rezaian30) data were evaluated in four studies. TNF-α (Reference Moertl, Hammer and Steiner4,Reference Zhao, Shao and Teng27–Reference Nodari, Triggiani and Campia29,Reference Kohashi, Nakagomi and Saiki31) , IL-6(Reference Moertl, Hammer and Steiner4,Reference Zhao, Shao and Teng27–Reference Nodari, Triggiani and Campia29) and Hs-CRP(Reference Moertl, Hammer and Steiner4,Reference Zhao, Shao and Teng27,Reference Kohashi, Nakagomi and Saiki31,Reference Campos-Staffico, Costa and Carvalho32) were evaluated from data extracted from 5, 4, 4 studies, respectively. Eleven trials (1804 participants) assessed cardiac function using echocardiography, while 1 study (358 participants)(Reference Heydari, Abdullah and Pottala16) used cardiac MRI (cMRI). The aetiology of CHF participants was classified as ICM in four studies(Reference Heydari, Abdullah and Pottala16,Reference Radaelli, Cazzaniga and Viola26,Reference Kojuri, Ostovan and Rezaian30,Reference Campos-Staffico, Costa and Carvalho32) , DCM in three studies(Reference Moertl, Hammer and Steiner4,Reference Nodari, Metra and Milesi28,Reference Nodari, Triggiani and Campia29) and both ICM and DCM in four studies(Reference Ghio, Scelsi and Latini14,Reference Chrysohoou, Metallinos and Georgiopoulos24,Reference Zhao, Shao and Teng27,Reference Kohashi, Nakagomi and Saiki31) , respectively.
Table 1. Baseline characteristics of included trials are summarised
(Mean values and standard deviations; numbers and percentages)

DCM, dilated cardiomyopathy; ICM, ischaemic cardiomyopathy; N/A, not applicable.
Administration details of n-3 PUFA and placebo groups
In all included studies, the daily dosage of n-3 PUFA varied from 1 to 5·2 g/d, with duration ranged from 3 to 12 months. The daily dosage of EPA ranged between 360 and 1860 mg and DHA ranged from 240 to 1500 mg, compared with a recommended dietary intake of 250–2000 mg/d for each(33). Eleven trials evaluated the combined effect of n-3 PUFA supplementation(Reference Moertl, Hammer and Steiner4,Reference Ghio, Scelsi and Latini14,Reference Heydari, Abdullah and Pottala16,Reference Chrysohoou, Metallinos and Georgiopoulos24–Reference Kojuri, Ostovan and Rezaian30,Reference Campos-Staffico, Costa and Carvalho32) while one trial assessed independent effect of EPA(Reference Kohashi, Nakagomi and Saiki31). The placebo composition included olive oil(Reference Skou, Toft and Christensen25,Reference Nodari, Metra and Milesi28,Reference Nodari, Triggiani and Campia29) , linoleic acid(Reference Heydari, Abdullah and Pottala16) or no pills(Reference Higgins, Thompson and Deeks23). The supplements of placebo groups were not mentioned in the remaining seven studies(Reference Moertl, Hammer and Steiner4,Reference Ghio, Scelsi and Latini14,Reference Radaelli, Cazzaniga and Viola26,Reference Zhao, Shao and Teng27,Reference Kojuri, Ostovan and Rezaian30–Reference Campos-Staffico, Costa and Carvalho32) .
Study quality assessment
The quality assessment of randomised controlled trials was analysed by Cochrane Collaboration’s Tool and generally of good quality (online Supplementary Fig. S1). In the Revised JADAD’s Scale, four studies scored 4(Reference Skou, Toft and Christensen25–Reference Nodari, Metra and Milesi28), four studies scored 5(Reference Ghio, Scelsi and Latini14,Reference Chrysohoou, Metallinos and Georgiopoulos24,Reference Nodari, Triggiani and Campia29,Reference Kojuri, Ostovan and Rezaian30) and the other two studies scored 7(Reference Moertl, Hammer and Steiner4,Reference Heydari, Abdullah and Pottala16) (online Supplementary Table S1a). The two prospective studies were assessed by the Newcastle–Ottawa Scale, and the result showed one study scored 7(Reference Kohashi, Nakagomi and Saiki31) while the other scored 6(Reference Campos-Staffico, Costa and Carvalho32) (online Supplementary Table S1b).
Effects of n-3 PUFA supplementation on ventricular remodelling
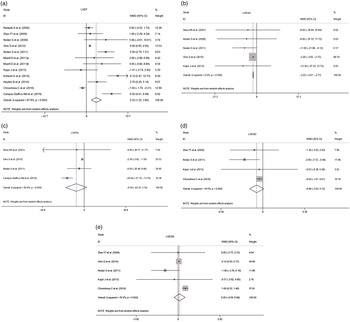
Compared with placebo groups, n-3 PUFA supplementation improved LVEF by a WMD of 2·52 (95 % CI 1·25, 3·80, I 2 = 87·8 %, Fig. 2(a)). Additionally, n-3 PUFA supplementation significantly decreased LVESV (WMD = –3·22, 95 % CI –3·67, –2·77, I 2 = 0·0 %, Fig. 2(b)). The pooled results indicated that the differences were not statistically significant for LVEDV (Fig. 2(c)), LVESD (Fig. 2(d)) or LVEDD (Fig. 2(e)). The I 2 value for studies assessing LVEF changes was 87·8 %, indicating a significant heterogeneity across the studies.

Fig. 2. Forest plot indicated the effects of n-3 PUFA supplementation on cardiac function. (a) Effect of n-3 PUFA supplementation on LVEF. (b)–(e) Effect of n-3 PUFA supplementation on LVESV, LVEDV, LVESD and LVEDD. I 2 indicated the degree of studies heterogeneity; WMD, weighed mean difference; LVEF, left ventricular ejection fraction; LVESV/LVESD, left ventricular end systolic volume/diameter; LVEDV/LVEDD, left ventricular end diastolic volume/diameter.
Next, the subgroup analysis was conducted to explore the sources of the heterogeneity (Table 2). In American College of Cardiology and American Heart Association guidelines, HF patients can be categorised by LVEF, including HFpEF (LVEF ≥ 50 %), HF with borderline EF (LVEF 41 % to 49 %) and HFrEF (LVEF ≤ 40 %)(Reference Yancy, Jessup and Bozkurt2). In the current study, LVEF improved by 2·02 % in HFrEF patients taking n-3 PUFA (WMD = 2·02, 95 % CI 0·70, 3·34, I 2 = 87·0 %), while it increased by 4·59 % in HFpEF group (WMD = 4·59, 95 % CI 0·87, 8·31, I 2 = 78·1 %). In the aetiology subgroup, the LVEF was prominently increased in DCM patients (WMD = 4·49, 95 % CI 2·72, 6·26, I 2 = 0·0 %), while no significant improvement could be observed in the ICM and ICM/DCM subgroups. Subgrouping according to BMI showed no significant improvement in LVEF in either normal weight patients (BMI = 18·5–24·9 kg/m2) or pre-obesity patients (BMI = 25·0–29·9 kg/m2). Based on the regions, results from studies in the western countries revealed a significant association between improvement in LVEF and n-3 PUFA intake (WMD = 2·19, 95 % CI 0·92, 3·47, I 2 = 86·8 %).
Table 2. Subgroup analysis of n-3 PUFA in CHF patients
(Numbers; 95 % confidence intervals)

WMD, weighted mean difference; LVEF, left ventricular ejection fraction.
Dosage accumulation effect of n-3 PUFA on left ventricular ejection fraction improvement
Further subgroup analysis was performed to examine whether the efficacy on LVEF was associated with the dosage of n-3 PUFA supplementation (Table 2). Average daily dosage, duration and total dosages for all included studies are summarised in Table 1. Subgrouping according to average daily intake showed n-3 PUFA at a dosage of 1–3 g/d (WMD = 3·60, 95 % CI 0·86, 6·33, I 2 = 87·5 %) and ≥ 3 g/d (WMD = 2·96, 95 % CI 1·25, 3·80, I 2 = 0·0 %) had beneficial effects in LVEF, whereas ≤ 1 g/d showed no significant improvement. Duration seemed to have effects on LVEF improvement, as a trend favoured a longer n-3 PUFA duration with a better LVEF improvement. Although short duration (≤ 6 months) had no significant effects on LVEF scores, the improvement was significant in long duration (> 6 months) (WMD = 4·53, 95 % CI 0·02, 3·80, I 2 = 95·2 %) intervention subgroup. Of note, subgroup analysis according to total n-3 PUFA intake showed a dosage-dependent accumulation effect on LVEF improvement: there was no significant improvement in LVEF at a dosage of ≤ 300 g and a high dosage 300–600 g, while a much higher dosage (≥ 600 g) achieved a greater benefit in LVEF scores (WMD = 5·23, 95 % CI 2·31, 8·15, I 2 = 77·1 %).
Effects of n-3 PUFA intake on circulating inflammatory mediators
Increased secretion of circulating inflammatory mediators was associated with the pathogenesis and progression for cardiac mortality and ventricular remodelling(Reference Hamzic-Mehmedbasic34,Reference Farnsworth, Bailey and Jaffe35) . Results showed that both TNF-α (WMD = –3·48, 95 % CI –4·67, –2·30, I 2 = 97·9 %, Fig. 3(a)) and IL-6 (WMD = –3·85, 95 % CI –6·05, –1·64, I 2 = 94·2 %, Fig. 3(b)) levels significantly decreased in n-3 PUFA treated group compared with placebo groups. Hs-CRP was also decreased in n-3 PUFA treated group (WMD = –0·23, 95 % CI –0·41, –0·05, I 2 = 80·8 %, Fig. 3(c)).

Fig. 3. Forest plot of the effect of n-3 PUFA on inflammatory cytokines. (a) The effect of n-3 PUFA on TNF-α. (b) The effect of n-3 PUFA on IL-6. (c) The effect of n-3 PUFA on Hs-CRP. Hs-CRP, hypersensitive c-reactive protein.
Publication bias and sensitivity analysis
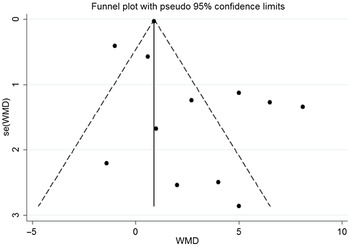
As shown in the funnel plot, there was no publication bias in the effects of n-3 PUFA on LVEF (Fig. 4). Sensitivity analysis showed that none of the studies changed the overall effect of n-3 PUFA supplements on LVEF improvements.

Fig. 4. Funnel plot of the effect of n-3 PUFA on LVEF. LVEF, left ventricular ejection fraction.
Discussion
This meta-analysis of twelve studies had several findings: first, n-3 PUFA treatment contributed to improve the LVEF and LVESV. Second, the benefits of n-3 PUFA mediated LVEF improvement seemed to be dependent on the accumulated dosage, which reflects combined effects of daily intake and duration. The benefits became more prominent when the accumulated dosage reached 600 g. Third, n-3 PUFA supplementation reduced the levels of circulating TNF-α, IL-6 and Hs-CRP. Collectively, these results supported that n-3 PUFA supplementation had a positive association with cardiac function and support its current recommendation in CHF patients(Reference Siscovick, Barringer and Fretts36).
LV remodelling is generally accepted as a critical factor in the progression of CHF. During the process of ventricular remodelling, structural and functional changes can be evaluated by a series of image examinations(Reference Aimo, Gaggin and Barison37), among which echocardiography and cMRI are most frequently used(Reference Bhat, Ashwath and Rosenbaum38). Compared with echocardiography, cMRI displays a better performance in cardiac remodelling assessment due to its clear contrast resolution and high reproducibility(Reference Barison, Grigoratos and Todiere39). However, only one study used cMRI in the current study, which demonstrated that n-3 PUFA significantly inhibited the LVESV index and myocardial fibrosis(Reference Heydari, Abdullah and Pottala16). Further large-scale studies using cMRI are needed to evaluate the effect of n-3 PUFA on cardiac remodelling.
n-3 PUFA have attracted interest as a possible addition to available lifestyle measures and medications for the prevention of CVD(Reference De Caterina40). Previous study showed the cardioprotective mechanisms of n-3 PUFA against HF, including anti-inflammatory; intervention of cardiac energy metabolism; modification of cardiac ion channels; improvement of vascular endothelial and modulation of autonomic nervous system activity(Reference Pepe and Mclennan41–Reference Sakamoto, Saotome and Iguchi43). The American Heart Association advisory board recommended n-3 PUFA supplementation (1 g/d, 2 years) in CHF patients for the secondary prevention of CVD death(Reference Siscovick, Barringer and Fretts36). Meanwhile, several animal studies provide solid evidence that n-3 PUFA supplementation not only prevents diastolic and systolic dysfunction but also improves abnormal ventricular remodelling(Reference Duda, O’Shea and Tintinu44,Reference Chen, Shearer and Chen45) . A previous meta-analysis performed in 2012 indicated n-3 PUFA supplementation of CHF patients led to a significantly increase in LVEF and a reduction in LVESV(Reference Xin, Wei and Li15), while a recent meta-analysis performed in 2016 produced inconsistent conclusions(Reference Wang, Xiong and Huang46). The current study provided an updated analysis about the effects of n-3 PUFA in CHF patients and suggested that n-3 PUFA supplementation could lead to improvement in LVEF and reduction in LVESV.
Additionally, our results showed the recovery of LVEF by n-3 PUFA supplementation in both HFpEF and HFrEF patients. Though HFpEF subgroup is relatively small (two trials), the LVEF improvement was evident. As a disease with limited evidence-based treatment options, our data support the application of n-3 PUFA supplementation in HFpEF patients. For HFrEF patients suffering from a progressive LVEF decay, n-3 PUFA supplementation could help to preserve LVEF level with slight but significant improvements. Furthermore, recent research showed that n-3 PUFA may determine long-term change in weight and the high dosage of n-3 PUFA intake can alleviate the genetic associations with changes in BMI(Reference Huang, Wang and Heianza47). However, no remarkable improvement in LVEF was found in either normal weight patients or pre-obesity patients in our study. Apart from the above discussed factors, there seemed to be regional disparity in n-3 PUFA mediated LVEF improvements. Dietary patterns in different regions might partly explain the disparity. Limited access to marine fish, which is the main dietary source of n-3 PUFA, might contribute to a severe deficiency of n-3 PUFA(Reference Lahoz, Castillo and Mostaza48). Hence, we considered that different dietary intake might contribute to the conflicting results between the western and the eastern countries. Especially, the Japan, which had large fish consumption(Reference Kohashi, Nakagomi and Saiki31), showed a favourable trend on LVEF in eastern subgroup.
n-3 PUFA can have a broad range of effects on inflammation, oxidation and stability of phospholipid membranes(Reference Nelson, True and Le49). The American and European guidelines have stated that prescription of n-3 PUFA (EPA + DHA or EPA-only) at a dose of 4 g/d is an effective and safe option for reducing TAG as monotherapy or as an adjunct to other lipid-lowering agents(Reference Skulas-Ray, Wilson and Harris50,Reference Mach, Baigent and Catapano51) . However, clinical trials reported inconsistent benefits from n-3 PUFA on cardiovascular events, even in trials using the same high dose of n-3 PUFA (4 g/d)(Reference Nicholls, Lincoff and Garcia52,Reference Bhatt, Steg and Miller53) . A proper dosage has been raised as one of the possible reasons for the contradictory results(Reference Jo, Han and Kim54,Reference Sharma, Martin and Blumenthal55) . The improvement in adverse LV remodelling during infarct convalescence remains the strongest favourable risk predictor and serves as a common mechanistic pathway that reduces mortality, sudden cardiac death and heart failure incidence(Reference White, Norris and Brown56). However, the cardiac remodelling is a long-term process and its improvement might be vague within short-term observation period, especially for the patients who received standard medical care of CHD. Our data suggested a longer intervention period of n-3 PUFA correlated with a favourable effect on LVEF. Additionally, it should be noted that the two included studies in the ≥ 3 g/d subgroup had a relative short duration (3–6 months), while five out of seven included studies in the 1–3 g/d subgroup had a longer duration (6–12 months). As both daily dose and duration might be influencing factors for the effects of n-3 PUFA, we speculated that the accumulated dosage, indicative for the two factors, might serve as a comprehensive parameter to evaluate the effects of n-3 PUFA. Though the included trials were limited, our data suggested that a sufficient accumulated dosage (≥ 600 g) was essential for n-3 PUFA mediated LVEF improvements (Table 2).
But, the high heterogeneity was the major challenge to clarify the associations of n-3 PUFA supplementation with LVEF improvements. We conducted meta-regression, sensitivity and subgroup analysis to identify potential sources of heterogeneity. The meta-regression and sensitivity analysis could not reduce the heterogeneity. Subgroup analyses revealed that several variables contributed to the heterogeneity of this meta-analysis including LVEF classification, aetiology, daily intake, duration and accumulated dosages.
Apart from structural and functional changes, evidences had shown that inflammatory response played an important role in ventricular remodelling(Reference Mishra, Srivastava and Mittal57). The induction of cytokines (such as IL-6, TNF-α) may be involved in the pathogenesis of adverse remodelling, cardiac dysfunction and ultimately HF. Persistent dysregulated inflammation response might induce cardiac hypertrophy, damage myocardial contractility and finally contribute to LV remodelling(Reference Aukrust, Yndestad and Waehre58). Clinically, patients with higher degrees of inflammation, as measured by circulating N-terminal-pro-type B natriuretic peptide and Hs-CRP, had increased morbidity and mortality(Reference Li, Chen and Gan59). Daily intake of n-3 PUFA could attenuate inflammatory response, which further prevent the progression of HF or ST-elevation MI patients(Reference Zhao, Shao and Teng27,Reference Campos-Staffico, Costa and Carvalho32,Reference Mehra, Lavie and Ventura60) . In HF patients, n-3 PUFA can reduce the circulating level of the pro-inflammatory cytokines activated by nuclear transcription factor kappa B, such as TNF-α, IL-1 and IL-6(Reference Duda, O’Shea and Stanley61). In a rat model of MI, increasing expression of IL-6, TNF-α and IL-1 in myocardium was significantly associated with increased LVEDD(Reference Ono, Matsumori and Shioi62). Our results suggested that n-3 PUFA supplementation significantly reduced the expression of inflammation cytokines including TNF-α, IL-6 and Hs-CRP. These effects might explain how n-3 PUFA supplementation attenuated the cardiac remodelling.
Limitation
Our study had some potential limitations. First, due to limited trials, the heterogeneity of LVEF scores remained significant even after stratification by the LVEF classification, aetiology, BMI and regions. Second, these were no reports about the effect of n-3 PUFA on patients with HF with borderline EF yet, so the present meta-analysis could not give any evidence on n-3 PUFA supplementation in this population. Third, other measurements and circulating inflammatory mediators such as left atrial volumes and NT-pro-B natriuretic peptide might possibly link to ventricular remodelling and need further investigation.
Conclusion
This meta-analysis demonstrated that n-3 PUFA supplementation was associated with a substantial improvement of LV function and remodelling in patients subjected to CHF. n-3 PUFA intake also decreased the levels of circulating pro-inflammatory factors in CHF patients. The accumulated dosage of n-3 PUFA consumption is vital for its cardiac protective role.
Acknowledgements
The present study was supported by the National Nature Science Foundation of China (NSFC; grant nos. 81670458, 81500207, 81470393 and 81370434), Shanghai Municipal Health and Family Planning Commission (grant nos. ZY(2018–2020)-FWTX-2007), Shanghai Key Medical Discipline for Critical Care Medicine (grant nos. 2017zz02017), The National Key Research and Development Program of China (grant nos. 2017YFA0105600), The Science and Technology Commission of Shanghai Municipality (grant nos. 17431906600), Three-year plan on TCM of Pudong Health Bureau of Shanghai (grant nos. PDZY-2018–0603) and Major Program of Development Fund for Shanghai Zhangjiang National Innovation Demonstration Zone (grant nos. ZJ2018-ZD-004).
The authors’ contributions were as follows. X. Z. and X. L. contributed to the conception and design of the study. J. L., Q. M., L. Z., P. Y., H. H., R. Z., X. G. and Z. L. contributed to the acquisition, analysis and interpretation of the data. J. L. wrote the M. S. Q. M., L. Z., P. Y., H. H., R. Z., X. G., Z. L., X. L. and X. Z. revised the manuscript. All authors read and approved the final manuscript.
The authors have no conflicting interest associated with this manuscript.
Supplementary material
For supplementary material accompanying this paper visit https://doi.org/10.1017/S0007114521004979