The metabolic syndrome (MetS) is a constellation of metabolic abnormalities that includes central obesity, low HDL concentrations, high fasting glucose and TAG concentrations, and elevated blood pressure (BP), which is fast becoming a major public health challenge(1). This combination of cardiometabolic risk factors has received an increasing level of attention due to its strong association with the development of diabetes and increased risk of cardiovascular morbidity and mortality(Reference Alberti, Eckel and Grundy2, Reference Mottillo, Filion and Genest3). According to Ford et al. (Reference Ford, Giles and Dietz4), the age-adjusted prevalence of the MetS in the US population is 23 %, yet many of the mechanisms underlying its development remain unknown. Given the prevalence of the MetS, there is increasing importance to obtain a greater understanding of the modifiable factors that may mitigate or moderate the progression of events leading towards its development.
Among these modifiable factors, there has only recently been interest in examining the effect of alcohol consumption on the development of the MetS. Alcohol consumption is an accepted part of the American culture, as an estimated 76 % of American men engage in alcohol consumption over the course of a year, with 33 % of men reporting alcohol consumption on any given day(5). Previous studies have established strong evidence for a reduced risk of CHD(Reference Hvidtfeldt, Tolstrup and Jakobsen6), stroke(Reference Sacco, Elkind and Boden-Albala7) and mortality(Reference Di Castelnuovo, Costanzo and Bagnardi8) with light-to-moderate levels of alcohol consumption, with heavier levels of consumption resulting in negative health consequences. Moderate-to-high levels of alcohol consumption have been associated with hypertension, liver disease, peptic ulcers, certain types of cancers, pregnancy complications and brain damage(9).
Given the popularity of alcohol and its diverse effects on health and chronic disease, it is important to obtain a clearer understanding of its association with the MetS. The extent of this association is controversial as the existing literature is filled with conflicting findings. A recent meta-analysis concluded that light-to-moderate drinking provides a beneficial metabolic effect(Reference Alkerwi, Boutsen and Vaillant10). However, individual studies have described the relationship between alcohol consumption and the MetS as being J-shaped(Reference Yoon, Oh and Baik11), U-shaped(Reference Wakabayashi12), an inverse relationship(Reference Park, Zhu and Palaniappan13, Reference Freiberg, Cabral and Heeren14), a linear relationship(Reference Fan, Russell and Naimi15, Reference Gigleux, Gagnon and St-Pierre16) or having no relationship at all(Reference Buja, Scafato and Sergi17, Reference Wilsgaard and Jacobsen18). Yet, only a handful of these studies have prospectively examined alcohol consumption and the incidence of the MetS(Reference Gigleux, Gagnon and St-Pierre16–Reference Carnethon, Loria and Hill20), and, of these, only two have been conducted in North America(Reference Gigleux, Gagnon and St-Pierre16, Reference Carnethon, Gidding and Nehgme21). Contributing to this controversy, there appear to be sex differences as have been found by investigators: the prevalence of the MetS to be higher in women than in men who abstained from drinking(Reference Park, Zhu and Palaniappan13); a positive association of alcohol consumption in women only(Reference Wilsgaard and Jacobsen18); a similar effect of moderate alcohol consumption in both sexes(Reference Yoon, Oh and Baik11); no association in either sex(Reference Buja, Scafato and Sergi17). A final weakness in previous studies involves the adjustment for physical activity levels. Studies examining the relationship between cardiorespiratory fitness and the MetS have identified an inverse association(Reference LaMonte, Barlow and Jurca22), whereas a study using self-reported physical activity exposure was equivocal(Reference Laaksonen, Lakka and Salonen23). Cardiorespiratory fitness is a stronger predictor of health than self-reported physical activity, and may therefore serve as a more accurate modifying variable(Reference Lee, Sui and Ortega24).
Therefore, the purpose of the present study was to examine the prospective association between alcohol consumption and the incidence of the MetS in a population of US men, and to determine whether this relationship is modified by cardiorespiratory fitness, BMI, age and baseline health status.
Experimental methods
Study population
Participants were 20–100-year-old men (mean 43·4 (sd 9·0) years) who had preventive medical examinations at the Cooper Clinic (Dallas, TX, USA) between 1979 and 2005 and who were enrolled in the Aerobics Center Longitudinal Study. Criteria for inclusion in the present analysis required individuals to have had at least two clinical examinations with complete measurements for each MetS variable and a baseline cardiorespiratory fitness assessment (n 12 551). Individuals were excluded based on pre-existing cases of the MetS (n 2419), CVD (including heart attack or stroke), cancer or an abnormal resting or exercise electrocardiogram (n 1282) at baseline. In addition, those not achieving ≥ 85 % of age-predicted maximal heart rate on the treadmill test at baseline (n 88) or who had less than 1 year of follow-up (n 1279) were excluded. The remaining 7483 men met all criteria for the present study and were included in the final analysis. The study was conducted according to the guidelines laid down in the Declaration of Helsinki, and all procedures involving human subjects/patients were approved by the Cooper Institute Institutional Review Board. Written informed consent was obtained from all subjects/patients for the baseline clinical examination and follow-up study.
Definition of the metabolic syndrome
The outcome variable of the MetS was defined, following the guidelines of the National Cholesterol Education Program – Adult Treatment Panel III (NCEP ATP III), as meeting three or more of the following criteria: abdominal obesity (waist circumference >102 cm); TAG ≥ 15 mg/l; HDL-cholesterol (HDL-C) < 4 mg/l; resting systolic or diastolic BP of ≥ 130 or ≥ 85 mmHg, respectively; a fasting blood glucose ≥ 10 mg/l(1). Self-reported history of physician-diagnosed hypertension and diabetes was included in the definition of abnormal BP and glucose, respectively, as done in other epidemiological studies investigating the incidence of the MetS(Reference Carnethon, Gidding and Nehgme21).
Alcohol exposure
At their initial clinical visit, as part of the Personal Habits section of their Medical History Questionnaire, individuals were asked a series of ten questions regarding their alcohol use, specifically whether they drank alcoholic beverages and how many drinks per week they consumed of beer, wine and hard liquor. Alcohol consumption was quantified as the number of drinks per week, with a drink standardised to 12 ounces (360 ml) of beer, 5 ounces (150 ml) of wine or 1·5 ounces (22.5 ml) of liquor. Individuals were then classified into five categories of alcohol consumption (non-drinkers: 0 drinks; light: 1–3 drinks/week; moderate: 4–7 drinks/week; moderate–heavy: 8–13 drinks/week; heavy: 14+ drinks/week), with those who reported no recent alcohol consumption (including both former drinkers and abstainers) serving as the reference group. These classifications are in accordance with current USDA Dietary Guidelines(1) that defines moderate alcohol consumption in men as two drinks per d and heavy or high-risk drinkers consuming more than four drinks per d or fourteen drinks per week. Since few individuals limited their drinking to only one type of alcohol, individuals were categorised as a beer, wine or liquor drinker if they reported consuming any of these alcohol beverages.
Clinical examination
The clinical examination followed a 12 h fast as described previously(Reference Blair, Kohl and Paffenbarger25, Reference Blair, Kampert and Kohl26). Briefly, participants completed a medical questionnaire consisting of demographic questions, lifestyle habits, and past and present chronic disease history. In addition, they underwent a clinical evaluation that included body composition assessment, blood chemistry analysis, BP measurement and a physical examination by a physician. Waist circumference was taken at the level of the umbilicus with an inelastic tape and classified as ≤ 102·0 or >102·0 cm according to clinical guidelines(27). Resting BP was measured by standard auscultatory methods after at least 5 min of seated rest and was recorded as the average of two or more readings separated by 2 min(Reference Pickering, Hall and Appel28). Concentrations of TAG, HDL-C and glucose were measured from the blood obtained from the antecubital venous blood and serum was analysed using automated bioassays in the Cooper Clinic Laboratory according to the Centers for Disease Control and Prevention's Lipid Standardization Program standards(Reference Pickering, Hall and Appel28). BMI was calculated from measured weight and height, and individuals were categorised as normal weight (18·5–24·9 kg/m2), overweight (25·0–29·9 kg/m2) and obese ( ≥ 30 kg/m2)(27). Smoking status and a personal history of hypertension, diabetes, heart attack, stroke and cancer were ascertained through a standardised medical questionnaire. Individuals were also stratified according to the number of MetS risk factors present at baseline to allow for the examination of alcohol consumption in groups with and without co-morbidities.
Cardiorespiratory fitness
Participants completed a maximal exercise treadmill test to assess their cardiorespiratory fitness. Cardiorespiratory fitness was quantified as the duration of the maximal treadmill exercise test using a modified Balke protocol(Reference Blair, Kohl and Paffenbarger25). Participants were encouraged to reach their maximal effort and those who did not achieve 85 % of their age-predicted maximal heart rate were excluded, as it was assumed that they probably had subclinical medical problems and less than near-maximal effort would lead to an underestimation of fitness. The duration of this treadmill test is highly correlated with measured maximal oxygen uptake in men (r 0·92)(Reference Pollock, Bohannon and Cooper29). Classification of fitness level was determined on the basis of fifths of treadmill time, with those having fitness levels in the lowest 20 % classified as the ‘unfit’ and the remainder classified as the ‘fit’. This cut point was used as there is no consensus for the clinical definition of being ‘unfit’ and because it has been used as a similar guideline in previous Aerobics Center Longitudinal Study investigations, which have shown low fitness to be an independent risk factor for various morbidity and mortality outcomes(Reference Sui, LaMonte and Laditka30, Reference Lee, Sui and Church31).
Statistical analyses
Baseline characteristics were summarised across the levels of alcohol consumption and tests for linear trends were calculated using general linear models. MetS cases were defined as individuals who met the MetS definition at any clinical examination after baseline. The follow-up time was computed as the difference between the date of the baseline examination and the first follow-up event of the MetS or the last clinical examination through 2005. A Cox proportional hazards model was used to estimate the hazard ratios (HR) and 95 % CI of the MetS across the categories of alcohol consumption. Ordinal linear trends across the five categories of alcohol consumption were tested using Cox regression models. In Cox regression models, baseline age, examination year, current smoking status and total treadmill time were considered as potential confounders as in previous studies(Reference Carnethon, Gidding and Nehgme21, Reference Lee, Sui and Church31, Reference Sui, Hooker and Lee32). The proportional hazards assumption was tested by examining the log–log survival plots grouped on exposure categories.
Further multivariate analyses were conducted based on stratification by age ( < 55 or ≥ 55 years), BMI ( < 25 or ≥ 25 kg/m2), number of pre-existing MetS risk factors (0, 1 or 2) and fitness level (fit or unfit). In the multivariate analysis for type of alcoholic beverage (beer, wine and liquor), there were insufficient numbers of drinkers who consumed a single beverage type exclusively. Therefore, this analysis was conducted separately for all individuals who consumed each type of alcoholic beverage while adjusting for the remaining types of alcohol consumed. Finally, the association between alcohol consumption and the individual components of the MetS was evaluated. To calculate multivariate HR, Cox proportional hazards models containing each of the components of the MetS as binary-dependent variables were fitted to the same data which were utilised for the association between alcohol consumption and the incidence of the MetS. The SAS (SAS 9.1; SAS Institute, Inc.) program was used to conduct statistical analyses. All P values were two-sided, and P< 0·05 was considered as statistically significant.
Results
From 1979 to 2005, 7483 eligible men were followed for an average of 6·0 (sd 5·2) years for a total of 44 943 person-years of follow-up. Descriptive characteristics of the study participants are presented in Table 1. Significant differences in baseline characteristics were observed between men in the different categories of alcohol consumption except for abdominal obesity. In general, heavier drinkers were more likely to be older, current smokers, and have a higher BMI, systolic and diastolic BP, fasting glucose concentrations, total cholesterol, HDL-C and TAG concentrations.
Table 1 Baseline characteristics of the sample population (Mean values and standard deviations)

C, category; FBG, fasting blood glucose; MetS, metabolic syndrome; HDL-C, HDL-cholesterol.
* Defined as a waist circumference >102 cm.
† Defined as a systolic blood pressure ≥ 130 mmHg, a diastolic blood pressure ≥ 85 mmHg or a history of physician-diagnosed hypertension.
‡ Defined as a FBG ≥ 10 mg/l or a history of physician-diagnosed diabetes.
§ Defined as an HDL concentration < 4 mg/l.
∥ Defined as TAG concentrations ≥ 15 mg/l.
During an average of 6·0 years of follow-up, 1578 new cases of the MetS were observed. Overall, the incidence rate of the MetS was 35·1 per 1000 person-years. The HR of the MetS across the five categories of alcohol consumption levels are presented in Table 2. Significant linear trends were observed across the five categories of alcohol consumption (P< 0·002). In model 1, with adjustment for age, year of examination and smoking, all levels of alcohol consumption showed a significant inverse association with the incidence of the MetS compared with non-drinkers. After additional adjustment for fitness level (model 2), all associations between the levels of alcohol consumption and the incidence of the MetS remained significant.
Table 2 Hazard ratios of the metabolic syndrome across the levels of alcohol consumption (Hazard ratios and 95 % confidence intervals)
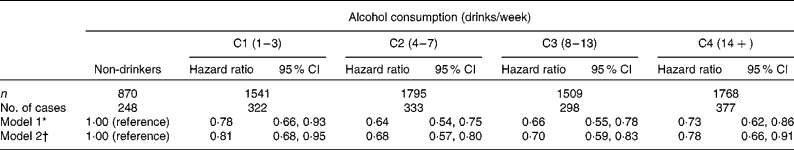
C, category.
* Adjusted for age (in years), year of examination, smoking (current smoker or not).
† Additionally adjusted for maximal treadmill time (min).
Table 3 presents the HR of the individual components of the MetS across the different categories of alcohol consumption. No effect of alcohol consumption was observed on central obesity, high TAG concentrations or hypertension. Moderate (28 %), moderate–heavy (22 %) and heavy (56 %) levels of drinking were associated with a higher risk of high fasting glucose concentrations. A significant positive association was observed between HDL-C and all levels of alcohol consumption in a dose–response manner with greater levels of alcohol consumption associated with a lower risk of low HDL-C.
Table 3 Hazard ratios of the individual components of the metabolic syndrome across the levels of alcohol consumption* (Hazard ratios and 95 % confidence intervals)
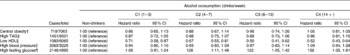
C, category.
* Adjusted for age (in years), year of examination, smoking status (current smoker or not) and maximal treadmill time (min).
† Defined as a waist circumference >102 cm.
‡ Defined as a serum TAG ≥ 15 mg/l.
§ Defined as < 4 mg/l.
∥ Defined as a systolic blood pressure ≥ 130 mmHg, a diastolic blood pressure ≥ 85 mmHg or a history of physician-diagnosed hypertension.
¶ Defined as ≥ 11 mg/l or a history of physician-diagnosed diabetes.
In Table 4, the HR of the MetS across the five categories of alcohol consumption are presented after stratification by age, BMI, pre-existing MetS components, fitness level and type of alcohol beverage consumed. Among individuals less than 55 years of age, inverse associations between alcohol consumption and the risk of the MetS were observed with all categories except light consumption, while similar inverse associations were seen in the light, moderate and moderate–heavy categories in men 55 years of age and older. Among individuals with a BMI ≥ 25 kg/m2, all levels of alcohol consumption resulted in a lower incidence of the MetS; however, among those with a BMI < 25 kg/m2, a positive association was seen only among moderate consumers. A separate sub-analysis (not shown) was conducted for individuals with BMI ≥ 30 kg/m2, but results were inconclusive due to the small number of cases in this category. Moderate, moderate–heavy and heavy alcohol consumption all resulted in an inverse association in individuals who had two pre-existing components for the MetS, while no significant associations were seen in men with no pre-existing MetS components at baseline. Significant inverse associations were observed in both fit and unfit individuals in the moderate, moderate–heavy and heavy alcohol consumption categories. Table 4 also presents the HR of the MetS across the five levels of alcohol consumption after stratification by type of alcohol beverage. Across each type of alcoholic beverage, there were similar significant inverse associations, except with heavy levels of liquor consumption.
Table 4 Hazard ratios of the metabolic syndrome across the levels of alcohol consumption after stratification by age, BMI, pre-existing metabolic syndrome (MetS) components, fitness level and beverage type* (Hazard ratios and 95 % confidence intervals)
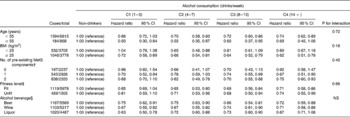
C, category.
* All stratified variables were adjusted for age (in years), year of examination, smoking (current smoker or not), number of pre-existing MetS components and maximal treadmill time (min), except variables within their same level of stratification.
† Defined as any of the following conditions: systolic/diastolic blood pressure ≥ 140/90 mmHg or history of hypertension; fasting glucose ≥ 12·6 mg/l or history of diabetes; dyslipidaemia (total cholesterol ≥ 24 mg/l, TAG ≥ 20 mg/l or HDL-cholesterol < 4 mg/l).
‡ Defined as the most fit (80 %) and the least fit (20 %) based on the distribution of maximal treadmill times from the study cohort.
§ Alcohol beverage stratification was created based on the distribution of use of each alcohol type with all remaining types of alcohol included as covariates in the model. No interaction test was performed between the type of alcohol and alcohol consumption, as alcohol consumption was categorised within each type of alcohol consumption.
Discussion
In the present prospective study of US men, we observed that all levels of alcohol consumption provided a significant inverse association with the development of the MetS after taking into account several potential confounding factors. In particular, the present study showed a significantly lower risk of the MetS in individuals who had a greater number of MetS risk factors present at baseline and were overweight/obese. The benefits of alcohol also remained significant regardless of the type of alcoholic beverage consumed.
The main finding of the present study that alcohol consumption was associated with a lower risk of MetS incidence expands upon previous epidemiological studies indicating that light-to-moderate levels of alcohol consumption are associated with a lower risk of the MetS in men(Reference Alkerwi, Boutsen and Vaillant10, Reference Freiberg, Cabral and Heeren14, Reference Djousse, Arnett and Eckfeldt33, Reference Zhu, St-Onge and Heshka34). However, few studies examining the relationship between alcohol consumption and the MetS have been conducted in prospective studies(Reference Gigleux, Gagnon and St-Pierre16, Reference Baik and Shin19, Reference Carnethon, Loria and Hill20), and only one(Reference Gigleux, Gagnon and St-Pierre16) has shown that moderate-to-heavy alcohol consumption is associated with a significantly lower risk of the MetS, as seen in the present study. Although this inverse association with alcohol consumption was seen in moderate drinkers, the present data also indicate that these benefits also extend to moderate–heavy and heavy levels of alcohol consumption. However, these results must be interpreted with caution as these findings do not take into account the numerous health hazards associated with heavy alcohol use (e.g. gastrointestinal and liver diseases, cancer, intentional injuries, etc.(Reference Room, Babor and Rehm35)).
There are many reasons for the varying results observed when examining the association between alcohol consumption and the MetS, including factors such as sex and race/ethnicity(Reference Carnethon, Loria and Hill20). There are several racial and ethnic biological differences that influence alcohol metabolism(36), as well as racial/ethnic variations in drinking quantities, patterns and alcohol abuse disorders(Reference Chartier and Caetano37). In contrast to the present study, Baik & Shin(Reference Baik and Shin19) found a linear trend between alcohol consumption and the incidence of the MetS, with the quartile of heaviest drinkers displaying a 63 % increase in relative risk. However, this cohort consisted of Korean men and women who have different alcohol metabolism rates(Reference Agarwal, Harada and Goedde38). Therefore, appropriate consideration must be given to race, sex and ethnicity when making comparisons between existing studies.
Another major confounding factor is the definition of the MetS itself. Wakabayashi(Reference Wakabayashi12) found no significant associations between alcohol consumption and the prevalence of the MetS using International Diabetes Federation guidelines(Reference Alberti, Zimmet and Shaw39), but did find a significant association in heavy drinkers when using the National Cholesterol Education Program – Adult Treatment Panel III guidelines(1). Other factors, such as quantification of drinking patterns(Reference Lee, Park and Kang40), frequency(Reference Mukamal, Jensen and Gronbaek41) and lifetime alcohol consumption(Reference Fan, Russell and Naimi15), may also have a differential impact on the development of the MetS, yet are rarely assessed and/or controlled for in a standardised manner when examining this relationship.
Effect of alcohol on individual metabolic syndrome components
In addition to the direct effect that alcohol consumption has on the development of the MetS, there may be an indirect effect through a series of relationships with each individual component of the MetS. Understanding these individual relationships may be of particular use when individualising guidance for patients who may be at risk for some components of the MetS and not others. In the present study, we observed no increase in the risk of hypertension with any level of alcohol consumption. This result is consistent with other prospective reports that have indicated no increase in the incidence of hypertension with light-to-moderate levels of alcohol consumption in white men(Reference Fuchs, Chambless and Whelton42, Reference Sesso, Cook and Buring43). However, these prospective studies, as well as other cross-sectional studies, have shown a higher risk of hypertension with moderate-to-heavy levels of alcohol consumption in comparison with non-drinkers(Reference Wakabayashi12, Reference Baik and Shin19, Reference Djousse, Arnett and Eckfeldt33). In the present study, there was no observed effect of alcohol consumption on either central obesity or serum TAG concentrations. Several studies, in agreement with the present findings, have demonstrated that alcohol consumption is not associated with increased waist circumference(Reference Wakabayashi12, Reference Freiberg, Cabral and Heeren14, Reference Djousse, Arnett and Eckfeldt33). A limited number of investigators have demonstrated an increased risk of moderate and heavy drinking on serum TAG concentrations(Reference Wakabayashi12, Reference Baik and Shin19). In contrast, the present results suggest that no observed level of alcohol consumption has a negative impact on serum TAG, a finding supported by others(Reference Freiberg, Cabral and Heeren14). Although alcohol consumption has been associated with decreased serum glucose concentrations, particularly in light and moderate drinkers(Reference Freiberg, Cabral and Heeren14), the present results indicate that moderate, moderate–heavy and heavy levels of drinking are associated with an increased risk of hyperglycaemia, which agrees with a recent study showing increased risk in heavy drinkers(Reference Baik and Shin19). Paradoxically, alcohol consumption has been related to enhanced insulin sensitivity and lower plasma insulin concentrations(Reference Wannamethee, Shaper and Perry44, Reference Facchini, Chen and Reaven45), providing a possible explanation for the lower incidence of type 2 diabetes in alcohol consumers compared with abstainers(Reference Gigleux, Gagnon and St-Pierre16, Reference Koppes, Dekker and Hendriks46). One area of consistent agreement involves the present findings of a positive dose–response relationship between alcohol consumption and the risk of low HDL-C, which is in agreement with others(Reference Wakabayashi12, Reference Baik and Shin19, Reference Wannamethee, Shaper and Perry44). Thus, it appears that the possible beneficial effect of alcohol consumption is that it is associated with a lower risk of low HDL-C, and may offset the deleterious effect of an increased risk of high fasting glucose concentrations. It appears that there is a need for further studies to clarify the physiological mechanisms and relationship between alcohol consumption and the individual components of the MetS.
Stratification based on BMI, age, fitness level and baseline health status
All levels of alcohol consumption, except light consumers, resulted in a reduced risk of the MetS in individuals < 55 years. In individuals ≥ 55 years of age, this association was seen in light, moderate and moderate–heavy drinkers. These results appear to indicate that moderate and moderate–heavy alcohol consumption have a positive impact on the incidence of the MetS across all ages in men. There was a lower risk of MetS incidence in men who consumed moderate, moderate–heavy and heavy levels of alcohol and who were positive for two MetS components at baseline. However, in men with no pre-existing MetS components at baseline (i.e. those who might be considered healthier), there was no lower risk with any level of alcohol consumption. This result is consistent with others who found the relationship between alcohol consumption and the MetS the strongest among individuals with a higher number of MetS components(Reference Djousse, Arnett and Eckfeldt33) and in men with an increased risk of diabetes(Reference Wannamethee, Shaper and Perry44). A lower risk of the MetS was also seen in individuals with a BMI ≥ 25 kg/m2 at all levels of alcohol consumption. This finding is in discord with others who observed that heavy drinking in overweight individuals was associated with a 37–115 % increase in the risk of the MetS(Reference Baik and Shin19, Reference Zhu, St-Onge and Heshka34). Finally, all levels of alcohol consumption, except for light drinkers, were associated with a lower risk of MetS incidence in both the fit and unfit men in the study. Previous literature has established a significant inverse relationship between cardiorespiratory fitness and the MetS in similar cohorts(Reference Carnethon, Gidding and Nehgme21, Reference LaMonte, Barlow and Jurca22). However, the mechanisms behind the lower risk of the MetS in unfit individuals with higher levels of alcohol consumption are unclear, and additional work is needed to further clarify this association.
Beverage-specific effects on the metabolic syndrome
Across each type of alcoholic beverage, similar lower risks of the MetS were observed within each category of alcohol consumption, except with high levels of liquor consumption. The present results support those of Djousse et al. (Reference Djousse, Arnett and Eckfeldt33) who found that consumption of wine only, beer only, spirits only and any combination of beverage type was associated with risk reductions in the MetS of 68, 58, 43 and 44 %, respectively. These findings are in contrast to those reporting that heavy liquor consumption results in a significant increase in the MetS(Reference Baik and Shin19), and that beer and wine consumption, but not liquor, resulted in a lower prevalence of the MetS(Reference Freiberg, Cabral and Heeren14). The present finding is of particular interest given that previous studies have primarily demonstrated that the beneficial effect of alcohol consumption has been limited to wine drinkers, individuals who are more likely to engage in healthier lifestyles(Reference Rosell, De Faire and Hellenius47).
Strengths and limitations
The strengths of the present study include the use of a comprehensive physical examination and an extensive follow-up period in one of the largest cohort studies to investigate the incidence of the MetS by alcohol consumption, as well as our ability to stratify and adjust our models using cardiorespiratory fitness rather than self-reported physical activity. Furthermore, this is one of the first prospective studies examining the relationship between alcohol consumption and the MetS in a US male population, enhancing the internal validity of the present findings. The limitations of the present study include: limited external validity beyond Caucasian males of a higher socio-economic status; possible under-reporting and subsequent misclassification of alcohol consumption; the potential misclassification of the reference group as former drinkers and lifetime abstainers have very different alcohol consumption histories. Additionally, in the stratified analyses, the relatively small number of incident MetS cases in the older, normal-weight (BMI < 25 kg/m2), healthy and least fit groups may have affected the results in these groups.
Conclusion
The results of the present study suggest that alcohol consumption may protect against the development of the MetS. These findings may be particularly useful for health professionals in providing individualised guidance to their patients with regard to alcohol consumption and its effect on the MetS or its individual components, and in assisting with the future establishment of guidelines regarding the benefits and risks of alcohol consumption.
Acknowledgements
We thank the Cooper Clinic physicians and technicians for collecting the baseline data, and staff at the Cooper Institute for the data entry and data management. The present study was supported by the National Institutes of Health grants (AG06945, HL62508 and DK088195), and an unrestricted research grant from The Coca-Cola Company. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The authors' contributions are as follows: S. B. designed the research; S. B. and S. H. conducted the research; D.-c. L. analysed the data; X. S., V. H., T. P. and M. S. wrote the paper; M. S. and S. B. had primary responsibility for the final content. All authors read and approved the final manuscript. None of the authors has any conflict of interest to disclose.






