INTRODUCTION
Clostridium difficile-associated disease (CDAD) is a common cause of nosocomial diarrhoea and is associated with substantial morbidity and mortality [Reference Ricciardi1]. Recent population-based data reveal an increase in the prevalence and severity of CDAD in the United States [Reference Ricciardi1, Reference McDonald, Owings and Jernigan2]. Changes in the epidemiology of C. difficile may be secondary to the emergence of epidemic strains in many areas of the United States and Canada [Reference Loo3, Reference Warny4]. In addition, others have demonstrated reduced efficacy of usual medical therapies, such as metronidazole and vancomycin, although resistance to these agents has not been demonstrated [Reference Pepin5, Reference Musher6]. A number of authors have sought to define patient-level risk factors that predispose patients to CDAD. Most such analyses have examined patient demographics [Reference Pepin7–Reference McFarland9], comorbid conditions [Reference Changela10–Reference McFarland, Surawicz and Stamm12], clinical variables [Reference Pepin7, Reference Brbut and Petit8], and antibiotic treatment characteristics [Reference Pepin7, Reference Brbut and Petit8, Reference Brown13]. Patient-level risk factors have been used to help develop treatment algorithms [Reference Carling14], and may also be useful in developing prevention and control measures.
Most cases of CDAD are transmitted while patients are in the hospital, often through contamination of the hands of hospital personnel caring for them [Reference McFarland9]. A recent county-level evaluation in Sweden revealed that hospital-acquired CDAD is 1300 times more common than community-acquired infections [Reference Noren15]. Thus, effective prevention and control measures are needed to reduce acquisition of CDAD. An evaluation of hospital-level characteristics may be more useful than a focus solely on patient-level factors. For this reason, an analysis of hospitals with the highest proportion of CDAD and their risk factors is needed. In this study, high rates of CDAD were compared to hospital admission, personnel, and service characteristics for hospitals with high rates of CDAD and compared to those with fewer CDAD cases.
METHODS
Data sources
Hospital in-patient discharge data, which contain greater than 95% of all discharge abstracts of non-government in-patient facilities, were obtained for California, Arizona, and Minnesota. These data provided information on patient demographics, socioeconomic factors, admission profiles, hospital profiles, state codes, discharge diagnoses, procedure codes, total charges, and vital status at hospital discharge. Data use agreements are held by the respective state data collectors, so all study protocols were considered exempt by the University of Minnesota institutional review board.
The American Hospital Association (AHA) Annual Survey of Hospitals database for the time period January 2000–December 2003 was obtained in order to determine hospital structural and service lines associated with high rates of CDAD. The AHA database contains hospital-specific information on over 6000 hospitals and over 450 health-care systems, including 700 data elements [Reference Health16]. The purpose of the AHA database is to generate a comprehensive and inclusive overview of hospitals while permitting the tracking of hospital performance over time. AHA data have been extensively used to study hospital-based outcomes [Reference Elting17], hospital policies [Reference Powner, Hernandez and Rives18], and reimbursement [Reference Irvin, Fox and Pothoven19].
Hospital infection rates
Diagnostic codes from the International Classification of Diseases (ICD-9) were used to identify all patients who were discharged with a diagnosis of C. difficile-pseudomembranous colitis (ICD-9 code 8.45) [Reference Ricciardi1, Reference McDonald, Owings and Jernigan2] from January 2000 to December 2003. Patients with either a primary or secondary diagnosis of CDAD were summed to calculate yearly hospital rates/1000 discharges. Hospitals were divided into two groups: Those whose CDAD rates were in the 90th percentile of all hospitals studied (‘high CDAD’ hospitals) and the remaining hospitals. Psychiatric facilities, chemical dependency facilities, and hospitals with incomplete information were excluded.
To test the association between CDAD and other infections, previously published methods of identifying common infections as well as an estimate of other bacterial infections were used. First, the proportion of patients with discharge diagnoses of other commonly treated infections, i.e. pneumonia [Reference Aronsky20], urinary tract infections [Reference Taub21], endocarditis [Reference Abbott22], cellulitis [Reference Ellis Simonsen23], bloodstream infections [Reference Dombrovskiy24], and osteomyelitis [Reference Upchurch25] for the entire cohort, for each state, and for each hospital was determined. Next, the proportion of admissions for each of these infections to the total of individual hospital discharges was calculated. In addition, the rate of discharges for other common bacterial infections (Escherichia coli, Pseudomonas, Streptococcus, and Staphylococcus) was calculated for the entire cohort, for each state, and for each hospital.
AHA variables
Survey results from the AHA files were used to include the time period from January 2000 to December 2003. The hypothesis tested was that hospitals with high rates of CDAD can be characterized by particular hospital characteristics, specifically hospital structural, personnel, and service-line data. These characteristics were chosen because of the ease of obtaining these variables and because high institutional rates of CDAD have been described in particular types of facilities [Reference Morris26, Reference Dallal27] and these files were merged with the administrative hospital data described previously. First, hospital structural characteristics and service lines were evaluated. Staffing characteristics were determined in terms of the number of full-time-equivalent medical doctors, registered nurses, and medical residents as well as other hospital factors such as distinction as a critical access hospital for hospitals with <25 beds, presence of an emergency department, and total number of annual surgical procedures.
Statistical analysis
A correlation analysis was used to assess associations between the rates of CDAD and the rates of other bacterial infections. Similarly, the rates of CDAD were compared with rates of six common infections (pneumonia, urinary tract infections, endocarditis, cellulitis, bloodstream infections, and osteomyelitis). The mean rate of these infections and of four other bacterial species (E. coli, Pseudomonas, Streptococcus, and Staphylococcus) was determined for ‘high CDAD’ hospitals and all other hospitals. χ2 tests were used to compare categorical variables (AHA data) and analysis of variance for continuous variables (mean rates of infection).
A logistic regression model was constructed to evaluate the relationship between ‘high CDAD’ hospitals in the 90th percentile of CDAD infections (categorical variable) and AHA characteristics. For this analysis, variables from the AHA files and the hospital location were included, while excluding infection variables because of the strong correlations between CDAD, other bacterial pathogens, and other common infections. The AHA categorical variables included were: characterization as a trauma facility, critical access hospital, patient burn beds, residents in training, haemodialysis, neurology, orthopaedic, palliative care, transplant, emergency department, cardiac intensive care, and rehabilitation services. AHA continuous variables were number of full-time medical doctors, registered nurses, hospital beds and surgical procedures in tertiles. The total annual hospital discharge figure was divided by total hospital beds in order to estimate patient bed turnover by facility. A comparatively high bed turnover ratio indicates a shorter length of stay (variable entered into model as a continuous variable). A similar analysis was performed for ‘high CDAD’ hospitals for each state.
RESULTS
Cohort characteristics
This analysis reflects the characterization of 20292499 discharges over the 4-year study period. Of these discharges, 80 619 patients had CDAD codes giving a rate of 3.97 CDAD infections/1000 discharges. The rate of CDAD for each metropolitan statistical area is presented in the Figure. Hospitals in the most populous localities had the highest rates of CDAD.
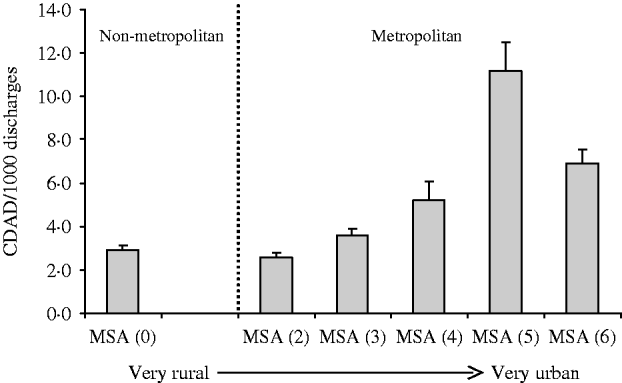
Fig. Mean number (with s.e.m.) of C. difficile colitis cases (number of cases/1000 hospitalized patients) by metropolitan statistical area (MSA). Code: MSA 0, non-metropolitan area; MSA 1, <100 000 population; MSA 2, 100 000–250 000 population; MSA 3, 250 000–500 000 population; MSA 4, 500 000–1 000 000 population; MSA 5, 1 000 000–2 500 000 population; MSA 6, >2 500 000 population.
Hospital-level analysis
Hospital characteristics
Data were available for 2363 acute care hospitals in the three states. CDAD was noted in 2075 hospitals (87·8% of the total). The mean hospital rate (±s.d.) for CDAD was 6·3±18·5 infections/1000 discharges. The mean hospital rate for the other bacterial infections was greatest for E. coli (13·5±9·4 infections/1000 discharges), followed by Staphylococcus, Streptococcus, and Pseudomonas (Table 1). Other infections were noted in the majority of hospitals. Almost all hospitals treated at least one patient with a urinary tract infection (98·9%), pneumonia (98·7%), cellulitis (98·5%), bloodstream infection (97·0%), and osteomyelitis (90·3%); only 71·4% of hospitals treated at least one patient with endocarditis. The mean hospital rate for the other infections was highest for pneumonia (93·0±72·3 cases/1000 discharges), followed by urinary tract infections, cellulitis, bloodstream infections, osteomyelitis, and endocarditis (Table 1).
Table 1. Hospital characteristics (for bacterial isolates and commonly treated infections)
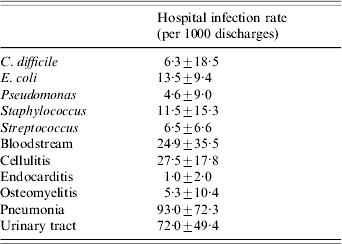
Values are mean±s.d.
Hospital characteristics were obtained from the AHA data for the majority of hospitals. The data revealed that the hospitals averaged 176·6±155·2 beds, 5248±5208 annual surgical procedures, and 8270±8533 discharges. A minority of hospitals admitted trauma patients (20·6%), provided transplant services (7·4%), or had beds assigned for burn patients (3·3%). Only 35·7% provided cardiac intensive care services (Tables 2 and 3).
Table 2. Hospital characteristics (proportion of hospitals with identified service offering)
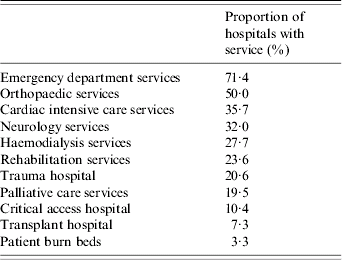
Table 3. Hospital characteristics (hospital beds, staffing levels, and bed turnover)

Values are mean±s.d.
Infection correlations
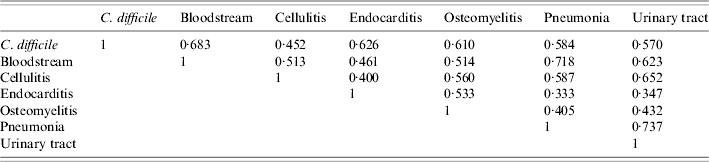
Statistically significant correlations for hospital rates of CDAD and the other bacterial pathogens were noted. The correlation between CDAD and Pseudomonas was highest, followed by Staphylococcus, Streptococcus, and E. coli (Table 4). Statistically significant correlations were also found between CDAD and bloodstream infections, followed by endocarditis, osteomyelitis, pneumonia, urinary tract infections, and cellulitis (Table 5).
Table 4. Bacterial correlations [correlation values (r) for individual pathogens in identified infections]

Table 5. Infection correlations [correlation values (r) for common infections]
‘High CDAD’ hospitals and infections
A group of hospitals was identified as having a disproportionately higher rate of CDAD, a plot of CDAD for each facility revealed an inflection point at which the rate of cases increased disproportionately. This inflection point of CDAD was identified at the 90th percentile of all infections. ‘High CDAD’ hospitals had on average ten times more CDAD cases than all other hospitals (Table 6). A comparison of the rates of bacterial pathogens and other common infections between ‘high CDAD’ hospitals and all other facilities (Table 6) revealed that the former were more likely to have E. coli, Staphylococcus, Streptococcus, and Pseudomonas infections (Table 6) and were also more likely to discharge patients with bloodstream infections, endocarditis, osteomyelitis, pneumonia, urinary tract infections, and cellulitis (Table 6). Hospitals in the 95th or 80th percentile of CDAD also showed similar associations.
Table 6. Hospital characteristics by CD colitis rate [differences in mean rates (±s.d.) of bacterial and infection characteristics between ‘high CDAD’ hospitals and all other facilities]
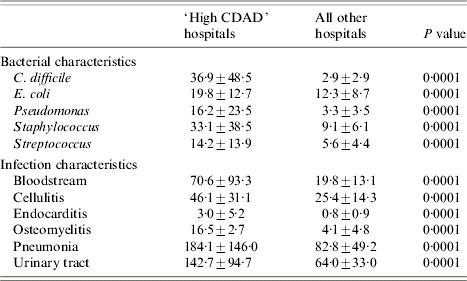
Values are mean rate/1000 discharges±s.d.
‘High CDAD’ hospitals and hospital variables
A univariate analysis of hospital variables for ‘high CDAD’ hospitals (90th percentile of C. difficile infections) compared to others revealed that the former were less likely to provide neurology, orthopaedic, and palliative care services; in addition, they had fewer nurses and residents, performed fewer operations, discharged fewer patients and were characterized by slower bed turnover. Lastly, ‘high CDAD’ hospitals were not more likely to be classified as critical access facilities (Table 7).
Table 7. Hospital characteristics by CD colitis rate (differences in hospital characteristics between ‘high CDAD’ hospitals and all other facilities)

State analysis
The median number of cases of CDAD cases was similar for California and Arizona and lowest for Minnesota. Despite the small difference in the median number of cases across the three states, the mean case rate (±s.d.) across states/1000 discharges were Arizona 11·1±30·1 cases; California 6·5±18·4 cases; Minnesota 2·9±3·2 cases. In addition, the rate of CDAD among the hospitals in the 90th percentile of C. difficile cases was highest for Arizona (83·7 cases/1000 discharges), followed by California (36·5 cases), and lowest for Minnesota (10·3 cases).
Comparison of ‘high CDAD’ hospitals (in the 90th percentile) for each state revealed patterns similar to those in the complete cohort analysis described above. With few exceptions, there were significant correlations between CDAD rates and isolates of other pathogens as well as other common infections for hospitals in the state of California, Arizona, or Minnesota and results for individual hospital reproduced those for the larger cohort. ‘High CDAD’ hospitals had fewer full-time registered nurses, fewer medical residents, fewer hospital beds, and performed fewer surgical procedures. There was also a significantly slower bed turnover (total discharges per bed) as found in the wider cohort analysis.
Multivariate analysis
Logistic regression analysis revealed that hospitals with transplant services [odds ratio (OR) 2·36, 95% confidence interval (CI) 1·15–4·93] were more likely to be in the 90th percentile of CDAD cases. Hospitals with emergency department services (OR 0·52, 95% CI 0·32–0·84) or trauma services (OR 0·29, 95% CI 0·13–0·62) were less likely to be in the 90th percentile and so were hospitals with faster bed turnover (OR 0·96, 95% CI 0·95–0·97). After adjusting for the above factors, the state in which the hospital was located and other AHA data variables were also not associated with high CDAD rates. Given that the majority of the latter hospitals were located in California and Arizona, a cohort of such hospitals was constructed for each state. A new cohort of hospitals that represented the 90th percentile of CDAD cases for each state was abstracted and the same multivariate analysis was conducted. This analysis revealed similar findings of increased risk of CDAD in hospitals with transplant services but decreased risk of CDAD in hospitals with emergency department services, trauma services, and faster bed turnover as well as an additional increased risk in hospitals providing haemodialysis services (OR 1·87, 95% CI 1·09–3·21).
DISCUSSION
This study examined hospital-level predictors of high rates of CDAD in over 2300 hospitals across California, Arizona, and Minnesota. The analysis of hospital characteristics is particularly useful, since most CDAD cases are acquired while patients are hospitalized [Reference Noren15]. Significant correlations between ‘high CDAD’ hospitals and facilities that admit large numbers of patients with other bacterial pathogens and other common infections was noted. In addition, although ‘high CDAD’ hospitals were more likely to provide transplant services, they were less likely to provide emergency department services or trauma services. The analysis by state revealed that the highest rates of CDAD occurred in Arizona; the lowest rates, in Minnesota. Thus, the present study revealed large variations in CDAD cases, with a clustering of common infections at ‘high CDAD’ hospitals.
Prior to this study, attempts to identify risk factors for CDAD have been conducted at the patient level. Pepin and colleagues found that older patients with multiple comorbidities were more likely to develop CDAD [Reference Pepin7] as were patients treated with proton pump inhibitors [Reference Dial28] or tube feedings [Reference Bliss29]. Most have described an association between antibiotics, particularly the use of cephalosporins, clindamycin, or fluoroquinolones and CDAD [Reference Pepin7, Reference Aronsson, Mollby and Nord30–Reference Pear34]. There were large differences in CDAD cases across hospitals, metropolitan statistical areas, and states. These differences are probably due to clustering of infections at particular facilities as was evident in the state analyses. State rates of CDAD demonstrated considerable difference between median and mean case rates and these were probably due to significant outbreaks of infection at particular types of facilities. This is further reinforced by the fact that ‘high CDAD’ hospitals had more than 10 times the number of CDAD cases than other hospitals while treating a significantly larger proportion of other common infections. Given this clustering of CDAD, it seems particularly important that prevention and control measures be applied in such high-risk hospitals. At this time a number of methods have been developed to reduce CDAD cases, including education, infection control measures (e.g. contact precautions or patient isolation), rigorous cleaning and disinfection protocols, and antibiotic restrictions [Reference Cartmill35, Reference Tedesco, Barton and Alpers36]. Although all hospitals should attempt to reduce outbreaks of CDAD, those with high numbers of other common infections are most in need of prevention and control measures.
In addition to hospital structural and personnel characteristics, the present study revealed that hospitals with high numbers of Pseudomonas and Staphylococcus infections were much more likely to diagnose and treat CDAD. ‘High CDAD’ hospitals also had high numbers of endocarditis and bloodstream infections which generally require prolonged hospital stay and antibiotic use, leading to a potentially higher CDAD risk. These findings are in agreement with the bed turnover ratio results, indicating that those facilities with a slower turnover of patients are at higher risk of CDAD. Hospitals with slower bed turnover rates and therefore increased CDAD rates may potentially have increased comorbidity or patient severity of illness. Others have described similar associations when comparing patient-level risk factors for CDAD [Reference Kyne11–Reference Brown13].
The association between CDAD and other infections is probably due to the mechanism by which C. difficile causes disease, i.e. by reducing normal colonic flora with antibiotics used to treat another infection, followed by patients' inoculation with C. difficile spores [Reference Gurwith, Rabin and Love33, Reference Pear34]. Despite the long-held view of CDAD pathophysiology, a recent systematic review investigated the association of antibiotics with CDAD in order to summarize the strength of the evidence for this relationship [Reference Thomas, Stevenson and Riley37]. These researchers found that most studies demonstrate a correlation between CDAD and antibiotics, yet none were able to define a causal relationship. They concluded that well-designed studies grounded in epidemiological principles are needed to identify true risk factors for CDAD and to provide reliable estimates of the strength of the association [Reference Thomas, Stevenson and Riley37]. Although we agree with those researchers, the analysis presented here of hospital-level risk factors provides alternative data regarding CDAD risk.
The present study has a number of strengths and limitations that are specific to the use of administrative data. Hospital in-patient discharge files do not provide clinical details commonly found in the medical record, such as culture results and treatment details. More information regarding culture results and treatment patterns would allow us to verify diagnostic codes while examining other associations. Despite the inability to confirm the presence of CDAD, others have used ICD-9 codes to evaluate trends in CDAD infections [Reference McDonald, Owings and Jernigan2] while a recent study demonstrated 75% sensitivity for this diagnosis [Reference Dubberke38]. In addition, the identified variability in CDAD may have been influenced by the variable presence of infection control teams leading to detection bias for those ‘high CDAD’ hospitals [Reference Zoutman and Ford39]. These hospitals, however, had strikingly close correlations with other infections; thus, the data demonstrate reliable and significant correlations between ‘high CDAD’ hospitals and each of the infections queried in both the larger cohort and across the individual states. Thus, we were able to analyse risk factors that had not been previously studied in smaller single-institution studies. The strength of the study is grounded on the representative nature of a large cohort of hospitalized patients with CDAD in three states across different geographic regions of the United States.
In conclusion, the present study revealed high rates of CDAD in hospitals that discharge large numbers of patients with other bacterial pathogens and other common infections. The analysis revealed large differences in CDAD cases across metropolitan statistical areas and states and that ‘high CDAD’ hospitals were more likely to provide transplant services and have slower turnover of beds, reflecting longer hospital lengths of stay. These findings provide more insight into the epidemiology of C. difficile and indicate marked differences in the burden of CDAD across localities.
ACKNOWLEDGEMENTS
The authors thank Franci Livingston for her assistance with the attainment of Minnesota hospital discharge data. The work is funded in part by a Minnesota Medical Foundation Grant.
DECLARATION OF INTEREST
None.










