The prevalence of obesity in women has more than doubled during the past four decades(1). It has been demonstrated in several studies that being overweight or obese increases the risk of developing gestational diabetes (GDM) by 2–10-fold(Reference Poston, Harthoorn and Van Der Beek2,Reference Farrar, Simmonds and Bryant3) . Maternal obesity and GDM are independently associated with a variety of health problems to the mother and her child during pregnancy, delivery and in later life(Reference Poston, Harthoorn and Van Der Beek2–Reference Aviram, Hod and Yogev4). Moreover, overweight and obese women tend to gain more weight than recommended during pregnancy, which in turn has been linked to adverse pregnancy outcomes(Reference Gaillard, Durmus and Hofman5–Reference Pellonpera, Koivuniemi and Vahlberg8). In the search for new means to improve pregnancy outcomes, it is important to determine the factors that lie behind these risk factors, including body composition.
Body composition reflects nutritional status and provides more precise information about the adiposity of the body than the widely used BMI(Reference Prentice and Jebb9). It has been found that body composition and fat distribution correlate better with the individual’s insulin sensitivity than BMI in both pregnant and non-pregnant individuals(Reference Kahn, Prigeon and Schwartz10–Reference Gur, Ince and Turan12). There is a marked inter-individual variation in the gains of both fat mass (FM) and fat-free mass (FFM) during pregnancy, emphasising the importance of measuring body composition, particularly when the potential health risks related to obesity are considered(Reference Marshall, Murphy and King13). GDM has been repeatedly associated with adiposity, mainly defined as high pre-pregnancy BMI, but little is known about the actual body composition of women diagnosed with GDM, or how their body composition develops during pregnancy compared with women without GDM. Furthermore, means to regulate body adiposity during pregnancy have been rarely investigated, even though it is the degree of adiposity rather than the mere weight gain which is likely to be associated with the onset of pregnancy complications.
One novel means to influence body composition could involve the consumption of certain dietary supplements. The consumption of fish oil (n-3 PUFA) has been proposed to modestly reduce weight and body fat percentage (BF%) in non-pregnant individuals(Reference Bender, Portmann and Heg14). Previous literature also suggests that the consumption of probiotics may help overweight adults in weight loss and FM loss, especially certain strains of Lactobacillus and Bifidobacterium (Reference Crovesy, Ostrowski and Ferreira15,Reference Borgeraas, Johnson and Skattebu16) . Nonetheless, the effects of these supplements on gestational weight gain (GWG) and body composition during pregnancy are largely unknown.
The primary objective of our previously published randomised placebo-controlled trial was to examine the effects of fish oil and probiotics on the risk of GDM and maternal glucose concentrations, and we found no intervention effect(Reference Pellonpera, Mokkala and Houttu17). In the present study, we investigated the effects of a fish oil and/or probiotic intervention on the GWG and body composition of overweight and obese women during pregnancy. This study is a pilot study directing the planning and execution of the future studies in pregnant women. Second, we evaluated whether GWG or body composition is different in women who develop GDM in comparison with women with normal glucose tolerance.
Subjects and methods
We studied GWG and body composition within an on-going trial designed to investigate the effects of fish oil and/or probiotic dietary supplements on maternal glycaemic control and child health. Details of the research design and methods have been previously described(Reference Pellonpera, Mokkala and Houttu17). Briefly, this study was conducted in Turku University Hospital and the University of Turku in Finland. The recruitment took place between October 2013 and July 2017 (ClinicalTrials.gov identifier: NCT01922791 https://clinicaltrials.gov/ct2/show/NCT01922791). This study was executed according to the guidelines of the Declaration of Helsinki as revised in 2013, and the protocol was approved by the Ethics Committee of the Hospital District of Southwest Finland (115/180/2012). Written informed consent was obtained from all subjects. At the first study visit in early pregnancy, eligible women were randomly assigned to one of the four parallel groups: fish oil + placebo (i.e. placebo for probiotics), probiotics + placebo (i.e. placebo for fish oil), fish oil + probiotics or placebo + placebo (placebo for probiotics and placebo for fish oil). Subjects were allocated into intervention groups according to mother’s parity and history of GDM (primipara; multipara; multipara with previous GDM). The stratified randomisation was performed with random permuted blocks of 4, and randomisation lists of the three blocks were generated by a statistician who was not involved in either study recruitment or its execution. Women were assigned to the intervention groups according to the randomisation list in their order of recruitment on the first study visit. Both study personnel and participants remained blinded to the intervention. Women visited the study unit twice during pregnancy (mean 13·9 (sd 2·1) and 35·2 (sd 0·9) gestational weeks) when their weight and body composition were measured. Supplements were consumed from the first study visit throughout the pregnancy. Women were instructed to take two fish oil capsules (a total of 2·4 g of n-3 PUFA of which 1·9 g DHA and 0·22 g EPA, Croda Europe Ltd) and one probiotic capsule (Lactobacillus rhamnosus HN001 and Bifidobacterium animalis ssp. lactis 420, each 1010 colony-forming units per capsule, ATCC SD5675 and DSM 22089; Dupont Nutrition & Health) every day. Placebo capsules for fish oil contained medium-chain fatty acids (capric acid C8 54·6 % and caprylic acid C10 40·3 %), while placebo for the probiotics consisted of microcrystalline cellulose. Placebo capsules were identical in size, shape and colour compared with their respective intervention capsules. Subjects were instructed not to consume any other probiotic or n-3 long-chain PUFA products during the trial.
The inclusion criteria were self-reported pre-pregnancy BMI ≥ 25 kg/m2, <18 gestational weeks and absence of chronic diseases (asthma and allergies were allowed). Exclusion criteria were diabetes before pregnancy (HbA1c ≥6·5 % (48 mmol/mol) or fasting glucose ≥ 7·0 mmol/l at randomisation); multifetal pregnancy; chronic diseases impacting on metabolic and gastrointestinal health including inflammatory bowel diseases; refusal to stop the intake of other probiotic or fish oil supplements; diagnosis or history of coagulopathy and anticoagulant medication.
On the first study visit, we measured the participants’ height with a wall stadiometer to the nearest 0·1 cm, and pre-pregnancy BMI was calculated using the height and self-reported pre-pregnancy weight obtained from medical records. Air displacement plethysmography and an electronic scale (the Bod Pod system, software version 5.4.0, COSMED Inc.) were used to measure the weight and the volume of the body according to the manufacturer’s instructions on both study visits. FM and FFM in kg were calculated from density using the formulas devised by van Raaij et al. (Reference van Raaij, Schonk and Vermaat-Miedema18), which take into account the gestational weeks and the presence of marked general swelling if applicable. When possible, thoracic gas volume was measured (n 385/438 in early gestation and n 341/369 in late gestation) to lower the error in the determination of body composition(Reference Pellonpera, Koivuniemi and Vahlberg19), and this value was applied in the calculations of FM and FFM, otherwise the predicted thoracic gas volume was used in body composition calculations. After overnight fasting and emptying their bladder, women entered the measurement chamber wearing a tight cap and tight underwear. They were instructed not to exercise or to shower in the morning of measurements.
Weight gain was evaluated at three different time periods: (1) from the first study visit to the second study visit, (2) from the randomisation to the end of pregnancy, that is, the period of nutritional intervention (last weight in the third trimester measured either at a maternity welfare clinic or at the second study visit, whichever visit was the latest, minus weight measured at the first study visit) and (3) during the whole pregnancy (last measured weight minus self-reported pre-pregnancy weight). Women were classified into groups of excess, ideal and inadequate GWG according to the recommendations issued by the Institute of Medicine(20) for overweight and obese women for the whole pregnancy. Additionally, we calculated the weekly GWG rate between the last gestational weight measurement and the first study visit and categorised the results according to the Institute of Medicine guidelines. In these calculations, the actual measured weight gain between the first study visit and the last measured weight before delivery was compared with recommended minimum and maximum weight gains over the same period and point of gestation.
GDM was diagnosed on the basis of a 2-h 75 g oral glucose tolerance test (OGTT) if one or more values were at or above the threshold concentration: 0 h ≥ 5·3, 1 h ≥ 10·0, 2 h ≥ 8·6 mmol/l in line with the Finnish Current Care guidelines(21). OGTT was offered by maternal welfare clinics to all women between 24 and 28 weeks and to high-risk women also at 12–16 gestational weeks (BMI ≥ 35, previous GDM, glucosuria, polycystic ovarian syndrome or family risk of diabetes). Regardless of the timing of OGTT, treatment for GDM was offered soon after diagnosis by health care services independent of the research protocol and in accordance with the national guidelines. In our analysis, we defined GDM positivity in two ways: (1) abnormal OGTT at any stage of pregnancy and (2) in the further analyses, the GDM diagnosis set only at the latter OGTT, that is, in these analyses, early pregnancy OGTT positive women were excluded.
Women filled in questionnaires concerning their health, education, smoking habits, obstetric medical history and family history of diabetes. Physical activity was also assessed by a questionnaire(Reference Mansikkaniemi, Juonala and Taimela22). Women were asked to report the intensity, frequency and duration of their habitual leisure time physical activity during the preceding week. A metabolic equivalent index for leisure time physical activity (MET-index) was calculated from the product of intensity x frequency x duration of activity (MET h/week) on both study visits. The coefficients for the intensity of physical activity were estimated from the existing tables(Reference Ainsworth, Haskell and Leon23).
Three-day food diaries (two weekdays and one weekend day) were recorded during the week preceding the study visits. Mean daily intakes of energy and energy yielding nutrients were calculated by using computerised software (AivoDiet 2.0.2.3; Aivo) utilising the food composition database provided by the Finnish National Institute for Health and Welfare (www.fineli.fi).
Statistical analyses
The pre-specified outcomes were body composition and GWG. At the time when the study was planned, there were no a priori data for the effects of probiotics or fish oil on body composition during pregnancy, the secondary outcomes of the trial, thus power calculations for these outcomes could not be performed.
The normality of the data was checked visually from histograms. The data were summarised as frequencies and percentages for categorical variables and as means and standard deviations for normally distributed continuous variables. The comparisons of baseline characteristics among the intervention groups were conducted by one-way ANOVA for continuous variables and χ 2 test or Fisher’s exact test for categorical variables, when applicable. GDM and non-GDM women were compared at baseline with two-sample t test, χ 2 test or Fisher’s exact test, when applicable.
The effect of the intervention on GWG and body composition was analysed using one-way ANOVA and χ 2 test. We also regrouped the data to compare the two bigger entities: women in the groups receiving fish oil were combined (fish oil + placebo and fish oil + probiotics) and compared with women who did not receive fish oil (probiotics + placebo and placebo + placebo). Similarly, women in the groups receiving probiotics were combined (probiotics + placebo and fish oil + probiotics) and compared with women who did not receive probiotics (fish oil + placebo and placebo + placebo combined). In these additional analyses, the effect of fish oil and probiotics on GWG and body composition was analysed by two-way ANOVA and multinomial logistic regression including the main effects of the fish oil and probiotics and the fish oil × probiotics interaction effect.
The energy consumption among the intervention groups was compared with one-way ANOVA. Physical activity was measured with the MET-index, which was not normally distributed and hence median with interquartile range was calculated and Kruskal–Wallis test applied when the intervention groups were compared.
When GWG and body composition between GDM women and non-GDM women were compared, for continuous variables, we used two-sample t test and linear model adjusted for variables that differed between the groups significantly at baseline and that were significantly associated with the measured outcome. Likewise, categorical variables were analysed with χ 2 test and, in addition, with logistic regression adjusted for confounding variables. As a result, adjustments were made for age, pre-pregnancy BMI, previous GDM, intervention group and, in the GWG analyses, also for gestational weeks at last weight measurement.
Possible associations between lifestyle variables and change in body composition measures were assessed using partial Pearson’s correlation test. Correlations of at least a medium effect size (r ≥ 0·3) were considered notable(Reference Cohen24).
A P value < 0·05 was considered significant. All analyses were performed using SAS software (version 9.4, SAS Institute Inc.).
Results
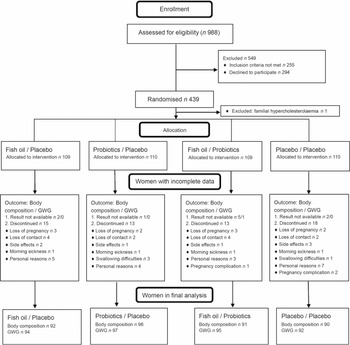
A total of 988 women from Southwest Finland were screened for eligibility, and 439 women were randomised to the intervention (Fig. 1). A total of 59 (13·5 %) women withdrew from the study before the second visit, and these women were evenly distributed among the intervention groups (P = 0·74). Additionally, ten body composition results and one GWG result were unavailable because these women gave birth before the second visit or the measurement was unsuccessful.

Fig. 1. Flow diagram. GWG, gestational weight gain.
Characteristics of participating women are presented in Table 1. Only the number of women with a family history of diabetes differed significantly among the intervention groups, the largest number being in the fish oil + placebo group.
Table 1. Characteristics of the pregnant women in the intervention groups
(Mean values and standard deviations; numbers and percentages)

GDM, gestational diabetes.
* One-way ANOVA.
† Two-sample t test.
‡ χ 2 test.
§ Significantly different from probiotics/placebo (P = 0·025) and placebo/placebo (P = 0·002).
The mean GWG from pre-pregnancy to the last measurement (1·6 (sd 1·6) weeks before delivery) was 13·0 (sd 6·3) kg, and Institute of Medicine recommendations for whole pregnancy GWG were exceeded by 64·3 % of women (Table 2). The mean GWG from the first visit to the last gestational measurement was 11·9 (sd 4·9) kg, and consequently, the recommended weekly GWG rate was excessive in 84·1 % of women (Table 2). On average, between the study visits, FM increased by 1·7 (sd 3·5) kg and FFM by 7·6 (sd 2·2) kg, thus BF% decreased by 2·4 (sd 2·6) percentage points. Compared with obese women, overweight women gained significantly more weight (12·8 (sd 4·7) kg v. 10·4 (sd 4·9) kg, P < 0·001) and FM (2·5 (sd 3·2) kg v. 0·4 (sd 3·5) kg, P < 0·001). The proportion of body fat decreased in both overweight and obese women, but significantly more in obese women (−1·8 (sd 2·6) v. −3·4 (sd 2·3) percentage points, P < 0·001).
Table 2. Gestational weight gain (GWG) and body composition in all women and in the different intervention groups*
(Mean values and standard deviations; numbers and percentages)

∆, Change.
* Women were divided into different GWG classes according to the recommendations issued by the Institute of Medicine(20).
† One-way ANOVA.
‡ χ 2 test.
Fish oil and/or probiotics intervention
Gestational weight gain was not significantly influenced by the fish oil and/or probiotic intervention (Table 2). The proportions of women either exceeding, falling below or adhering to the GWG recommendations were also essentially the same in all four groups. Additionally, we found no significant differences among the intervention groups in body composition at the first or the second study visit, or in the change of body composition between the visits (Table 2). When the groups receiving fish oil were combined and compared with the combined non-fish oil group, no significant difference in the body composition or GWG was detected (P > 0·08 in all comparisons, online Supplementary Table S1). Similarly, when the groups receiving probiotics were combined and compared with the combined non-probiotics group, no significant difference in body composition or GWG was found (P > 0·09 in all comparisons, online Supplementary Table S1). The change in physical activity or the dietary intake of energy did not differ significantly among the intervention groups (P > 0·3 in both comparisons, data not shown).
Impact of gestational diabetes
Characteristics of the pregnant women according to the GDM status are presented in Table 3. Altogether 119 (30 %) women developed GDM at some stage of their pregnancy; the other 278 (70 %) women remained normoglycaemic. Compared with the women without GDM, the women with GDM were significantly older, less well educated, had a higher pre-pregnancy weight and BMI, had more often a history of GDM and parents with diabetes. Additionally, women with GDM had their last weight measurement before delivery significantly earlier in gestation than non-diabetic women. These differences in the characteristics of women were taken into account in the adjustments of the results. Women with GDM gained significantly less weight than women without GDM between the first study visit and the last weight measurement during pregnancy (P < 0·001, Table 4). The proportion of women with an excessive weekly weight gain was significantly higher in the group of healthy women than in women with GDM. Furthermore, the proportion of women with inadequate weekly weight gain was significantly lower in the group of normoglycaemic women compared with women with GDM.
Table 3. Characteristics of the pregnant women according to gestational diabetes (GDM) status
(Mean values and standard deviations; numbers and percentages)

* Two-sample t test.
† χ 2 test.
‡ Fisher’s exact test.
Table 4. Gestational weight gain (GWG) and body composition in women diagnosed with gestational diabetes (GDM) at any stage of pregnancy and in normoglycaemic women*
(Mean values and standard deviations; numbers and percentages; adjusted mean values with their standard errors; adjusted mean difference and 95% confidence intervals; odds ratios)

∆, Change.
* Women were divided into different GWG classes according to the recommendations issued by the Institute of Medicine(20).
† Two-sample t test.
‡ Linear model adjusted for age, prepregnancy BMI, previous GDM and intervention group. GWG analyses have also been adjusted to gw at the last weight measurement.
§ χ 2 test.
|| Logistic regression adjusted for age, prepregnancy BMI, previous GDM, intervention group and gestational weeks of the last weight measurement.
¶ Significant difference between inadequate v. ideal GWG (P = 0·014) and inadequate v. excess GWG (P < 0·001).
At the first study visit, women who would be diagnosed with GDM at any stage of their pregnancy had significantly more FM and greater BF% than women without GDM (Table 4). However, after adjusting for confounding factors, especially for pre-pregnancy BMI, the differences were no longer statistically significant. On the second visit in late gestation, women with an established GDM had significantly less FM and lower BF% than women without GDM (adjusted P = 0·003 and P = 0·026 respectively, Table 4). The change in body composition from early to late pregnancy was also significantly different between women with GDM and normoglycemic women: less FM was gained and BF% reduced more in women with GDM (P < 0·001 and P = 0·011, respectively), while no significant difference between the groups was found in the change of FFM.
When the early gestation OGTT-positive women were excluded from the analyses (n 27), the results remained essentially same, that is, the body composition at the first visit did not significantly differ between women who remained normoglycaemic or who later developed GDM (online Supplementary Table S2). The only difference was that in late gestation, the BF% of women with GDM did not differ significantly from women without GDM.
When the women receiving metformin for the treatment of GDM were excluded from the analyses, the difference in change in FM and the change in BF% remained significant between women with GDM and women without GDM (P < 0·03 in all comparisons, data not shown).
No correlations were detected between lifestyle variables (change in energy intake, carbohydrates, fat, protein and change in physical activity assessed by MET-index) and change in body composition (r < 0·21).
Discussion
Supplementation with fish oil and/or probiotics did not influence the body composition during pregnancy or GWG of overweight and obese women. Women diagnosed with GDM gained less weight and FM than their normoglycaemic counterparts. In addition, their weekly weight gain was less frequently found to be excessive and conversely it was more often inadequate as compared to normoglycaemic women. Moreover, we did not observe a significant difference in the early pregnancy body composition between healthy women and those who would later be diagnosed with GDM.
Two recent systematic reviews/meta-analyses focusing on overweight or obese non-pregnant adults have found that probiotics are beneficial with regard to weight reduction and FM loss(Reference Crovesy, Ostrowski and Ferreira15,Reference Borgeraas, Johnson and Skattebu16) . In pregnant women, there are no previous studies that have assessed body composition in conjunction with a probiotic intervention. However, one RCT that investigated the effects of probiotics on weight and anthropometrics found no differences compared with placebo during pregnancy, but detected beneficial effects of the consumption of probiotics on waist circumference and biceps skinfold thickness in the 12 months’ postpartum period(Reference Ilmonen, Isolauri and Poussa25). Trials assessing weight gain, change in BMI or anthropometrics during pregnancy as a secondary outcome also revealed no differences between probiotic supplementation and placebo(Reference Wickens, Barthow and Murphy26–Reference Badehnoosh, Karamali and Zarrati28). All in all, our findings support the results of the previous studies.
Although consumption of fish oil has been speculated to reduce weight and BF% in non-pregnant individuals and in animals(Reference Bender, Portmann and Heg14,Reference Albracht-Schulte, Kalupahana and Ramalingam29) , there is a paucity of data related to pregnant women. Some studies have described a gestational weight change during an n-3 PUFA intervention as a secondary outcome, but found no significant difference compared with controls(Reference Bosaeus, Hussain and Karlsson30,Reference Haghiac, Yang and Presley31) . The information on the effects of n-3 PUFA on body composition of pregnant women is almost non-existent, as only one small study (n 35) has reported that nutritional counselling to increase fish intake did not affect gains in either FM or FFM during pregnancy in comparison with a control group(Reference Bosaeus, Hussain and Karlsson30). Our results from the present trial provide new information; they indicate that the provision of fish oil supplementation with the current dose and composition confers no benefits on the regulation of adiposity in overweight or obese pregnant women.
Proposed mechanisms by which n-3 PUFA and probiotics could work to improve body composition include alleviating adipose tissue inflammation and altering epigenetic mechanisms(Reference Crovesy, Ostrowski and Ferreira15,Reference Albracht-Schulte, Kalupahana and Ramalingam29,Reference Vahamiko, Laiho and Lund32) . It could be that the metabolic burden related to the pregnancy and obesity was too severe to be overcome by the nutritional supplements in this study. Other causes for the absence of an intervention effect could be related to the timing and duration of intervention, the probiotic strains used and to the dose and ratio of DHA and EPA in the supplements.
Body composition of women with GDM has also not been widely investigated. Some studies have assessed the possibility that the body composition in early pregnancy can predict the development of GDM. These studies have suggested that ultrasonographically measured visceral fat thickness would be associated with GDM or higher glucose values(Reference Gur, Ince and Turan12,Reference Bartha, Marin-Segura and Gonzalez-Gonzalez33–Reference De Souza, Berger and Retnakaran35) . Similarly, a number of trials conducted with bioimpedance analysis have reported that both truncal fat gains between 15 and 28 gestational weeks or FM and BF% measured at 21–24 gestational weeks are associated with the onset of GDM(Reference Sommer, Morkrid and Jenum11,Reference Xu, Gao and Li36) . Our results indicate that after adjustment for BMI, body composition measured with air displacement plethysmography in early pregnancy is not different between those women who will later develop GDM and those who will remain healthy.
Regarding late pregnancy, our data on body composition of women with GDM are novel. Our results suggest that in late pregnancy, the adiposity of diabetic women falls below that of healthy women. The reason for this finding remains unresolved. The most logical explanation would be that the treatment of GDM would result in positive lifestyle changes and improved body composition. However, we could not detect any correlation between lifestyle changes and body composition changes in women with or without GDM. The metformin medication for GDM was found not to have an effect either. Ehrenberg et al. (Reference Ehrenberg, Huston-Presley and Catalano37) have conducted a small study investigating the relationship of GDM and changes of body composition over pregnancy. They used hydrodensitometry to measure body composition before conception, at 12–14 gestational weeks and at 33–36 gestational weeks. Although the trial included only nineteen patients with GDM and thirty-three controls, the gains of FM tended to be smaller and BF% became reduced in diabetic women (P = 0·08 and P = 0·07, respectively), which is in line with our results. Whatever the reason behind the greater weight gain in normoglycaemic women as compared with diabetic women, it seems clear that the excess weight gained is mainly FM and not FFM.
This was a well-conducted prospective trial with a small drop-out rate and, considering the lack of existing data in the field involved, a large sample size. Regarding the nutritional intervention, this was also a double-blind, placebo-controlled trial. Nonetheless, we acknowledge some limitations. Since this was an analysis of secondary outcomes of a trial designed to evaluate the effect of the nutritional intervention on the incidence of GDM, power was not calculated for the intervention effect on body composition or GWG. However, when inspecting the results with their high P values and extensive variance in body composition, it is unlikely that even a considerably larger sample size would have yielded statistically significant differences. All in all, studies investigating body composition during pregnancy are small both in number and in sample size. As there is indication from past studies that fish oil and probiotics may have beneficial effects on body adiposity, and synergetic effects of these supplements during pregnancy have not been previously investigated, we assessed that the results of these secondary outcomes were worth reporting. In this respect, this study may be considered a pilot study directing the planning and execution of the potential future studies. Further studies with adequate power are also needed to clarify the effect of GDM on maternal body composition, and moreover, the role of body composition in the onset of GDM in both normal weight and in overweight/obese women. Follow-up visits of this cohort are planned, and it is yet to be seen, if the findings of lesser weight and FM gain in women with GDM compared with normoglycaemic women persist postpartum.
Limitations also relate to the method of measuring body composition. With air displacement plethysmography, like most other methods for body composition analysis during pregnancy, fetal tissues cannot be distinguished from maternal tissues. Nevertheless, air displacement plethysmography has been stated to be a valid method for measuring adiposity in overweight and obese non-pregnant women(Reference Wingfield, Smith-Ryan and Woessner38), and it has also been claimed to be the preferred method for assessing maternal FM in late pregnancy(Reference Marshall, Murphy and King13).
In conclusion, fish oil and/or probiotic supplementation did not affect weight gain or adiposity of overweight and obese pregnant women. Furthermore, it was found that women with GDM gained less weight and FM than healthy women. More studies are needed to evaluate the impact of GDM on GWG and body composition.
Acknowledgements
The authors thank Päivi Isaksson, University of Turku, for assistance with execution of the study visits and Ewen MacDonald for the English language revision.
This work was supported by the Academy of Finland (no. 258606), State research funding for university-level health research of the Turku University Hospital Expert Responsibility Area, Diabetes Research Foundation, Juho Vainio Foundation, Business Finland (no. 3486/31/2015), the Finnish Medical Foundation (personal support to O. P.), University of Turku Graduate School (personal support to O. P.). The probiotic and placebo capsules were provided by DuPont Nutrition & Health (Niebüll, Germany), and fish oil and placebo capsules were provided by Croda Europe Ltd (Leek, England). These funding sources had no role in the design, execution, analyses and interpretation of the data or in the decision to submit this article for publication.
K. L. designed the original clinical study and directed the project. T. R. and K. T. commented on the design. O. P. wrote the paper with support from K. L., O. P., K. M., N. H. and E. K. contributed to data acquisition. T. V. performed the statistical analyses. O. P. and K. L. interpreted the results. All authors read, commented and approved the final version of the paper. K. L. is the guarantor taking responsibility for the contents of the article.
There are no conflicts of interest.
Supplementary material
For supplementary materials referred to in this article, please visit https://doi.org/10.1017/S0007114520004407