Introduction
Diverse plant germplasm, including seeds, pollen and fern spores, have different functional traits that influence whether they survive drying (DT, desiccation tolerant) and how long they survive in the dry state (longevity). These traits are critical for successful ex situ conservation of germplasm (Wyse et al., Reference Wyse, Dickie and Willis2018; Ballesteros et al., Reference Ballesteros, Hill, Lynch, Pritchard and Walters2019; Colville and Pritchard, Reference Colville and Pritchard2019; Pence et al., Reference Pence, Ballesteros, Walters, Reed, Philpott, Dixon, Pritchard, Culley and Vanhove2020). The ability to survive desiccation is a common trait among seeds, pollen and fern spores. An estimated 90% of angiosperm species produce DT seeds (Wyse and Dickie, Reference Wyse and Dickie2017), DT appears to be widespread among pollen of most plant families studied (Hoekstra, Reference Hoekstra, Black and Pritchard2002; Franchi et al., Reference Franchi, Piotto, Nepi, Baskin, Baskin and Pacini2011; Nebot et al., Reference Nebot, Pritchard and Ballesteros2018) and most fern spores are DT (López-Pozo et al., Reference López-Pozo, Fernández-Marín, García-Plazaola, Ballesteros and Fernández2018).
DT is often characterized as survival to moisture levels <0.1 g H2O gDW−1 or drying to relative humidity (RH) <50% (Leprince and Buitink, Reference Leprince and Buitink2010). Germplasm lifespan when stored dry (i.e. longevity) varies with storage conditions, specifically the surrounding temperature and RH (Ellis and Roberts, Reference Ellis and Roberts1980; Buitink et al., Reference Buitink, Walters, Hoekstra and Crane1998; Ballesteros et al., Reference Ballesteros, Hill and Walters2017, Reference Ballesteros, Hill, Lynch, Pritchard and Walters2019) and possibly gaseous environment (Gonzalez-Benito et al., Reference Gonzalez-Benito, Perez-Garcia, Tejeda and Gomez-Campo2011; Groot et al., Reference Groot, de Groot, Kodde and van Treuren2015). Lethal ice does not form in germplasm dried to these levels and so freezer storage is widely used with the aim of preserving living germplasm for the long-term (e.g. FAO, 2014; Ballesteros and Pence, Reference Ballesteros, Pence and Fernández2018).
However, life cannot be preserved indefinitely during dry/cold storage, and seeds, pollen and fern spores eventually die. Seeds from numerous crops might be expected to survive for nearly 100 years (Walters et al., Reference Walters, Wheeler and Stanwood2004, Reference Walters, Wheeler and Grotenhuis2005c). Long surviving germplasm provides an opportunity to understand how drying and low-temperature interact with and affect the functional stability of cells and tissues. Rapid ageing was detected in about 26% of seed accessions from wild species stored for over 20 years under conventional freezer conditions at Kew's Millennium Seed Bank (i.e. dried at 15°C and 15% RH then placed at −20°C) (Probert et al., Reference Probert, Daws and Hay2009), and many crop seed accessions have a relatively short lifespan in dry and cold storage (Walters et al., Reference Walters, Wheeler and Grotenhuis2005c; Li and Pritchard, Reference Li and Pritchard2009; Colville and Pritchard, Reference Colville and Pritchard2019). In addition, significant ageing was detected within 25 years for dry seeds and fern spores stored at liquid nitrogen (LN) temperatures (Walters et al., Reference Walters, Wheeler and Stanwood2004; Ballesteros and Pence, Reference Ballesteros and Pence2017; Ballesteros et al., Reference Ballesteros, Hill, Lynch, Pritchard and Walters2019), despite early supposition that the low temperature of LN could extend viability ‘forever’.
Short lifespans are worrisome because aged germplasm must be regenerated or recollected, substantially reducing the efficacy and efficiency of gene banking operations for agriculture and conservation (Walters et al., Reference Walters, Wheeler and Grotenhuis2005c, Reference Walters, Ballesteros and Vertucci2010; Li and Pritchard, Reference Li and Pritchard2009; Probert et al., Reference Probert, Daws and Hay2009; Nagel and Börner, Reference Nagel and Börner2010; Hay and Probert, Reference Hay and Probert2013; Colville and Pritchard, Reference Colville and Pritchard2019). Some crop seeds, such as lettuce, celery and peanut, are known for relatively short shelf life compared to longer surviving species such as wheat or peas (Walters et al., Reference Walters, Wheeler and Grotenhuis2005c).
Considerable variation in seed longevity occurs within and among species, and even seed lots harvested in different years. DT seeds of some species, such as orchids, poplars, willows and elm deteriorate especially quickly (Pritchard and Seaton, Reference Pritchard and Seaton1993; Ballesteros and Pence, Reference Ballesteros and Pence2017; Davies et al., Reference Davies, Dickie and Ballesteros2018); pollen (Hoekstra, Reference Hoekstra2005) and some fern spores (Ballesteros et al., Reference Ballesteros, Hill, Lynch, Pritchard and Walters2019) are notorious for fast ageing. This is important as it has recently been suggested that relatively short seed lifespan seems to be a common trait in a majority of species in diverse collections and storage conditions (Colville and Pritchard, Reference Colville and Pritchard2019).
The characteristics within dry cytoplasm that result in differences in ageing rate among diverse species and cell types are poorly understood. In this paper, we take a molecular and biophysical perspective to investigate the status of life in dry cytoplasm of DT germplasm in order to postulate about the processes responsible for ageing and why there is a variation of lifespans among cells. We aim to explore whether there are specific physicochemical characteristics in some dry seeds, fern spores and pollen that make them predisposed to fast ageing. Elucidating these traits can help to predict short lifespans and ultimately devise post-harvest procedures and banking options that extend germplasm longevity.
Acquisition of DT and longevity
DT and longevity are determined in seeds during the mid and late stages, respectively, of the embryogenic development programme (Walters et al., Reference Walters, Ballesteros and Vertucci2010; Dekkers et al., Reference Dekkers, Costa, Maia, Bentsink, Ligterink and Hilhorst2015; Sano et al., Reference Sano, Rajjou, North, Debeaujon, Marion-Poll and Seo2016; Leprince et al., Reference Leprince, Pellizzaro, Berriri and Buitink2017, and references within). Embryo cells accumulate specific compounds that are associated with cells’ ability to tolerate extreme water stress; for example, low molecular weight antioxidants, complex carbohydrates such as oligosaccharides of the raffinose family for species within Fabaceae, late embryogenesis abundant proteins (LEAs), and heat-shock proteins (HSPs). Moreover, structures within cells either dismantle or fortify during development and maturation. Changes to cell structures include folded cell walls, condensed chromatin and dismantled thylakoids of chloroplasts (Webb and Arnott, Reference Webb and Arnott1982; Vertucci and Farrant, Reference Vertucci, Farrant, Kigel and Galili1995; Hoekstra, Reference Hoekstra2005; Carranco et al., Reference Carranco, Espinosa, Prieto-Dapena, Almoguera and Jordano2010; Nagel et al., Reference Nagel, Kranner, Neumann, Rolletschek, Seal, Colville, Fernández-Marín and Börner2015; Sano et al., Reference Sano, Rajjou, North, Debeaujon, Marion-Poll and Seo2016; Arif et al., Reference Arif, Nagel, Lohwasser and Börner2017; Leprince et al., Reference Leprince, Pellizzaro, Berriri and Buitink2017; Pereira Lima et al., Reference Pereira Lima, Buitink, Lalanne, Rossi, Pelletier, da Silva and Leprince2017; López-Pozo et al., Reference López-Pozo, Fernández-Marín, García-Plazaola, Ballesteros and Fernández2018). DT cells tend to be undifferentiated, having relatively small if any vacuoles and considerable deposits of dry matter reserves (Farrant et al., Reference Farrant, Pammenter, Berjak and Walters1997; Pérez et al., Reference Pérez, Hill and Walters2012; Walters, Reference Walters2015). These structural changes presumably reduce metabolic activity while also mitigating mechanical stress of cell shrinkage incumbent when cells lose water (Walters, Reference Walters2015).
The molecular and structural changes to embryonic cells during maturation appear guided by suites of genes that are activated or inhibited in a highly regulated co-expression network (Leprince et al., Reference Leprince, Pellizzaro, Berriri and Buitink2017, and references therein). Gene regulation and longevity are also influenced by maternal effects, such as environmental cues and growing conditions of the parent plant (Carranco et al., Reference Carranco, Espinosa, Prieto-Dapena, Almoguera and Jordano2010; Nagel et al., Reference Nagel, Kranner, Neumann, Rolletschek, Seal, Colville, Fernández-Marín and Börner2015; Sano et al., Reference Sano, Rajjou, North, Debeaujon, Marion-Poll and Seo2016; Arif et al., Reference Arif, Nagel, Lohwasser and Börner2017; Leprince et al., Reference Leprince, Pellizzaro, Berriri and Buitink2017; Pereira Lima et al., Reference Pereira Lima, Buitink, Lalanne, Rossi, Pelletier, da Silva and Leprince2017).
Germplasm that acquires DT survives water removal and the consequent changes in structure and function within cells (Walters et al., Reference Walters, Ballesteros and Vertucci2010; Walters, Reference Walters2015; Sano et al., Reference Sano, Rajjou, North, Debeaujon, Marion-Poll and Seo2016; Leprince et al., Reference Leprince, Pellizzaro, Berriri and Buitink2017). As cellular constituents compress when cells dehydrate, reacting substrates concentrate and molecules are no longer dispersed within the cell. Without protection, there is greater opportunity to interact with neighbouring molecules to destabilize important cellular machinery through oxidations, cross-linking and structural relaxations (Walters et al., Reference Walters, Ballesteros and Vertucci2010; Ballesteros and Walters, Reference Ballesteros and Walters2011, Reference Ballesteros, Hill, Lynch, Pritchard and Walters2019; Fleming et al., Reference Fleming, Richards and Walters2017). The ability of dry cytoplasm to avoid or ameliorate ageing reactions, maintain function and survive becomes crucial and is a critical feature of longevity – not dying while dry. The properties of the cytoplasm that forms, vis-a-vis the amount of molecular movement that is allowed and the reactivity of adjacent molecules, defines the persistence of dried germplasm (Walters et al., Reference Walters, Ballesteros and Vertucci2010; Ballesteros and Walters, Reference Ballesteros and Walters2011, Reference Ballesteros and Walters2019; Walters, Reference Walters2015).
Biochemistry of seed ageing
Ageing-associated degradation is mostly characterized by an accumulation of oxidized molecules (e.g. Kranner et al., Reference Kranner, Birtić, Anderson and Pritchard2006; Rajjou et al., Reference Rajjou, Lovigny, Groot, Belghazi, Job and Job2008). Reactions involving free radicals (FR) or reactive oxygen species (ROS) are often implicated in ageing (Wang et al., Reference Wang, Li, Xue, Pritchard and Wang2015; Nagel et al., Reference Nagel, Seal, Colville, Rodenstein, Un, Richter, Pritchard, Börner and Kranner2019). These ubiquitous molecules are particularly hungry for electrons, abstracting them from other organic molecules, and becoming reduced while the molecule that lost an electron is oxidized. Antioxidants are particularly generous and readily donate electrons, which prevents ROS from seeking electrons and damaging molecules critical to cell machinery. The diversity of products, such as volatile organic carbons (VOC), DNA and RNA fragments, loss in antioxidant redox potential and carbonylated proteins, supports growing evidence that all cellular constituents are potential substrates or targets for ageing reactions and that an initial oxidation event can cascade into molecular fragmentation or cross-linking (Fig. 1; Walters et al., Reference Walters, Ballesteros and Vertucci2010; Colville et al., Reference Colville, Bradley, Lloyd, Pritchard, Castle and Kranner2012; Nagel et al., Reference Nagel, Kranner, Neumann, Rolletschek, Seal, Colville, Fernández-Marín and Börner2015; Mira et al., Reference Mira, Hill, González-Benito, Ibáñez and Walters2016; Fleming et al., Reference Fleming, Richards and Walters2017, Reference Fleming, Patterson, Reeves, Richards, Gaines and Walters2018).
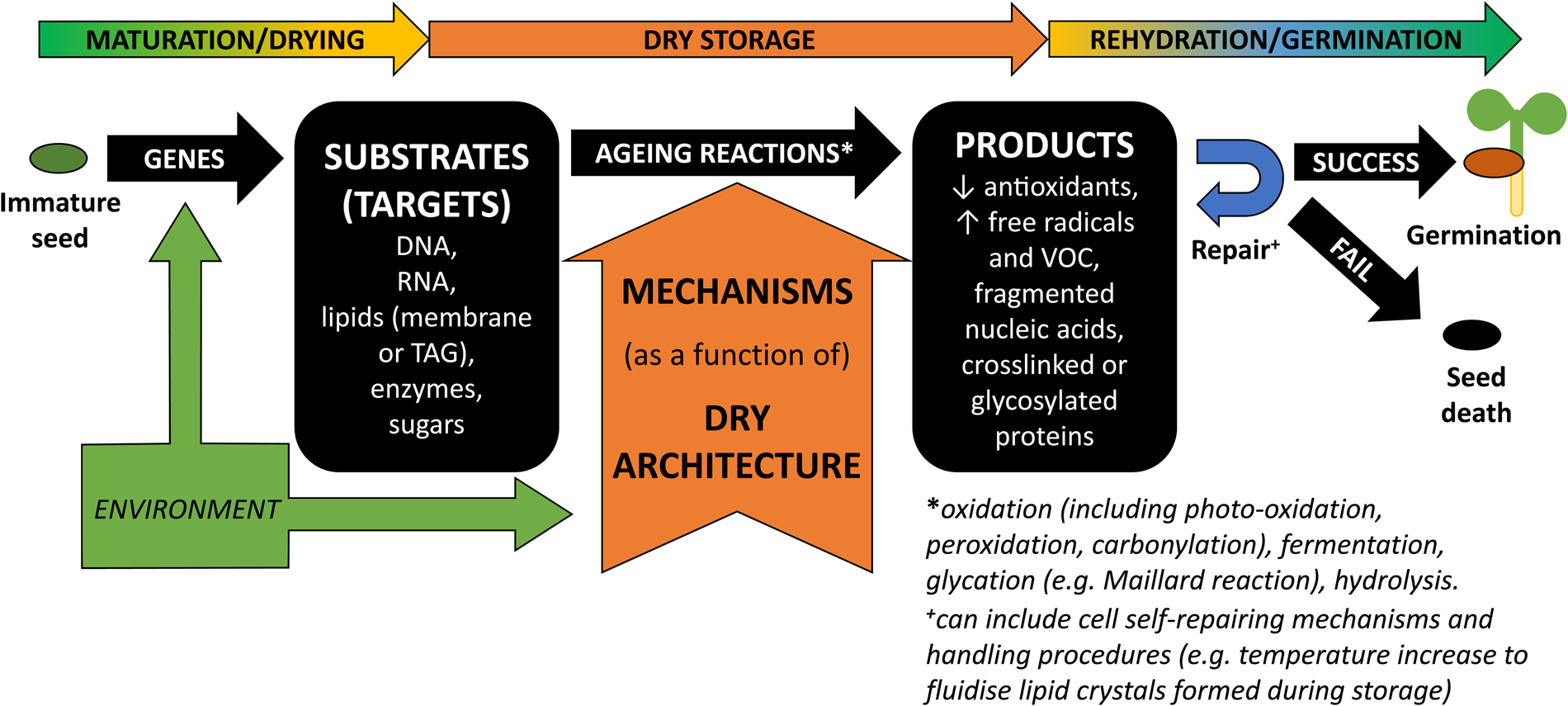
Fig. 1. Schematic representation of the substrate-dependent ageing mechanisms that generate products impacting on whether a seed can or cannot germinate after storage. During seed development and maturation/drying, diverse specific biochemical compounds are accumulated in the cells. These are determined by activation/inhibition of a variety of genes but are also influenced by environmental cues (Nagel et al., Reference Nagel, Kranner, Neumann, Rolletschek, Seal, Colville, Fernández-Marín and Börner2015; Sano et al., Reference Sano, Rajjou, North, Debeaujon, Marion-Poll and Seo2016; Arif et al., Reference Arif, Nagel, Lohwasser and Börner2017; Leprince et al., Reference Leprince, Pellizzaro, Berriri and Buitink2017; Pereira Lima et al., Reference Pereira Lima, Buitink, Lalanne, Rossi, Pelletier, da Silva and Leprince2017). Some of these biochemical compounds are correlated to the acquisition of seed longevity and will be the cell's substrates or targets for the ageing reactions, which include oxidation (including photo-oxidation, peroxidation and carbonylation), fermentation, glycation (e.g. Maillard reaction) and hydrolysis (Rajjou et al., Reference Rajjou, Lovigny, Groot, Belghazi, Job and Job2008; Kranner et al., Reference Kranner, Chen, Pritchard, Pearce and Birtić2011; Colville et al., Reference Colville, Bradley, Lloyd, Pritchard, Castle and Kranner2012; Nagel et al., Reference Nagel, Kranner, Neumann, Rolletschek, Seal, Colville, Fernández-Marín and Börner2015; Mira et al., Reference Mira, Hill, González-Benito, Ibáñez and Walters2016; Sano et al., Reference Sano, Rajjou, North, Debeaujon, Marion-Poll and Seo2016; Leprince et al., Reference Leprince, Pellizzaro, Berriri and Buitink2017). Ageing reactions lead to the formation of diverse ‘products’ that have been used to probe and predict seed longevity (e.g. Kranner et al., Reference Kranner, Birtić, Anderson and Pritchard2006; Mira et al., Reference Mira, Hill, González-Benito, Ibáñez and Walters2016; Fleming et al., Reference Fleming, Patterson, Reeves, Richards, Gaines and Walters2018). During dry storage, environmental factors such as moisture and temperature are going to be the main drivers for ageing in DT seeds and the maintenance of a solid cytoplasm (a.k.a. glassy state) is the basis for seed bank strategies (FAO, 2014; Walters, Reference Walters2015).
The reduction–oxidation reactions associated with ageing are usually not catalysed by enzymes and so require the presence of highly energetic (i.e. mobile) molecules that can diffuse through the cytosol and reach relatively distant targets (Roudaut et al., Reference Roudaut, Simatos, Champion, Contreras-Lopez and Le Meste2004). Alternatively, neighbouring molecules may be so confined that the close proximity allows enhanced interaction, as is the case of oxidation of proteins by auto-oxidized lipids in microencapsulated oils (Velasco et al., Reference Velasco, Dobarganes and Márquez-Rui2003), deamidation of proteins (Manning et al., Reference Manning, Chou, Murphy, Payne and Katayama2010) and reduction of disulphide bonds of amino acids by nearby photo-excited tryptophan (Miller et al., Reference Miller, Hageman, Thamann, Barron and Schöneich2003). Given a constant pressure of oxidation, the placement of antioxidants adjacent to core machinery or to block the pathway of diffusing oxidizers is critical (Halliwell and Gutteridge, Reference Halliwell and Gutteridge1999; Walters et al., Reference Walters, Ballesteros and Vertucci2010).
Diverse reactions are increasingly reported in dry, viable germplasm before a change in physiology (i.e. mortality). For example, VOC, mostly indicative of lipid peroxidation (Mira et al., Reference Mira, González-Benito, Hill and Walters2010, Reference Mira, Hill, González-Benito, Ibáñez and Walters2016), fragmented nucleic acids (Kranner et al., Reference Kranner, Chen, Pritchard, Pearce and Birtić2011; Fleming et al., Reference Fleming, Richards and Walters2017, Reference Fleming, Patterson, Reeves, Richards, Gaines and Walters2018), decrease in pH, likely due to membrane deterioration (Nagel et al., Reference Nagel, Seal, Colville, Rodenstein, Un, Richter, Pritchard, Börner and Kranner2019) and loss of glutathione reducing capacity (Kranner et al., Reference Kranner, Birtić, Anderson and Pritchard2006), occur in seeds that maintain germination potential. Loss of antioxidant capacity of glutathione appears to trigger irreversible, deleterious changes that have been correlated with programmed cell death (PCD) (Schafer and Buettner, Reference Schafer and Buettner2001; Kranner et al., Reference Kranner, Birtić, Anderson and Pritchard2006). Oxidized molecules of DNA, lipids, carbohydrates and proteins are readily found in dry cells that have lost viability and it is unclear whether these changes are a cause or consequence of mortality (Walters, Reference Walters1998; McDonald, Reference McDonald1999; Rajjou et al., Reference Rajjou, Lovigny, Groot, Belghazi, Job and Job2008; Kalemba and Pukacka, Reference Kalemba and Pukacka2014; Mira et al., Reference Mira, Hill, González-Benito, Ibáñez and Walters2016; Nagel et al., Reference Nagel, Seal, Colville, Rodenstein, Un, Richter, Pritchard, Börner and Kranner2019). Dead plant cells can be distinguished from live ones by glutathione half-cell reduction potential increasing to up to −180 to −160 mV (Kranner et al., Reference Kranner, Birtić, Anderson and Pritchard2006; Nagel et al., Reference Nagel, Seal, Colville, Rodenstein, Un, Richter, Pritchard, Börner and Kranner2019), which likely signals the cell's inability to buffer an oxidizing environment.
Eventually, dry germplasm loses germination potential and the question arises about the cause of the discontinuity between viable and inviable. In other words, is lost function caused by a culmination of minor damage that eventually has a major effect, or by a sudden weakening of protective mechanisms that lead to failure of critical cellular machinery? Are all molecules in the cell equally susceptible to degradation or are some molecules more prone to damage, and what damage has greatest impact on viability? Indeed, the period that viability is maintained (i.e. longevity) may be highly dependent on the variation in susceptibility of different molecules to ageing reactions and to the impact of different damaged molecules on function. Therefore, the organization and chemical composition of the cytoplasm is likely to have profound effects on the longevity trait (Priestley, Reference Priestley1986; Horbowicz and Obendorf, Reference Horbowicz and Obendorf1994; Hoekstra, Reference Hoekstra2005; Walters et al., Reference Walters, Wheeler and Grotenhuis2005c, Reference Walters, Ballesteros and Vertucci2010; Probert et al., Reference Probert, Daws and Hay2009; Nagel et al., Reference Nagel, Kranner, Neumann, Rolletschek, Seal, Colville, Fernández-Marín and Börner2015; Walters, Reference Walters2015). The mechanism of the protection is likely to vary depending on whether ageing reflects specific or random events, and this distinction might provide insight into the unexplained variation of longevity within and among cell types, species and growth environments.
The role of glassy matrices in survival and deterioration of dried germplasm
The quiescence of dry organisms makes it difficult to pinpoint the moment of death from desiccation or ageing as well as the particular reaction that caused mortality. This quiescence is a consequence of being dry, which leads to a metabolic shutdown as the cytoplasm transitions from fluid to solid. Solidification limits diffusion of molecules that comprise the matrix thereby inhibiting most reactions, especially those that are catalysed by enzymes (Roudaut et al., Reference Roudaut, Simatos, Champion, Contreras-Lopez and Le Meste2004; Fernández-Marin et al., Reference Fernández-Marín, Kranner, Sebastián, Artetxe, Laza, Vilas, Pritchard, Nadajaran, Minguez, Becerril and García-Plazaola2013; Nagel et al., Reference Nagel, Seal, Colville, Rodenstein, Un, Richter, Pritchard, Börner and Kranner2019).
Diffusion is limited in solids because molecules are pressed together when water is removed, entrapping each other and slowing down movement (Fig. 2). At some point, the flow of molecules stops. Molecules within the cytosol or cytoplasm of cells are diverse (Fig. 2a). About 50% of the dry matter is protein, of which half could be storage proteins and up to 20% of the protein in DT cells may contain intrinsically disordered proteins (IDPs), such as LEA and HSPs. Soluble carbohydrates (such as sugars including trehalose, raffinose and stachyose) comprise up to 5–10% of the dry mass, and ions comprise about 2%. The cytosol also contains other diverse macromolecules, such as antioxidants and RNAs. Upon drying, the cytosol and internal compartments of organelles thicken and cellular constituents compress and interact with each other and with membrane surfaces (Fig. 2b), essentially locking them into position. Eventually, the matrix stabilizes into a structure that holds its shape and, in DT cells, preserves function of lipid bilayers, organelles and cytosolic macromolecules (Fig. 2c; Koster et al., Reference Koster, Lei, Anderson, Martin and Bryant2000; Wolkers et al., Reference Wolkers, McCready, Brandt, Lindsey and Hoekstra2001; Cicerone et al., Reference Cicerone, Tellington, Trost and Sokolov2003; Walters and Koster, Reference Walters, Koster, Jenks and Wood2007; Buitink and Leprince, Reference Buitink and Leprince2008; Thalhammer et al., Reference Thalhammer, Hundertmark, Popova, Seckler and Hincha2010, Reference Thalhammer, Bryant, Sulpice and Hincha2014; Walters et al., Reference Walters, Ballesteros and Vertucci2010; Ballesteros and Walters, Reference Ballesteros and Walters2011; Cornette and Kikawada, Reference Cornette and Kikawada2011; Hincha and Thalhammer, Reference Hincha and Thalhammer2012; Walters, Reference Walters2015; Bremer et al., Reference Bremer, Wolff, Thalhammer and Hincha2017; Boothby et al., Reference Boothby, Piszkiewicz, Mehta, Brozena, Tapia, Koshland, Holehouse, Pappu, Goldstein and Pielak2018). In contrast, in cells of DS seeds, the translocation PLDα1 to the membrane facilitates the production of phosphatidic acid (PA) and destabilization of the plasma membrane before the matrix stabilizes (Chen et al., Reference Chen, Yu, Pritchard and Li2017).

Fig. 2. Schematic representation of the glass formation in a plant cell during drying at diverse relative humidity (RH). Cell's water potential and volume change from initial are also indicated. (a) Cell cytoplasm before drying: the cytosol of the cells contains a mixture of sugars (trehalose, raffinose and stachyose: white hexagonal figures), proteins (e.g. LEAs and other IDP, blue lines) and other biochemical and metabolites (e.g. antioxidants, green triangles); organelles with fluid contents are immersed in this cytosol. (b) As cell dries <95% RH, cell volume decreases up to 75%, cell wall folds, LEAs modify their 3D structure, and with sugars and ions, by forming a gel, encapsulate other cell constituents and structures, such as cell organelles; organelle content also jellifies as water is removed. In this stage, voids form in the aqueous cellular matrix. (c) Cell cytoplasm after drying: the jelly cytosol vitrifies forming the glass and locking the cytoplasmic contents, containing among diverse biomolecules, vitrified (solid figures) or fluid (bubbly figure) organelles (Walters, Reference Walters2004, Reference Walters2015; Walters and Koster, Reference Walters, Koster, Jenks and Wood2007; Thalhammer et al., Reference Thalhammer, Hundertmark, Popova, Seckler and Hincha2010, Reference Thalhammer, Bryant, Sulpice and Hincha2014; Walters et al., Reference Walters, Ballesteros and Vertucci2010; Ballesteros and Walters, Reference Ballesteros and Walters2011; Cornette and Kikawada, Reference Cornette and Kikawada2011; Hincha and Thalhammer, Reference Hincha and Thalhammer2012; Boothby et al., Reference Boothby, Piszkiewicz, Mehta, Brozena, Tapia, Koshland, Holehouse, Pappu, Goldstein and Pielak2018).
When the diverse solutes of the cytosol compress during drying, the structure that forms is disorganized and comprises of pores having different shapes and sizes. Hence, dried cytoplasm is called a glass or glassy matrix, to distinguish it from crystalline solids in which molecules pack together in a regular pattern forming uniform-shaped pores. Solidification to glass is called a glass transition or vitrification, and the temperature at which this occurs is abbreviated T g. The reverse solid-to-fluid transition is called plasticization and water is a potent plasticizer of cytoplasmic glasses, meaning that it reduces T g by increasing pore size so that immobilized molecules can relax. At room temperature and moisture contents corresponding to about 50% RH, the cytoplasm is, on average, near the fluid-to-solid transition (Buitink and Leprince, Reference Buitink and Leprince2008; Ballesteros and Walters, Reference Ballesteros and Walters2011, Reference Ballesteros, Hill, Lynch, Pritchard and Walters2019). However, cell components are not uniformly dispersed within the cytoplasm. Localized differences in the nature and concentration of molecules can lead to localized differences in fluid-to-solid transitions. This was described for natural deep eutectic solvents (NADES), which maintain high fluidity in a concentrated solution (Choi et al., Reference Choi, van Spronsen, Dai, Verberne, Hollmann, Arends, Witkamp and Verpoorte2011). Furthermore, heterogeneity of pores can create mobility islands where the localized diffusional motion of small molecules and the relaxation of large molecules is allowed (Roudaut et al., Reference Roudaut, Simatos, Champion, Contreras-Lopez and Le Meste2004). Gaseous molecules, which are not part of the solid matrix, are still able to diffuse in a solid if pores are large enough. Pore size also affects short-range motion that persists in solids such as stretching, bending, rotation and vibration of atoms and ligands (Cicerone et al., Reference Cicerone, Tellington, Trost and Sokolov2003; Roudaut et al., Reference Roudaut, Simatos, Champion, Contreras-Lopez and Le Meste2004; Ballesteros and Walters, Reference Ballesteros and Walters2011). These short-range fast motions can lead to a reorientation of side chains of large molecules and ultimately intermolecular interactions or further relaxation of the structure (Walters, Reference Walters2004; Ballesteros and Walters, Reference Ballesteros and Walters2019). Therefore, both the composition and packing efficiency of molecules have profound effects on the ultimate physical and chemical stability of the matrix.
Moreover, the physical structure of non-water soluble constituents, like lipids, is hardly influenced by water. Oil droplets remain fluid within cells until exposed to low temperatures (Crane et al., Reference Crane, Miller, Van Roekel and Walters2003; Ballesteros et al., Reference Ballesteros, Narayan, Varghese and Sershen2018, Reference Ballesteros, Hill, Lynch, Pritchard and Walters2019). Cells with high triacylglycerol (TAG) components may be analogous to composite materials that are engineered from polymers and plastics. Combining components with different physical–chemical properties can improve strength, or reduce mass or cost. However, composite materials may be prone to failure when environmental conditions change, causing the components to separate. Co-existence of fluid and solid cellular regions could also have serious implications for the nature and kinetics of ageing reactions by facilitating long-range diffusion of ROS (Fryars et al., Reference Fryars, Limanton, Gauffre, Paquin, Lagrost and Hapiot2018), allowing catabolic reactions more typical of deterioration under fluid conditions (Velasco et al., Reference Velasco, Dobarganes and Márquez-Rui2003) and encouraging molecular realignment and pore reconfiguration (Ballesteros et al., Reference Ballesteros, Hill, Lynch, Pritchard and Walters2019).
Water tends to remain mobile in glassy systems (Roudaut et al., Reference Roudaut, Simatos, Champion, Contreras-Lopez and Le Meste2004) and can be removed by exposure to extremely low RH. Under very dry conditions (<10% RH), water can act as an anti-plasticizer to reduce molecular mobility and increase structural stability (Pittia and Sacchetti, Reference Pittia and Sacchetti2008). This duality of water as plasticizer or anti-plasticizer, depending on the proximity of conditions to T g, might explain increased ageing rates in seeds that have been excessively dried (Vertucci et al., Reference Vertucci, Roos and Crane1994). The mechanical properties of seeds appeared more stable at low temperatures in the presence of some water and complete drying tended to increase molecular mobility (Ballesteros and Walters, Reference Ballesteros and Walters2011). Other low weight molecules solutes, such as glycerol, dimethyl sulphoxide and ethylene glycol, can also act as anti-plasticizers to stabilize molecular relaxations and local motions (Cicerone et al., Reference Cicerone, Tellington, Trost and Sokolov2003).
The susceptibility and rate of degradation of diverse biological molecules differ in solid matrices and fluid systems owing to the nature and spatial scale of molecular motion and interactions (Cicerone et al., Reference Cicerone, Tellington, Trost and Sokolov2003; Velasco et al., Reference Velasco, Dobarganes and Márquez-Rui2003; Roudaut et al., Reference Roudaut, Simatos, Champion, Contreras-Lopez and Le Meste2004). Analogously, we may expect that the mechanisms that cause ageing and eventual mortality in dry seeds may differ from cells that are fluid and allow reactions that are dominated by long-range diffusive motion (Walters, Reference Walters1998; Ballesteros and Walters, Reference Ballesteros and Walters2011; Fernández-Marin et al., Reference Fernández-Marín, Kranner, Sebastián, Artetxe, Laza, Vilas, Pritchard, Nadajaran, Minguez, Becerril and García-Plazaola2013; Nagel et al., Reference Nagel, Seal, Colville, Rodenstein, Un, Richter, Pritchard, Börner and Kranner2019).
The predominant motions within glasses are short range, which increases the likelihood of cross-linking or fragmentation (Roudaut et al., Reference Roudaut, Simatos, Champion, Contreras-Lopez and Le Meste2004; Ballesteros and Walters, Reference Ballesteros and Walters2011, Reference Ballesteros and Walters2019; López-Pozo et al., Reference López-Pozo, Ballesteros, Laza, García-Plazaola and Fernández-Marín2019). For example, solidified peptides and proteins are susceptible to deamidation, which hydrolyses the amide side chains of asparagine and glutamine (Manning et al., Reference Manning, Chou, Murphy, Payne and Katayama2010). Cross-linking can occur between reducing sugars and peptides in close proximity (i.e. glycation) (Povey et al., Reference Povey, Perez-Moral, Noel, Parker, Howard and Smales2009). Light-induced oxidation of solidified protein mixtures is common and can result in a number of breakdown products (Miller et al., Reference Miller, Hageman, Thamann, Barron and Schöneich2003; Manning et al., Reference Manning, Chou, Murphy, Payne and Katayama2010). These reactions arise from slight molecular rearrangements within the molecule (Cicerone et al., Reference Cicerone, Tellington, Trost and Sokolov2003; Roudaut et al., Reference Roudaut, Simatos, Champion, Contreras-Lopez and Le Meste2004; Ballesteros and Walters, Reference Ballesteros and Walters2011).
The small movements of molecules within the glassy matrix can be a source of ‘physical ageing’ of the structure, which has been implicated in the deterioration of foods, plastics and pharmaceutical compounds (e.g. Cicerone et al., Reference Cicerone, Tellington, Trost and Sokolov2003; Roudaut et al., Reference Roudaut, Simatos, Champion, Contreras-Lopez and Le Meste2004; Kucera et al., Reference Kucera, Felton and McGinity2013; Cowie and Arrighi, Reference Cowie, Arrighi, Utracki and Wilkie2014). Over time, molecules that were entrapped by neighbouring molecules budge (or relax) and begin to fill in the pores. The rate of compression reflects molecular mobility within the matrix, which is determined by temperature, molecular packing, presence of plasticizers and anti-plasticizers and the innate flexibility and space-filling properties of constituents of the matrix (Cicerone et al., Reference Cicerone, Tellington, Trost and Sokolov2003; Roudaut et al., Reference Roudaut, Simatos, Champion, Contreras-Lopez and Le Meste2004; Walters, Reference Walters2004; Walters et al., Reference Walters, Ballesteros and Vertucci2010; Ballesteros and Walters, Reference Ballesteros and Walters2011, Reference Ballesteros and Walters2019).
Slow diffusion of oxygen or other gaseous ROS molecules through the large pores of glassy matrices is an important factor in the deterioration of polymers and biomolecules (Roudaut et al., Reference Roudaut, Simatos, Champion, Contreras-Lopez and Le Meste2004). Photodegradation of proteins depends on the presence of molecular oxygen (Miller et al., Reference Miller, Hageman, Thamann, Barron and Schöneich2003) and auto-oxidation is facilitated in the presence of mobile ROS (Minemoto et al., Reference Minemoto, Adachi and Matsuno2001; Velasco et al., Reference Velasco, Dobarganes and Márquez-Rui2003). Volatilized by-products of oxidized fatty acids can react with surrounding molecules, including proteins, which may lead to eventual impairment of catalytic function (Velasco et al., Reference Velasco, Dobarganes and Márquez-Rui2003).
To summarize, dehydrating cytoplasm eventually leads to a solid with heterogeneous properties and domains of high and low fluidity due to the diverse mixture of cellular constituents as well as the imperfect packing. DT organisms survive the immediate effects of cell shrinkage and molecular compression that caused solidification, but eventually, succumb with time to deteriorative reactions that are highly dependent on molecular mobility and proximity. We expect the nature of deteriorative reactions to differ in fluid and solid cytoplasm because restricted diffusive motion in structures affects the probabilities of different molecular interactions. Structure and mobility within a solid are inextricably related. Here, we make the case that the structure of the solid matrix is a critical factor in the long-term survival of DT organisms.
Dry architecture of dry germplasm
Solid matrices stabilize cytoplasm by slowing reactions, but the protection provided is not indefinite. Deterioration occurs and ultimately causes mortality. Duration of survival (i.e. longevity) varies considerably among organisms (Priestley, Reference Priestley1986; Hoekstra, Reference Hoekstra2005; Walters et al., Reference Walters, Hill and Wheeler2005a,Reference Walters, Wheeler and Grotenhuisc; Probert et al., Reference Probert, Daws and Hay2009; Nagel and Börner, Reference Nagel and Börner2010; Ballesteros et al., Reference Ballesteros, Hill, Lynch, Pritchard and Walters2019). The intrinsic differences in longevity among cell types and species suggest that chemical reactivity varies in glassy matrices or that cells have different tolerances to chemical reactivity.
Biochemical composition, in terms of total protein, lipid and carbohydrates, does not provide strong insights into the susceptibility of seeds to ageing, though some trends emerge that suggest that cell structures are an important factor in the longevity of dried germplasm. For example, long-lived Fabaceae seeds lack storage lipids and have highly degraded photosystems, for example, peas and beans (Farrant et al., Reference Farrant, Pammenter, Berjak and Walters1997; Walters et al., Reference Walters, Wheeler and Grotenhuis2005c). On the other hand, short-lived cells maintain well-developed photosystems, for example, chlorophyllous seeds and fern spores (Roqueiro et al., Reference Roqueiro, Facorro, Huarte, de Celis, Garcia, Maldonado and Maroder2010; Ballesteros et al., Reference Ballesteros, Hill, Lynch, Pritchard and Walters2019). In addition, some seeds and fern spores with high lipid content age faster in the freezer at −20°C than in the refrigerator at 5°C (Dussert et al., Reference Dussert, Chabrillange, Rocquelin, Engelmann, Lopez and Hammon2001; Crane et al., Reference Crane, Miller, Van Roekel and Walters2003; Hor et al., Reference Hor, Kim, Ugap, Chabrillange, Sinniah, Engelmann and Dussert2005; Ballesteros et al., Reference Ballesteros, Hill, Lynch, Pritchard and Walters2019; Fleming et al., Reference Fleming, Hill and Walters2019).
In this paper, we hypothesize that different structural conformations promote distinctive ageing mechanisms in dry cells (e.g. Ballesteros et al., Reference Ballesteros, Narayan, Varghese and Sershen2018), particularly under preservation conditions used by most seed banks (−20°C; FAO, 2014), and that this can lead to faster than expected ageing. Three main conformations of dry cells are presented to illustrate how chlorophyll content and storage lipids can affect physical–chemical properties: (1) non-chlorophyllous cells lacking TAG (Fig. 3a), (2) chlorophyllous cells lacking TAG (Fig. 3b) and (3) non-chlorophyllous cells with TAG (Fig. 3c).

Fig. 3. Schematic representation of three basic structural cell conformations before and after drying. During drying, aqueous vacuoles (V) are reduced and mostly disappear, organelles come in close proximity, some may shrink [e.g. the nucleus (N) and mitochondria (M)], and cell walls are slightly folded. (a) Non-chlorophyllous cells with very low TAG: cytoplasm is occupied by dry matter (D), including protein storage bodies, starch and sometimes small amount of lipid droplets (L). Internal membranes, such as the endoplasmic reticulum and the Golgi apparatus, are not represented for simplicity of the model but are considered as part of N and D, respectively. (b) Chorophyllous cells with low TAG: chloroplasts (C) are not degraded in the maturation phase and are present. (c) Non-chlorophyllous cells with high storage lipids: cytoplasm is occupied by dry matter (D), including some protein storage bodies and starch but mostly by lipid droplets (L).
Non-chlorophyllous cells with low TAG (Fig. 3a)
Photosynthetic pigments, including chlorophyll and carotenoids, become degraded during maturation and drying of DT cells (Vertucci and Farrant, Reference Vertucci, Farrant, Kigel and Galili1995; Leprince et al., Reference Leprince, Pellizzaro, Berriri and Buitink2017). During embryogenesis, DT cells also accumulate high amounts of protein, carbohydrates or TAG, replacing more than 50% of the cellular water with these dry matter reserves (Farrant et al., Reference Farrant, Pammenter, Berjak and Walters1997; Walters et al., Reference Walters, Ballesteros and Vertucci2010; Pérez et al., Reference Pérez, Hill and Walters2012; Walters, Reference Walters2015). About one-third of angiosperm taxa, including representatives in Poaceae and Fabaceae, favour protein and carbohydrate dry matter, synthesizing less than a 10% of accumulated dry matter as TAG (Fig. 4a); half of these accumulate less than 5% TAG (Royal Botanic Gardens Kew SID, 2018). The accumulated dry matter could be a plentiful substrate for oxidation within the glassy matrix, with proteins and starch possibly serving as scavengers of ROS (Fig. 5). TAG in these cells may be susceptible to peroxidation (Fig. 5), but the densely packed protein bodies and starch granules probably slow transport of oxidized lipid by-products from the small and dispersed lipid droplets.

Fig. 4. Lipid (TAG) composition in seeds from 2,865 species that are listed in the Kew Seed Information Database that are (a) orthodox (DT) and (b) considered ‘intermediate’ between orthodox and recalcitrant (not DT). In (c), a comparison of lipid composition is provided for seeds within Poaceae (black bars) and Fabaceae (white bars), both families producing predominantly DT seeds.

Fig. 5. A schematic model of ageing in dry cells that begins with accumulated ROS and FR from background levels or impaired metabolism during drying. With time, the efficiency of the antioxidant machinery declines and leads to autocatalytic reactions and damaged molecules. ROS and FR attack of abundant starch or storage proteins are not as deleterious as attack on genetic (DNA and RNA), structural (e.g. membranes, carbohydrates and soluble proteins) or metabolic (e.g. mitochondria, enzymes and membrane proteins) components (Davies, Reference Davies2005; Kranner et al., Reference Kranner, Birtić, Anderson and Pritchard2006; Bailly et al., Reference Bailly, El-Maarouf-Bouteau and Corbineau2008; Rajjou et al., Reference Rajjou, Lovigny, Groot, Belghazi, Job and Job2008; Manning et al., Reference Manning, Chou, Murphy, Payne and Katayama2010; Kalemba and Pukacka, Reference Kalemba and Pukacka2014; Mira et al., Reference Mira, González-Benito, Hill and Walters2010, Reference Mira, Hill, González-Benito, Ibáñez and Walters2016; Fleming et al., Reference Fleming, Richards and Walters2017, Reference Fleming, Patterson, Reeves, Richards, Gaines and Walters2018; Nagel et al., Reference Nagel, Seal, Colville, Rodenstein, Un, Richter, Pritchard, Börner and Kranner2019). Increased pH in the dry cytoplasm (Nagel et al., Reference Nagel, Seal, Colville, Rodenstein, Un, Richter, Pritchard, Börner and Kranner2019) may compromise rehydration/germination or alter the rate of protein glycation within the glassy matrix (Povey et al., Reference Povey, Perez-Moral, Noel, Parker, Howard and Smales2009). M, Mitochondria; N, Nucleus; L, lipid bodies; D, Dry matter (e.g. protein bodies and starch).
The determined or predicted lifespan of seeds with non-chlorophyllous cells and <10% TAG ranges broadly from <10 to >400 years for specific dry and cold storage conditions (Walters et al., Reference Walters, Wheeler and Grotenhuis2005c). We postulate that variation in longevity arises from different properties of the solid matrices among dried seeds. For example, a comparison of mechanical properties of cells within dried embryonic axes pea and soybean, both non-chlorophyllous containing <10% TAG, suggest that soybean, which ages faster, has a more fragile matrix. This means that molecules are less densely spaced in soybean and allow more molecular relaxation, especially in the presence of water (Ballesteros and Walters, Reference Ballesteros and Walters2019). Larger pore size and greater flexibility of molecules within the soybean matrix could allow greater diffusion of volatile ROS and greater local interactions between molecules to enhance cross-linking with neighbouring molecules, for example, glycation and carbonylation of LEAs and HSPs (Rajjou et al., Reference Rajjou, Lovigny, Groot, Belghazi, Job and Job2008; Kalemba and Pukacka, Reference Kalemba and Pukacka2014). Differences in the stability of solid structures can, therefore, affect biochemical reactivity and hence ageing rates. The composition of molecules within the glassy matrix surely contribute to fragility, and it is compelling to think that soluble carbohydrates and/or IDPs, which provided early foundations of glass formation in the biological literature, may protect cells by scaffolding a fragile matrix (e.g. Buitink et al., Reference Buitink, Hemminga and Hoekstra2000; Koster et al., Reference Koster, Lei, Anderson, Martin and Bryant2000; Wolkers et al., Reference Wolkers, McCready, Brandt, Lindsey and Hoekstra2001; Buitink and Leprince, Reference Buitink and Leprince2008; Hincha and Thalhammer, Reference Hincha and Thalhammer2012; Boothby et al., Reference Boothby, Piszkiewicz, Mehta, Brozena, Tapia, Koshland, Holehouse, Pappu, Goldstein and Pielak2018).
Chlorophyllous cells with low TAG (Fig. 3b)
Degradation of photosynthetic pigments sometimes does not occur in DT plant germplasm and dried cells retain active chlorophyll (Fig. 3b). About 16% of Australian dicotyledon species (from a sample of approximately 300 species) produced mature seeds with chlorophyllous cells (Wright et al., Reference Wright, Clifford, Kidson, Reed, Rice and Westoby2000). Fern spores from Equisetaceae, Hymenophyllaceae, Onocleaceae and Osmundaceae are chlorophyllous, as well as all grammatid ferns within Polypodiaceae, representing significant portions of the fern flora in Mesoamerica, the Neotropics and Africa-Madagascar (Sundue et al., Reference Sundue, Vasco and Moran2011). Dried seeds and spores with chlorophyllous cells also tend to have low TAG content (Fig. 3b; Lloyd and Klekowski, Reference Lloyd and Klekowski1970; Ballesteros et al., Reference Ballesteros, Hill, Lynch, Pritchard and Walters2019). Chlorophyllous cells tend to have intact chloroplasts and are distinguished from cells that are simply green. For example, dried pea cotyledons have one-tenth of the chlorophyll of Salix cotyledons (Cheng et al., Reference Cheng, Mcphee and Baik2004) and thylakoids are disassembled giving them lower capacity for electron transfer beyond light-harvesting pigments as a result of the reorganization of the chloroplast structure (Vertucci et al., Reference Vertucci, Ellenson and Leopold1985).
Seeds and spores with chlorophyllous cells often tend to age quickly compared to non-chlorophyllous germplasm. Some examples include seeds of Salix and Populus ssp., seeds from mutant lines of Arabidopsis and Medicago truncatula that retain chlorophyll, and chlorophyllous fern spores (Lloyd and Klekowski, Reference Lloyd and Klekowski1970; Maroder et al., Reference Maroder, Prego, Facciuto and Maldonado2000; Popova et al., Reference Popova, Han, Moltchanova, Pritchard and Hong2013; Ballesteros and Pence, Reference Ballesteros and Pence2017; Ballesteros et al., Reference Ballesteros, Hill and Walters2017, Reference Ballesteros, Hill, Lynch, Pritchard and Walters2019; Leprince et al., Reference Leprince, Pellizzaro, Berriri and Buitink2017). Ageing of dry cells and proteins is faster in the light compared to darkness (Vertucci et al., Reference Vertucci, Roos and Crane1994; Khan et al., Reference Khan, Hendry, Atherton and Vertucci-Walters1996; Manning et al., Reference Manning, Chou, Murphy, Payne and Katayama2010; Roqueiro et al., Reference Roqueiro, Facorro, Huarte, de Celis, Garcia, Maldonado and Maroder2010; Ballesteros et al., Reference Ballesteros, Narayan, Varghese and Sershen2018). Intact photosynthetic apparatus can enhance the presence of ROS and FR, especially in the presence of light (Fig. 6). Even under dry conditions, pigments can absorb light and transduce the excited state through Photosystem II and I (Vertucci et al., Reference Vertucci, Ellenson and Leopold1985). Photo-excited pigments release electrons that become strong oxidizers in the absence of water or antioxidant protection (Fig. 6a; Heber et al., Reference Heber, Lange and Shuvalov2006; Kranner et al., Reference Kranner, Beckett, Hochman and Nash2008; Roqueiro et al., Reference Roqueiro, Facorro, Huarte, de Celis, Garcia, Maldonado and Maroder2010; Ballesteros et al., Reference Ballesteros, Narayan, Varghese and Sershen2018; Verhoeven et al., Reference Verhoeven, García-Plazaola and Fernández-Marín2018). The light harvested by intact photosynthetic systems increases the production of FR which then begins attacking thylakoid membranes (Roqueiro et al., Reference Roqueiro, Facorro, Huarte, de Celis, Garcia, Maldonado and Maroder2010). Even in darkness, germplasm with chlorophyllous cells tend to age quickly, and we speculate that this can be attributed to highly efficient electron transfer among pigments and within thylakoids of Photosystem II (PSII) (Fig. 3b). Multiple double bounds of photosynthetic pigments are regions of high electron density that allow electron jumps, even under dry conditions (Vertucci et al., Reference Vertucci, Ellenson and Leopold1985; Lebkuecher, Reference Lebkuecher1997), but these electron-dense molecules are extremely susceptible to ROS and FR and can serve as ‘ground-zero’ for autocatalytic cascades. Chlorophyllous cells may have insufficient levels of molecular antioxidant defenses (Kranner et al., Reference Kranner, Beckett, Wornik, Zorn and Pfeifhofer2002, Reference Kranner, Minibayeva, Beckett and Seal2010; Roqueiro et al., Reference Roqueiro, Facorro, Huarte, de Celis, Garcia, Maldonado and Maroder2010; Ballesteros et al., Reference Ballesteros, Narayan, Varghese and Sershen2018). Failure to down-regulate light-harvesting machinery can exacerbate oxidation stress by increasing FR levels before cells desiccate (Vertucci and Farrant, Reference Vertucci, Farrant, Kigel and Galili1995; Bailly, Reference Bailly2004).
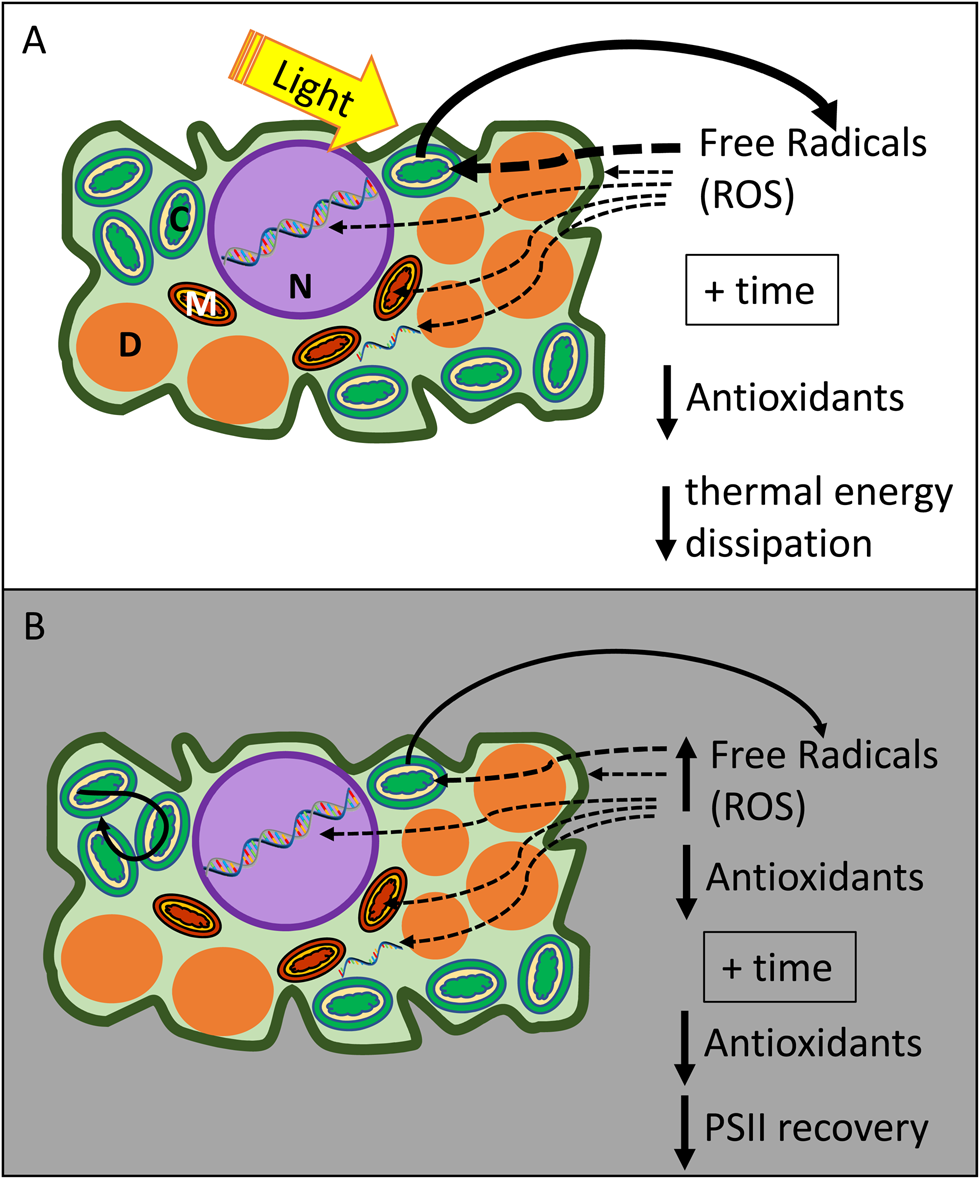
Fig. 6. A schematic model of the ageing mechanisms in the dry state for seeds and spores with chlorophyllous cells in the presence of light (a) or in the dark (b). In (a), light is absorbed by the pigments of the photosynthetic apparatus, and when water is absent, free radicals are produced and threaten to oxidize components of the photosynthetic apparatus and other cellular components such as membranes or the genetic material. This oxidative stress is evident over time as thermal energy dissipation mechanisms and antioxidant protection are reduced (Heber et al., Reference Heber, Lange and Shuvalov2006; Kranner et al., Reference Kranner, Beckett, Hochman and Nash2008, Reference Kranner, Minibayeva, Beckett and Seal2010; Roqueiro et al., Reference Roqueiro, Facorro, Huarte, de Celis, Garcia, Maldonado and Maroder2010; Ballesteros et al., Reference Ballesteros, Narayan, Varghese and Sershen2018; Verhoeven et al., Reference Verhoeven, García-Plazaola and Fernández-Marín2018). In (b), high accumulation of free radicals during maturation/drying threaten to oxidize diverse cellular components (including the PSII), particularly when antioxidant protection is initially reduced or is reduced over time (Roqueiro et al., Reference Roqueiro, Facorro, Huarte, de Celis, Garcia, Maldonado and Maroder2010; Ballesteros et al., Reference Ballesteros, Narayan, Varghese and Sershen2018). These mechanisms may be potentiated by a non-stable and highly mobile glassy matrix in the cytoplasm (Ballesteros and Walters, Reference Ballesteros and Walters2011; Ballesteros et al., Reference Ballesteros, Hill, Lynch, Pritchard and Walters2019) that facilitates cross-linking reactions within the photosynthetic apparatus and diffusion of ROS and other small molecules across the cell (Roudaut et al., Reference Roudaut, Simatos, Champion, Contreras-Lopez and Le Meste2004; Ballesteros and Walters, Reference Ballesteros and Walters2011; López-Pozo et al., Reference López-Pozo, Ballesteros, Laza, García-Plazaola and Fernández-Marín2019). M, Mitochondria; N, Nucleus; C, chloroplast; D, Dry matter (e.g. protein bodies and starch).
Formation of glassy structures that are fragile or allow a lot of local movement may also explain fast ageing of chlorophyllous seeds and fern (Walters, Reference Walters2004; Ballesteros and Walters, Reference Ballesteros and Walters2011; Ballesteros et al., Reference Ballesteros, Hill and Walters2017, Reference Ballesteros, Hill, Lynch, Pritchard and Walters2019; López-Pozo et al., Reference López-Pozo, Ballesteros, Laza, García-Plazaola and Fernández-Marín2019). However, parallel Arrhenius plots of ageing rate for chlorophyllous and non-chlorophyllous spores argue against differences in temperature-dependency of long-range motions (Ballesteros et al., Reference Ballesteros, Hill, Lynch, Pritchard and Walters2019).
Non-chlorophyllous cells with high storage lipids (Fig. 3c)
Many plant species accumulate TAG within cells of seeds, pollen or spores, likely as a main source of energy for germination (Lloyd and Klekowski, Reference Lloyd and Klekowski1970; Bewley and Black, Reference Bewley and Black1994; Graham, Reference Graham2008). Two-thirds of taxa listed in the Kew SID (Royal Botanic Gardens Kew Seed Information Database - SID, 2018) produce seeds with over 10% TAG, and 9% of these accumulate more than 40% dry matter as TAG. TAG is encapsulated in lipid droplets of diverse composition, size and number, depending on species (Tzen et al., Reference Tzen, Cao, Laurent, Ratnayake and Huang1993; Fig. 7a). TAG content in non-chlorophyllous fern spores ranges between 20 and 50% depending on species (Ballesteros and Walters, Reference Ballesteros and Walters2007; Ballesteros et al., Reference Ballesteros, Hill and Walters2017).

Fig. 7. A schematic model of the mechanisms of deterioration in the dry state for seeds and spores with non-chlorophyllous cells with high storage lipids. When dry seeds and spores (a) are exposed to low temperatures (i.e. −20°C) storage lipids (L) crystallize (b). Lipid crystallization (b) can be reverted (a) by warming to the melting temperature given by the specific lipid (triacylglycerol) composition (e.g. Crane et al., Reference Crane, Miller, Van Roekel and Walters2003, Reference Crane, Kovach, Gardner and Walters2006; Ballesteros et al., Reference Ballesteros, Narayan, Varghese and Sershen2018; PCP). When seeds or spores are stored at −20°C (b) crystallization of storage lipids continue progressing into the most stable lipid crystal forms (Ballesteros et al., Reference Ballesteros, Narayan, Varghese and Sershen2018; PCP). Lipid crystallization involves volume reduction, which could create pores and spaces between the solid cytoplasm and the compressed lipid droplet (c). These pores could facilitate lipid droplet restructuration and merging upon melting (represented by double arrows) but also could lead to structural collapse (red rays) and/or changes in the glassy properties of the dry cytoplasm (squared pattern) (Leprince et al., Reference Leprince, van Aelst, Pritchard and Murphy1998; Crane et al., Reference Crane, Kovach, Gardner and Walters2006; Shimada et al., Reference Shimada, Shimada, Takahashi, Fukao and Hara-Nishimura2008; Walters, Reference Walters2015). All these structural changes over time could also facilitate ROS diffusion and make cellular constituents more prone to oxidative stress. M, Mitochondria; N, Nucleus; D, Dry matter (e.g. protein bodies and starch).
There is no correlation between seed longevity and TAG content (Priestley, Reference Priestley1986; Walters et al., Reference Walters, Wheeler and Grotenhuis2005c; Probert et al., Reference Probert, Daws and Hay2009; Nagel et al., Reference Nagel, Kranner, Neumann, Rolletschek, Seal, Colville, Fernández-Marín and Börner2015; Mira et al., Reference Mira, Nadarajan, Liu, González-Benito and Pritchard2019). Nevertheless, TAG continues to be linked to poor storage quality. Historically, this association arises from rancidity problems of unsaturated oils extracted from seeds or known differences in keeping quality of pea and beans (low TAG) compared to soybean and peanuts (high TAG). Higher free fatty acid content and lost polyunsaturation of fatty acids are found in long-dead seeds (Walters, Reference Walters1998; McDonald, Reference McDonald1999) and VOC from lipid oxidation are detected before mortality (Mira et al., Reference Mira, González-Benito, Hill and Walters2010, Reference Mira, Hill, González-Benito, Ibáñez and Walters2016). Moreover, the physical properties of TAG, related to crystallization behaviour, change during storage (Vertucci, Reference Vertucci1992; Walters et al., Reference Walters, Landre, Hill, Corbineau and Bailly2005b; Ballesteros et al., Reference Ballesteros, Hill, Lynch, Pritchard and Walters2019). As is true for the suite of chemical changes detected in preserved germplasm, there is insufficient evidence to link changes of TAG specifically to the event(s) causing mortality (Walters, Reference Walters1998; Fleming et al., Reference Fleming, Patterson, Reeves, Richards, Gaines and Walters2018).
Despite unclear relationships between chemical changes of TAG and seed ageing, the different physical–chemical properties of TAG and the aqueous-based cytoplasm that surrounds lipid bodies presents a compelling chapter in the story of dry architecture. While water-soluble regions of the cell shrink and solidify during drying at room temperature, TAG does not, but rather maintain volume and stay fluid. TAG solidify when cooled, usually to sub-zero temperatures (e.g. Vertucci, Reference Vertucci1992; Crane et al., Reference Crane, Miller, Van Roekel and Walters2003; Walters et al., Reference Walters, Landre, Hill, Corbineau and Bailly2005b; Ballesteros and Walters, Reference Ballesteros and Walters2007; Ballesteros et al., Reference Ballesteros, Hill, Lynch, Pritchard and Walters2019; Mira et al., Reference Mira, Nadarajan, Liu, González-Benito and Pritchard2019). Especially with rapid cooling, TAG initially forms disordered crystals; but as annealing time increases, crystals recrystallize into more ordered, lower energy forms, creating ever denser, lower volume oil droplets (Metin and Hartel, Reference Metin, Hartel and Shahidi2005; Fig. 7b,c). As TAG crystals shrink, gaps between the oil droplets and solidified cytoplasm grow, potentially destabilizing the matrix or allowing larger ROS molecules to penetrate. Cytoplasm with regions of TAG might be considered as a ‘composite’ in materials sciences contexts. Composite materials are comprised of components with distinct properties, usually engineered to improve strength or reduce mass or cost of a structure. However, when the separate components have incompatible physical–chemical properties and temperature changes, components can separate and the composite structure fails.
An analogous structural failure has been used to explain the detrimental effects of −20°C in germplasm from diverse species (Crane et al., Reference Crane, Miller, Van Roekel and Walters2003, Reference Crane, Kovach, Gardner and Walters2006; Walters, Reference Walters2015). The syndrome has been used to explain ‘intermediate’ storage physiology, which was classically defined as seeds that do not survive the combined stress of desiccation and low temperature and so are inappropriate for conventional freezer storage (Ellis et al., Reference Ellis, Hong and Roberts1991, FAO, 2014). Seeds from some Coffea ssp. (Ellis et al., Reference Ellis, Hong and Roberts1990; Dussert et al., Reference Dussert, Chabrillange, Rocquelin, Engelmann, Lopez and Hammon2001; Eira et al., Reference Eira, Amaral da Silva, De Castro, Dussert, Walters, Bewley and Hilhorst2006), Cuphea ssp. (Crane et al., Reference Crane, Miller, Van Roekel and Walters2003, Reference Crane, Kovach, Gardner and Walters2006), Citrus ssp. (Hor et al., Reference Hor, Kim, Ugap, Chabrillange, Sinniah, Engelmann and Dussert2005), papaya (Ellis et al., Reference Ellis, Hong and Roberts1991) and orchids (Pritchard and Seaton, Reference Pritchard and Seaton1993), as well as spores from some fern species (Ballesteros, Reference Ballesteros, Kumar, Fernández and Revilla-Bahillo2011), can be damaged by exposing them to freezer temperatures. TAG is a usual feature in the cells of germplasm characterized as having ‘intermediate’ storage physiology. Seeds in over 80% of species categorized as ‘intermediate’ contain >20% TAG, and 57% of ‘intermediate’ species produce seeds with >40% TAG (Royal Botanic Gardens Kew SID, 2018). Most are from the tropical origin. Damage can be expressed with either short duration in the freezer (Crane et al., Reference Crane, Miller, Van Roekel and Walters2003, Reference Crane, Kovach, Gardner and Walters2006) or as storage time increases, and so takes on characteristics of ageing (Walters, Reference Walters2015; Ballesteros et al., Reference Ballesteros, Hill, Lynch, Pritchard and Walters2019; Fleming et al., Reference Fleming, Hill and Walters2019). A different interaction between water and crystallized or fluid TAG might exacerbate damage to imbibing cells (Crane et al., Reference Crane, Miller, Van Roekel and Walters2003, Reference Crane, Kovach, Gardner and Walters2006). Oleosins, amphiphilic proteins that surround and stabilize the lipid droplet (Leprince et al., Reference Leprince, van Aelst, Pritchard and Murphy1998; Shimada et al., Reference Shimada, Shimada, Takahashi, Fukao and Hara-Nishimura2008), likely reorganize or eject during contraction of the crystallized TAG (Leprince et al., Reference Leprince, van Aelst, Pritchard and Murphy1998; Walters, Reference Walters2015), which can lead to coalescence of the oil bodies upon hydration, deformation of nuclei, and ultimately defective germination and seed mortality (Shimada et al., Reference Shimada, Shimada, Takahashi, Fukao and Hara-Nishimura2008; Fig. 7c). The interrelationships between TAG properties, lipid body geometry and oleosin interactions with solidified cytoplasm will provide greater insight into ageing mechanisms of non-chlorophyllous cells with TAG (Huang, Reference Huang1996; Leprince et al., Reference Leprince, van Aelst, Pritchard and Murphy1998; Shimada et al., Reference Shimada, Shimada, Takahashi, Fukao and Hara-Nishimura2008, Reference Shimada, Hayashi and Hara-Nishimura2018; Walters et al., Reference Walters, Ballesteros and Vertucci2010; Ballesteros and Walters, Reference Ballesteros and Walters2011; Walters, Reference Walters2015).
Genebanking strategies to maintain germplasm viability
Strategies to preserve DT germplasm have historically involved controlling the moisture and temperature environment (Table 1) and have been modelled to illustrate, what we now understand, as the exponential relationships between these environmental factors and viscosity of the cytoplasm prior to solidification, that is, above T g (Harrington, Reference Harrington and Kozlowski1972; Ellis and Roberts, Reference Ellis and Roberts1980). Poignantly, Harrington's Hundred Rule (safe storage occurs when the sum of RH and temperature in Fahrenheit is less than 100) advocates storage conditions that nearly parallel water plasticization effects on cytoplasm (Walters, Reference Walters1998). However, once the cytoplasm has solidified, the relationships between viscosity and environmental factors of temperature and moisture are expected to change from those observed in the more fluid cytoplasm (Walters, Reference Walters2004). Moreover, within solids, or glasses, the mobility of molecules differs considerably with the environment (Ballesteros and Walters, Reference Ballesteros and Walters2011), and this is a factor of both the composition and organization of molecules within the dried cytoplasm (Walters, Reference Walters2015; Ballesteros and Walters, Reference Ballesteros and Walters2019). The diversity of solid-state structures that form in diverse cells and species during preservation is surely key to the variation in longevity that is observed in nature (e.g. Shen-Miller et al., Reference Shen-Miller, Mudgett, Schopf, Clarke and Berger1995; Sallon et al., Reference Sallon, Cherif, Chabrillange, Solowey, Gros-Balthazard, Ivorra, Terral, Egli and Aberlenc2020) and under controlled genebanking conditions (e.g. Walters et al., Reference Walters, Wheeler and Grotenhuis2005c; Nagel and Börner, Reference Nagel and Börner2010). New strategies to predict the longevity of preserved germplasm, and possibly to extend it, must account for stability and reactivity within solids.
Table 1. Genebanking strategies to maintain germplasm viability, their influence in the different dry architectures and suggestions to improve longevity

a CCLL, Chlorophyllous Cells with very Low or no storage Lipids; NCHL, Non-Chlorophyllous cells with moderate or High storage Lipids; NCLL, Non-Chlorophyllous cells with very Low or no storage Lipid.
b These modifications of the cellular properties to create more stable structures for preservation may diverge from genebanking principles to not alter genetic identity or may be particularly difficult for wild germplasm when there is a small window of opportunity to collect.
Developing advanced strategies to preserve germplasm requires a focus on both environmental controls as well as properties intrinsic to the cell (Table 1). For example, lowering the storage temperature to cryogenic temperatures is a good way to expand germplasm longevity beyond the time accrued by the standard seed storage temperatures (Walters et al., Reference Walters, Wheeler and Stanwood2004; FAO, 2014; Ballesteros and Pence, Reference Ballesteros and Pence2017; Ballesteros et al., Reference Ballesteros, Hill, Lynch, Pritchard and Walters2019). However, neither low moisture nor temperature will confer infinite stability within the solid (Dickie et al., Reference Dickie, Ellis, Kraak, Ryder and Tompsett1990; Walters, Reference Walters2004; Walters et al., Reference Walters, Hill and Wheeler2005a; Ballesteros and Walters, Reference Ballesteros and Walters2011, Reference Ballesteros, Hill, Lynch, Pritchard and Walters2019). Water is a plasticizer near T g, but an anti-plasticizer well below T g, which explains the dual effects manifested by faster ageing under extremely dry conditions (Vertucci et al., Reference Vertucci, Roos and Crane1994; Walters, Reference Walters1998; Walters et al., Reference Walters, Hill and Wheeler2005a; Ballesteros et al., Reference Ballesteros, Hill and Walters2017). With the current understanding of the diversity of structure and motion in cellular glasses, as well as the interaction of temperature, it would be reasonable to postulate that optimum moisture levels vary among organisms and storage temperature.
Temperature also has conflicting effects on structure and mobility within solids. Diffusive motion is certainly limited by cooling to cryogenic temperatures; however, there is a greater disparity between the structure formed with open pores at T g and the equilibrium structure that is far denser at lower temperatures, and this disparity serves as a driving force for molecular movement (Walters, Reference Walters2004). Moreover, the temperature has less effect on short-range motions, which are likely involved in continued deterioration of cryogenically stored germplasm (Walters, Reference Walters2004; Walters et al., Reference Walters, Wheeler and Stanwood2004, Ballesteros et al., Reference Ballesteros, Hill, Lynch, Pritchard and Walters2019). In other words, low temperature alone will not stop ageing reactions. Indeed, specific cold temperatures might facilitate ageing reactions through a change in the dry architecture brought about by lipid crystallization. That said, storage at cryogenic temperature provides a powerful tool to induce compatibility among aqueous and TAG components, that have very different physical–chemical properties at higher sub-zero temperatures.
In addition to temperature and moisture, modifications in the physicochemical environment where seeds are processed and stored to reduce the source of FR and ROS may also be beneficial to increase longevity (Table 1). Some examples are the reduction of the oxygen levels within the storage containers (Gonzalez-Benito et al., Reference Gonzalez-Benito, Perez-Garcia, Tejeda and Gomez-Campo2011; Groot et al., Reference Groot, de Groot, Kodde and van Treuren2015), or the reduction of the exposure to light across the post-harvest procedures (Roqueiro et al., Reference Roqueiro, Facorro, Huarte, de Celis, Garcia, Maldonado and Maroder2010; Ballesteros and Pence, Reference Ballesteros and Pence2017; Ballesteros et al., Reference Ballesteros, Narayan, Varghese and Sershen2018).
Modifying cellular properties to create more stable structures for preservation (Table 1) may diverge from genebanking principles to not alter genetic identity or may be particularly difficult for wild germplasm when there is a small window of opportunity to collect. Our greatest insights might come from studies of germplasm that are particularly long-lived. One solution may involve an early onset of quiescence to reduce high-energy metabolites that are a source of FR and ROS. Cellular constituents that increase the density of cytoplasm above T g may induce quiescence while also making glasses stronger and less likely to relax below T g. Additionally, anti-plasticizers stiffen molecules so they are less likely to move (Table 1). These can be engineered as ligands on large molecules that fill space or additions of small molecules like glycerol, dimethyl sulphoxide and ethylene glycol that quench molecular relaxations and local mobility (Cicerone et al., Reference Cicerone, Tellington, Trost and Sokolov2003).
Conclusions
The cells of preserved germplasm successfully transition from fluid to solid. The structure of the solid impedes molecular mobility, thereby, limiting chemical reactivity that leads to ageing. Nevertheless, ageing of preserved germplasm occurs, albeit slowly, and eventually leads to mortality. Here, we have reviewed the solidification process and have argued that structures formed as DT organisms dry can vary considerably among diverse organisms and that these differences can contribute to differences in how long preserved germplasm persists (i.e. longevity). We have identified some structural features that tend to reduce longevity. Chlorophyllous cells appear to retain intact photosynthesizing machinery, which possibly increases their risk to oxidative damage either by residual high-energy intermediates or by the high content of molecules particularly vulnerable to electron abstraction. Non-chlorophyllous cells with high levels of storage lipids are comprised of components with very different physical–chemical properties that make them prone to structural failure with changes in temperature. A temperature anomaly near −20°C, manifested by faster than expected mortality, may arise when the lipids crystallize, causing aqueous and lipid domains to rip apart. The diversity of solid cellular structures in preserved germplasm may require the implementation of additional treatments to stabilize the dry architecture of cells that are intrinsically unstable. A greater understanding of the variation in structural conformations in space and time will lead to improved strategies that increase germplasm longevity in cells with short lifespans.
Acknowledgements
The authors acknowledge Udi Liu (RBG Kew) for her help sourcing data from the SID for Fig. 4. Royal Botanic Gardens, Kew receives grant-in-aid from Defra, UK. Garfield Weston Foundation under the Global Tree Seed Bank Project funded the trip to the ISSS 2nd seed longevity workshop where the basis of this paper was presented for the first time.











