Many children with CHD are underweight for ageReference Unger, DeKleermaeker, Gidding and Christoffel 1 – Reference Ehlers 11 and/or stunted – even at an early age. Being significantly underweight for age and wasted has been associated with higher perioperative morbidity and mortality in children with CHD.Reference Anderson, Beekman and Border 12 In addition, the perioperative period is frequently complicated by difficulties with enteral nutritionReference Leite, Fisberg, Novo, Nogueira and Ueda 10 , Reference Schwalbe-Terilli, Hartman and Nagle 13 and potentially with increased nutritional loss associated with problems such as chylothorax.
There is a significant burden of childhood undernutrition in low- and middle-income countries where some 90% of the children born with CHD across the world live.Reference Tchervenkov, Jacobs and Bernier 14 The co-existence of CHD increases the likelihood and severity of undernutrition, and this adds to challenges for the management of CHD including resource constraints; late presentation and diagnosis; and perioperative infection.Reference Jenkins, Castaneda and Cherian 15 – Reference Bernier, Stefanescu, Samoukovic and Tchervenkov 18 The number of children, including infants and newborns being operated for CHD, has recently increased in these regions.Reference Jenkins, Castaneda and Cherian 15
This review seeks to summarise existing evidence on prevalence of undernutrition and CHD and its impact on surgical outcomes with a focus on implications for low- and middle-income countries. An effort has been made to identify specific gaps in knowledge as a basis for future multicentre studies.
Definition of malnutrition in CHD
In the context of CHD, it is important to reach a clear consensus on what is meant by malnutrition. Simple measurements, such as height, weight, skinfold thickness, or mid-upper arm circumference, and their relationships, for example, weight-for-height, have been the basis of most definitions of nutritional status. 19 It is not possible to exclude malnutrition on the grounds of normal anthropometry alone, as there may be children with acute malnutrition who fall within the “normal” ranges for standard anthropometry.Reference Van den Broeck, Meulemans and Eeckels 20 Green Corkins has recently highlighted the importance of a full clinical evaluation – versus simply viewing anthropometry – in the assessment of nutrition status in children.Reference Green Corkins 21
Mehta et al defined malnutrition as “an imbalance between nutrient requirement and intake, resulting in cumulative deficits of energy, protein, or micronutrients that may negatively affect growth, development, and other relevant outcomes”Reference Mehta, Corkins and Lyman 22 (see Fig 1). Ideally, any assessment of nutrition and investigations on the role of nutrition in patient management should include data regarding growth over a period of time: actual dietary intake available (and provided); evidence for malabsorption; evidence of inflammatory processes; and evidence of any underlying disease.Reference Becker, Carney and Corkins 23
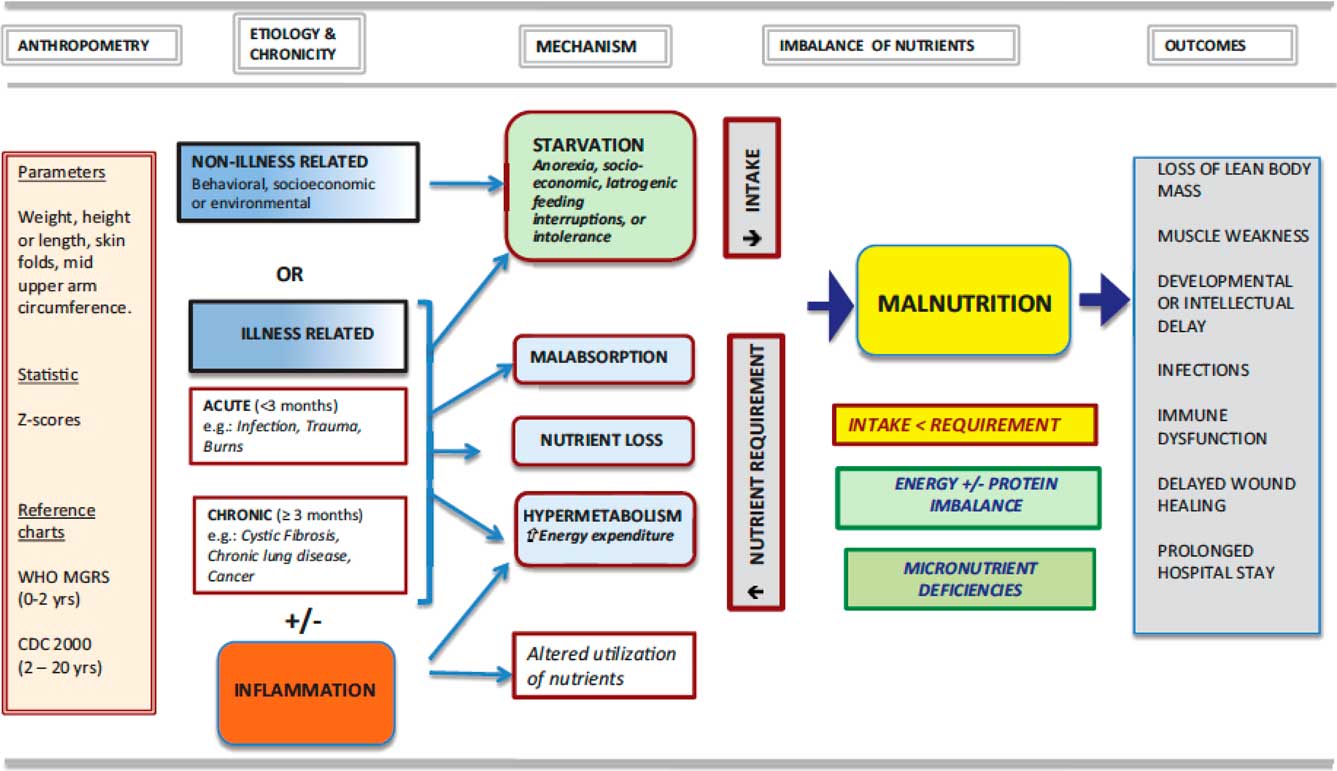
Figure 1 An approach to nutrition in the child with failure to grow or to gain weight, from Mehta et al.Reference Mehta, Corkins and Lyman 22
Failure to thrive in children with CHD
The basis of growth failure or underweight in CHD appears to be multifactorial and may differ in aetiology from patient to patient. It includes the underlying cardiac anomaly,Reference Blasquez, Clouzeau and Fayon 24 haemodynamic factors, hypoxaemia, inadequate calorie, or macronutrient intake,Reference Unger, DeKleermaeker, Gidding and Christoffel 1 , Reference Schwalbe-Terilli, Hartman and Nagle 13 , Reference Yahav, Avigad and Frand 25 – Reference Nicholson, Clabby, Kanter and Mahle 28 increased energy expenditure relative to intake,Reference Menon and Poskitt 29 – Reference Mitchell, Davies, Day, Pollock and Jamieson 31 increased inflammation,Reference Goulart, Schuh, Moraes, Barbiero and Pellanda 32 or associated comorbidities that include gut dysfunction,Reference Yahav, Avigad and Frand 25 , respiratory infections, associated genetic syndromes, and reduced growth potential.Reference Varan, Tokel and Yilmaz 7 , Reference Mehrizi and Drash 33 – Reference Matthiesen, Henriksen and Gaynor 37
A study of anthropometric data in children with CHD in India showed that recorded dietary intake was not associated with the probability of being underweight.Reference Vaidyanathan, Nair and Sundaram 6 Another study concluded that there was no reduction in intake in children with CHD relative to normal children, whereas “normal serum prealbumin and albumin in the infants with CHD ruled out protein–calorie malnutrition.”Reference Salzer, Haschke, Wimmer, Heil and Schilling 36 The plasma amino acid profile was normal preoperatively in a group of children with CHD, regardless of anthropometric values.Reference Villares, Leal, Diaz and Gonzalez 38 A study from Latin America of children with CHD showed that anthropometric measures of malnutrition were present in >80% of the children; albumin and prealbumin levels were lower than in normal controls, although transferrin levels were no different; and that failure to thrive was worse in children with pulmonary hypertension.Reference Leite, de Camargo Carvalho and Fisberg 39
In low- and middle-income countries with resource limitations, the prevalence of abnormal preoperative anthropometry is high owing to late presentation, delays in corrective intervention, and frequent hospitalisations related to respiratory infections.Reference Vaidyanathan, Nair and Sundaram 6 , Reference Vaidyanathan, Roth, Rao, Gauvreau, Shivaprakasha and Kumar 16 In a cohort of 100 consecutive infants undergoing ventricular septal defect closure, severe underweight (weight z-score <−3) was observed in 46% of patients.Reference Mehrizi and Drash 33 In a prospective study of 476 consecutive patients undergoing corrective intervention for CHD, Vaidyanathan et al reported z-scores <−2 in 59% (weight), 26.3% (height), and 55.9% (weight/height), respectively; z-scores <−3 were observed in 27.7% (weight), 10.1% (height), and 24.2% (weight/height).Reference Vaidyanathan, Nair and Sundaram 6 Congestive heart failure, older age at correction, and limited growth potential as suggested by lower birth weight for gestation, lower parental anthropometry, and genetic syndromes were identified as predictors of “malnutrition”. The International Quality Improvement Collaborative for Congenital Heart Surgery in low- and middle-income countries uses a registry to collect data from over 50 sites. An analysis of data from 15,049 patients with CHD revealed that >50% of children had weight z-scores of −3 or less and 12% had an emaciated appearance before their surgery.Reference Vaidyanathan, Roth, Rao, Gauvreau, Shivaprakasha and Kumar 16
Although anthropometric measurements in children with CHD are frequently abnormal, classical features of malnutrition such as skin changes, presence of oedema, hair changes, and so on are relatively rare in children with CHD. In the setting of malnutrition in Africa, a discrepancy between clinical signs of acute malnutrition and anthropometryReference Van den Broeck, Meulemans and Eeckels 20 may be an important issue to pursue.
Finally, there is evidence that micronutrient deficiencies may be relatively common in children with CHD.Reference Steier, Lopez and Cooperman 40 This has never been explored in low- and middle-income countries where it is even more likely to be an issue.
Supplementary Table 1 summarises data from publications on nutritional status in CHD from low- and middle-income countries.
Impact of preoperative state on postoperative outcome
Because of their lower protein and energy reserves, infants and newborns may be particularly vulnerable to the hypercatabolic state that is expected following heart surgery,Reference Finnerty, Mabvuure, Ali, Kozar and Herndon 41 , Reference Teixeira-Cintra, Monteiro, Tremeschin, Trevilato, Halperin and Carlotti 42 although hypercatabolism may not follow all paediatric cardiac operations.Reference Mehta, Costello and Bechard 43 This situation is further worsened by comorbidities such as lack of protein:calorie provision or major infections (Fig 1). When the acute phase of metabolic stress resolves, the anabolic phase begins, resulting in somatic growth, with decreasing concentrations of acute-phase reactants, proteins, and total urinary nitrogen values, and with increasing concentrations of visceral proteins.Reference Jones, Pierro, Hammond and Lloyd 44 The timing of transition to the anabolic phase is influenced by the extent of “surgical stress”, associated comorbidities, quality of nutritional support, and a number of other potential factors.
Within that framework, there has been concern that cardiac surgery in underweight children with CHD would be associated with worse outcomes. However, there is very little evidence to support the notion that lower weights are associated with poorer surgical outcomes. In a study published in 1992, Hardin et alReference Hardin, Muskett, Canter, Martin and Spray 45 compared two groups of patients undergoing closure of ventricular septal defects (>4 kg and <4 kg) and found no significant difference in outcomes.
In a study from southern India on 100 consecutive infants undergoing surgical closure of ventricular septal defects, 46% had weight z-scores of −3 or lower, although this did not affect postoperative mortality or morbidity, duration of mechanical ventilation, or length-of-stay in the ICU or hospital.Reference Vaidyanathan, Roth, Rao, Gauvreau, Shivaprakasha and Kumar 16 A more recent prospective study of 1028 infants from the same centre studied the impact of preoperative factors on postoperative outcomes after congenital heart surgery.Reference Reddy, Kappanayil and Balachandran 17 Weight z-scores <−3 and low birth weight (<2.5 kg) did not adversely affect mortality or morbidity. These results reinforce the strategy of early correction of CHDs irrespective of nutritional status. Preoperative optimisation of nutritional status through aggressive feeding is not necessary in most patients, although it may make sense to use this technique if there are ongoing delays in access to surgical repair.
Correction verses palliation
The question of correction versus palliation in a severely undernourished child with CHD is likely to be dictated by several factors that include the specific defect, the experience and expertise of the surgical and intensive care teams, and associated comorbidities. Relatively simple CHD that can be corrected through a single operation, such as a ventricular septal defect, should be closed surgically. A study in South Africa demonstrated that when the realities of a staged approach using pulmonary artery banding – including loss to follow-up, delays in definitive procedures, the challenges of two surgical procedures in a context with limited surgical time, and so on – were taken into account, mortality after a staged approach using pulmonary artery banding was higher than primary surgical closure of the ventricular septal defect.Reference Brooks, Geldenhuys, Zuhlke, Human and Zilla 46 Nonetheless, there are clearly situations where pulmonary artery banding may be a useful stage en route to full correction.
Management of undernutrition
Cardiac teams in low- and middle-income countries are frequently faced with children with CHD who are underweight, thin, and stunted. The first response should be to regard that as further evidence that the diagnosis of CHD is being delayed and to focus on interventions to improve early diagnosis and referral.
Preoperative assessment of nutritional status:
A full preoperative assessment should be obtained as it provides essential baseline information for further monitoring of progress following surgical correction. This would typically include accurate measurements of height, weight, and head circumference. The measurement of biceps or triceps skinfold thickness is a simple method for determining fat stores, which is not routinely performed during preoperative assessment, 19 but might provide useful data for follow-up, both for individual patients and for patient groups. Laboratory tests that include plasma levels of albumin, transferrin, prealbumin, and retinol-binding protein are seldom undertaken because they are thought to be of limited value.
Nutritional support
Significant caloric supplementation in children with CHD could lead to improved growthReference Jackson and Poskitt 47 and, in some, improved growth could be achieved using continuous enteral feeds.Reference Bougle, Iselin, Kahyat and Duhamel 48 – Reference Schwarz, Gewitz and See 50
Enteral nutrition is the preferred mode of nutritional support in paediatric ICUs.Reference Skillman and Mehta 51 Enteral nutrition is physiological, has a favourable effect on the intestinal mucosa, and has fewer complications compared with parenteral nutrition.Reference Zamberlan, Delgado, Leone, Feferbaum and Okay 52 , Reference Mehta 53 It has the additional benefit that it is considerably cheaper than parenteral nutrition and more easily available in low- and middle-income countries. Intragastric feeding is the most common route, and there are insufficient data to recommend routine use of post-pyloric feeding, unless there are particular concerns such as poor tolerance of gastric feeding or evidence of gastro-oesophageal reflux with aspiration. In a single randomised controlled trial in 74 critically ill children, a higher number of patients with post-pyloric feeding reached caloric goals.Reference Meert, Daphtary and Metheny 54
There are particular challenges with feeding of neonates before surgery, although in most cases it is possible to start enteral feeding – even while on prostaglandins. Many of these infants may continue to require gavage feeding postoperatively.Reference Natarajan, Reddy Anne and Aggarwal 55
Postoperative nutrition
Postoperative enteral feeds should be initiated as soon as possible, and parenteral nutrition should only be used if absolutely essential. Recent data suggest that early parenteral nutrition, within 7 days of admission, in the paediatric ICU is associated with worse patient outcomes, at least in high-income countries.Reference Fivez, Kerklaan and Mesotten 56
Initiation and advancement of feeds:
Guidelines for withholding feeds after paediatric cardiac surgery are not clearly defined, and there are extremely limited data available specific to low- and middle-income countries. The most common concern in early initiation of feeds is the potential for low cardiac output with gut hypoperfusion, especially in neonates with duct-dependent circulation, leading to necrotising enterocolitis.Reference Jeffries, Wells, Starnes, Wetzel and Moromisato 57 , Reference Giannone, Luce, Nankervis, Hoffman and Wold 58 With careful monitoring and slow advancement of feeds, early enteral nutrition is feasible in most neonates within the first 24 hours. In a retrospective review of 67 neonates, including 52 patients with duct-dependent circulation undergoing surgical repair, postoperative enteral feeds could be initiated within 3 days in 98.5% (n=66); 64 patients could reach full feeds at a median duration of 7.5 days following surgical correction.Reference Natarajan, Reddy Anne and Aggarwal 55
In neonates and young infants, feeds are typically started – within 12–24 hours of surgery – at 1 ml/kg/hour and advanced at the same rate every 4–6 hours to reach the goal volume. Although continuous feeds have not been shown to minimise aspiration or feed intolerance, it is sometimes resorted to in infants with poor weight gain and feeding complications.Reference Vanderhoof, Hofschire and Baluff 49 Feeds are withheld before extubation, around invasive procedures, in patients with haemodynamic instability, or with impending respiratory failure.
In the early postoperative phase, feed volume is dictated by the maintenance fluid rate. Fluid intake is usually restricted to 50–80% of maintenance rate in neonates and infants undergoing open-heart surgery.Reference Owens and Musa 59 On the basis of studies in patients with CHD, the resting energy expenditure is estimated to be 55–75 kcal/kg/day in the first 3–5 days.Reference De Wit, Meyer, Desai, Macrae and Pathan 60 This guides initial feeding, which can be escalated to 120–150 kcal/kg/day with transitioning to step down care to facilitate catch-up growth in infants.
Protein intake is crucial as protein catabolism could manifest as loss of respiratory muscle mass, failed weaning, poor weight gain, depressed immune function, and poor wound healing. In a small study from Brazil, patients became anabolic post-cardiac surgery with a calorie intake of 54 kcal/kg/day and a protein intake of 1.1 g/kg/day.Reference Teixeira-Cintra, Monteiro, Tremeschin, Trevilato, Halperin and Carlotti 42 The decline in C-reactive protein levels to values <2 is considered an indirect marker of the onset of the anabolic phase in critically ill patients; however, its relevance in post-cardiac surgical patients has not been proven.Reference Owens and Musa 59
For neonates and infants, particularly in low- and middle-income countries, human breast milk is the preferred form of enteral feeding, although the practicalities of maintaining breast milk production in this context may be challenging.Reference Torowicz, Seelhorst, Froh and Spatz 61 Breast milk is cost-effective, has immunological benefits, promotes better absorption of trace elements, and may even lower the risk of developing necrotising enterocolitis when used exclusively.Reference Sullivan, Schanler and Kim 62 In the setting where tight fluid restriction is required, it may be necessary to increase the energy density of feeds – both for formula and expressed breast milk. An energy density of ~1 kcal/ml is well tolerated. Further increases should be monitored carefully as high calorie density may precipitate osmotic diarrhoea. Cost-effective options to fortify formula feeds include additives such as coconut oil or medium-chain triglyceride oil.
A feeding gastrostomy or jejunostomy may be a reasonable option when long-term enteral nutrition is required in children.Reference Hofner, Behrens, Koch, Singer and Hofbeck 63 , Reference El-Sayed Ahmed, Alfares and Hynes 64 It may also be considered in patients with gastro-oesophageal reflux, aspiration, or severe failure to thrive. In a retrospective cohort of 54 patients who required gastrostomy tube placement after surgery for single-ventricle palliation, patients who underwent earlier placement of a gastrostomy tube had shorter ICU and hospital stays.Reference El-Sayed Ahmed, Alfares and Hynes 64
Monitoring enteral nutrition should include regular audits of weight, fluid and dietary intake, calories prescribed and calories achieved, gastrointestinal function, feeding tube integrity, and feeding complications. Undue attention to gastric residual volumes may be a deterrent to enteral feeding in critical care settings. Decisions to interrupt feeds should be considered with signs of feeding intolerance such as distention, vomiting, or diarrhoea, rather than relying on residual volumes alone.Reference Lee, Rogers and Chor 65 The most important aspect of monitoring enteral nutrition may be to detect and minimise interruptions in feeding. In a recent prospective study in a paediatric ICU, implementation of a stepwise enteral nutrition algorithm led to a significant decrease in the number of avoidable episodes of feeding interruptions (n=3 versus n=51, p=0.0001), shortened the time to reach energy goals from 4 days to 1 (p<0.0001), and resulted in a higher proportion of patients reaching their energy goals (99 versus 61%, p=0.01).Reference Hamilton, McAleer and Ariagno 66
Underfeeding is common in critically ill children even in most advanced units.Reference Martinez, Smallwood, Bechard, Graham and Mehta 67 – Reference Mehta, Bechard, Zurakowski, Duggan and Heyland 69 Algorithm-led nutritional therapy and attention to energy and protein goals by dedicated nutrition support teams has translated to better postoperative outcomes in high-income countries.Reference Mehta, Bechard and Cahill 68 ,69 However, in emerging economies there is a perceived lack of dedicated nutrition support teams. A viable option is to use existing personnel in monitoring and supervising nutrition delivery. The initial phase should focus on education of in-house staff to bridge knowledge gaps in feeding practices.Reference Martinez, Bechard and Mehta 70 As primary caregivers, intensive care nurses should be empowered to initiate, monitor, and maintain nutrition delivery in the postoperative phase. A nutrition algorithm can be formulated to serve as a clinical aid to guide the therapy (Fig 2). This can be successfully integrated into multidisciplinary ICU rounds as nurse-led nutrition rounds.Reference Balachandran, Nair, Gopalraj, Vaidyanathan and Kumar 71

Figure 2 Nutritional management in the early postoperative period: suggested algorithm for low-resource environments. EBM=expressed breast milk.
There are very limited data on recommendations for feeding children who were previously underweight and failing to thrive. We recommend that such children be started on the nutritional programme as outlined above, together with close monitoring of progress and weight gain. Failure to gain weight should stimulate renewed focus on adequacy of the cardiac repair; the presence of complications such as chylothorax, with associated nutritional deficits; and the possibility of other underlying conditions that may cause malabsorption of enteral feeds. In addition, there needs to be focus on optimisation of the diet available to the child, and provision of supplements of essential macronutrients and micronutrients.
Parenteral nutrition
The overall use of parenteral nutrition in critical care settings in low- and middle-income countries is reported to be very low,Reference Ramakrishnan, Shankar, Ranganathan, Daphnee, Bharadwaj and Venkataraman 72 primarily owing to prohibitive costs and lack of dedicated personnel to ensure safe prescription and delivery. In addition, there are the biological complications related to parenteral nutrition including infection, venous thrombosis, electrolyte imbalances, and parenteral nutrition-induced cholestasis.Reference Heine and Bines 73 Notwithstanding these concerns, the use of parenteral nutrition may be warranted in critically ill patients after congenital heart surgery in situations in which enteral feeding is contraindicated or is insufficient to promote adequate growth. Guidelines are available for prescription of parenteral nutrition in children.Reference Koletzko, Goulet, Hunt, Krohn and Shamir 74 Prescription of parenteral nutrition is a complex process with potential for errors, requiring appropriate precautions and systems when used.Reference Boullata, Gilbert and Sacks 75
Parenteral nutrition in the low-resource environment:
In paediatric ICUs of low- and middle-income countries, parenteral nutrition should be prescribed only if absolutely indicated. The paediatric intensivist in conjunction with the nurse leader and the hospital clinical pharmacist should be in charge of prescribing and ensuring its safe administration. The feasibility of re-introducing enteral nutrition should be constantly explored. Trophic feeds should be encouraged wherever feasible to prevent intestinal mucosal atrophy.Reference Heine and Bines 73
Nutritional recovery after corrective surgery
Studies from advanced nations have previously reported normalisation of somatic growth typically 6–12 months after corrective congenital heart surgery.Reference Weintraub and Menahem 34 , Reference Cheung, Davis, Wilkinson and Weintraub 76 Weintraub et alReference Weintraub and Menahem 34 reported that after surgical closure of ventricular septal defects in infancy, growth parameters were almost comparable to the reference population by 5.7 years; however, in a study from southern India, the nutritional recovery on follow-up after ventricular septal defect closure in infancy was sub-optimal, with weight and height z-scores <−2 in 42 and 27% of patients, respectively.Reference Vaidyanathan, Roth, Gauvreau, Shivaprakasha, Rao and Kumar 77 In a larger study including all forms of CHD, the same authors reported significant catch-up growth after correction, typically within 3–12 months postoperatively, followed by plateauing of growth curves after 1 year.Reference Vaidyanathan, Radhakrishnan, Sarala, Sundaram and Kumar 78 This reflects an immediate catch-up growth in the short term owing to correction of the haemodynamic derangement. Other determinants of growth such as dietary and constitutional factors play a more important long-term role. Sub-optimal nutritional recovery with persistent weight, z-score <−2 was observed in 27% of patients and this was predicted by lower weight z-score at surgery, lower birth weight, and lower parental anthropometry.Reference Vaidyanathan, Radhakrishnan, Sarala, Sundaram and Kumar 78 It is important to identify patients at risk of sub-optimal recovery – those with lower growth potential – before intervention so that targeted nutritional rehabilitation may be provided on follow-up.
Review post-surgery and surgical recovery
Following paediatric ICU discharge, close attention to nutrition is required, particularly in some subsets of patientsReference Medoff-Cooper and Ravishankar 79 such as those with Fontan circulations following complex CHD.Reference Glockler, Severin and Arnold 80 However, this may pose challenges in resource-limited settings.
Future directions
Ideally, nutritional support for children with CHD should be based on data that are appropriate to specific environments. Although there is a reasonable body of published evidence on the existence of failure of growth and weight gain in children with CHD in low- and middle-income countries (see Supplementary Table 1), there are very limited data available on the optimum way to address these deficits.
Much of the current data on children with CHD are limited to a relatively small spectrum of cardiac anomalies. As increasingly complex CHD is addressed in low- and middle-income countries, the issues related to nutrition may well change and require different approaches. The maintenance of regional databases of information and outcomes would provide an ideal basis for future research in this area.
Much work needs to be done in the areas of quality control and research implementation in order to carry through what is already known into clinical practice. There may need to be increased focus on supporting teams and structures that are implemented for clinicians who work with patients with CHD in low- and middle-income countries.
Conclusions
The overall management of children with CHD may be complex, and attention to nutrition is a critical element of that care. In general, nutritional support is relatively inexpensive. However, it relies on the existence of a structured system within the cardiac care environment to ensure appropriate recognition of the particular role of nutrition in a specific child. Repeated and accurate measurement of growth parameters together with detailed attention to nutritional intake and tolerance are crucial. It makes little sense to invest in the expense of cardiac surgery while ignoring the associated nutritional challenges.
Acknowledgement
None.
Financial Support
This research received no specific grant from any funding agency, commercial, or not-for-profit sectors.
Conflicts of Interest
None.
Supplementary material
To view supplementary material for this article, please visit https://doi.org/10.1017/S104795111700258X




