Burden of mental disorders and PM pollution
Mental disorders are highly prevalent in the population and have recently been identified as an essential contributor to global disease burden.1 In 2016, depressive disorder accounted for about 44 million disability-adjusted life-years (DALYs) and 34.1 million years lived with disability.2 Suicide, another mental health problem closely associated with depressive disorder, was the second leading cause of death among those aged 15−29 years and the 17th leading cause of death in the general population in 2015.3
Ambient particulate matter (PM) pollution – one of the most important environmental risk factors for human health – was responsible for 105.7 million DALYs globally in 2016, ranking sixth among behavioural, environmental and occupational, and metabolic risk factors.4 It was also among the top ten risk factors for attributable deaths in 195 countries and territories, including India and China, where it ranked the third and fourth place in 2016, respectively.4 Since the prevalence of depression and suicide has been increasing rapidly and effective treatments for depression are not easily accessible,1–3 it is important to investigate relevant risk factors and public prevention methods for these mental health issues.
Relevance and lack of epidemiological studies
Previous epidemiological and experimental studies have linked air pollution to diseases of the central nervous system, behaviour deficits, neuroinflammation and neuropathology in humans and animals.Reference Block and Calderón-Garcidueñas5–Reference Block, Elder, Auten, Bilbo, Chen and Chen7 There has been an increasing number of studies investigating the association between mental health problems and air pollutionReference Cho, Choi, Suh, Sohn, Kim and Cho8–Reference Kim, Ng, Chung, Kim, Honda and Guo21 following the discovery of a possible mechanism of inflammation and oxidative stress.Reference Anisman and Hayley22–Reference Campbell, Oldham, Becaria, Bondy, Meacher and Sioutas25
Therefore, we conducted the first meta-analysis for cohort and case-crossover studies on ambient PM and mental health (specifically depression and completed suicide) in the general population. We also investigated the potential pattern of association over different lag times and subgroups divided by study characteristics such as gender ratio, country/region, study design and study quality.
Methods
Search strategy
We identified relevant publications before 13 March 2018 by systematic searches in the following literature databases: PubMed, Medline, Embase, Ovid and Web of Science. We also searched the reference lists of all identified relevant publications and the following grey literature databases: SIGLE, Social Care Online, British National Bibliography for Report Literature and NTIS. We conducted a literature search for particulate matter, depression or suicide using ‘AND’ as the combining term for the two search themes (see details in Supplementary Table 1 available at https://doi.org/10.1192/bjp.2018.295).
Inclusion criteria
Two reviewers (S.W. and X.G.) collected articles eligible for further review by performing an initial screening of identified abstracts or titles, followed by a full-text review. Discrepancies were resolved by consensus. The broad inclusion criteria for articles were: (a) original studies and reporting on the association of PM and depression or suicide in a cohort study (prospective cohort or historical cohort) or case-crossover design for the general population; (b) results were reported in a quantitative exposure–response relationship manner (relative risk/odds ratio and 95% CIs); (c) daily air PM data were ambient and obtained from multiple air monitoring stations; (d) studies had outcomes for incidence of depression, depressive symptoms or completed suicide; and (e) efforts had been taken to control for important confounding factors including demographic variables (such as age, gender, jobs and income) and meteorological variables (such as temperature, humidity and sunshine duration).
Exclusion criteria
The exclusion criteria for articles were: (a) not original and other animal studies, toxicological studies, case reports, case series and duplicates; (b) inadequate information after contacting the authors; (c) not full-length articles or did not provide calculable or reported relative risk/odds ratio and 95% CIs; (d) the definition of depression or suicide was uncertain or suicide events were uncompleted; (e) study participants were selected from special population subgroups with high risk of neurological and psychological disease; and (f) study designs were cross-sectional designs or time-series designs.
Data extraction
Two reviewers (S.W. and X.G.) extracted the following information about the studies independently using a form developed for this review: study characteristics (study name, authors, publication year, journal, study design, location, population, follow-up years and sample size), participants' characteristics (mean age and gender ratio), PM exposure variable (PM2.5 or PM10), exposure concentration (mean/median), outcome measure (depression or suicide definition), effect estimates (relative risk/odds ratio), lag time, analytic strategy (statistical models and confounders included in the models) and funding information. Discrepancies were resolved by consensus and a third author (Q.L.) was available to determine eligibility if consensus could not be reached. When extracted information remained unclear after inspection of the full text, we contacted the relevant authors to seek clarity and details on this issue. If several effect estimates with different lag times were reported in the same article, we extracted all estimates for statistical analyses; if several statistical models were provided in the same article, we extracted effect estimates from the model which adjusted for the largest number of covariates in the relevant multivariate analyses.
Quality assessment
Quality assessment was performed using the Newcastle–Ottawa Scale, considering the following three general aspects: the selection of the study groups, the comparability of the groups and the ascertainment of either the exposure or outcome of interest for case-crossover or cohort studies, respectively (see details in Supplementary Table 2).Reference Wells, O'Connell, Peterson, Welch, Losos and Tugwell26 A study could be awarded, for each variable within each assessment domain, a possible maximum total score of nine (four for selection, two for comparability and three for exposure/outcome ascertainment). A study with a final score of more than six was regarded as high quality.Reference Yang, Zhang, Feng, Chen, Liu and Chen27 The quality assessment was conducted independently by two reviewers (Q.L. and X.G.) and the results were reconciled until a consensus was reached.
Statistical analyses
The odds ratio was used as the common measure of association across studies. The relative risk was considered equivalent to odds ratio because the depression and suicide prevalence in the general population was less than 5%. The odds ratios were pooled using the random-effects model. Forest plots were produced to visually assess the odds ratios and corresponding 95% confidence intervals across studies. The heterogeneity of odds ratios across studies was evaluated by the Q statistic and the I2 statistic. To evaluate the possibility of publication bias, we conducted funnel plot analysis and performed the Begg rank correlation test for funnel symmetry.
For studies that reported stratified risk estimates by lag patterns such as single-day lag (e.g. lag 0 means the present-day exposure of depression or suicide measurement, lag 1 means the day before, and so on) or cumulative lag (e.g. lag 0–1 means the moving average of the present day and the previous day, lag 0–2 means the moving average of the present day and the previous 2 days), each subgroup and lag time was included in our meta-analysis separately. If several lag estimates were reported in the same article, we chose the lag pattern with the largest estimate size used to assess overall risk estimates. In addition, we pooled the various estimates according to lag patterns (short-term exposure [<40 days] and long-term exposure [≥40 days]) and specific lag times separately and only pooled estimates where two or more estimates were available.
We also performed sensitivity analyses by omitting one study at a time and subgroup analyses stratified by participants' gender ratio, study location, study design, study quality and three categories of quality assessment (selection, comparability and exposure/outcome) to evaluate the impact of individual studies and study characteristics on the results.
We performed all analyses using R statistical software (R version 3.4.2 for Windows) with the package ‘metafor’. Final estimated effects were expressed as odds ratios associated with a fixed increment of PM (10 µg/m3 for PM2.5 or PM10), and a two-sided P-value of <0.05 was considered statistically significant.
Results
Literature search
The search strategy identified 1167 articles and 1 additional article was extracted from a reference list. After the first round of screening based on titles and abstracts using the inclusion/exclusion criteria, 26 articles remained for further evaluation as shown in Fig. 1. After screening the full text and contacting authors for more details, 14 articles met the inclusion criteria and were included in the meta-analysis,Reference Cho, Choi, Suh, Sohn, Kim and Cho8–Reference Kim, Ng, Chung, Kim, Honda and Guo21 with 7 articles for the depression outcomeReference Cho, Choi, Suh, Sohn, Kim and Cho8–Reference Wang, Liu, Li, Liu, Guo and Yuan14 and 7 articles for the suicide outcome.Reference Kim, Jung, Kang, Kim, Moon and Hur15–Reference Kim, Ng, Chung, Kim, Honda and Guo21
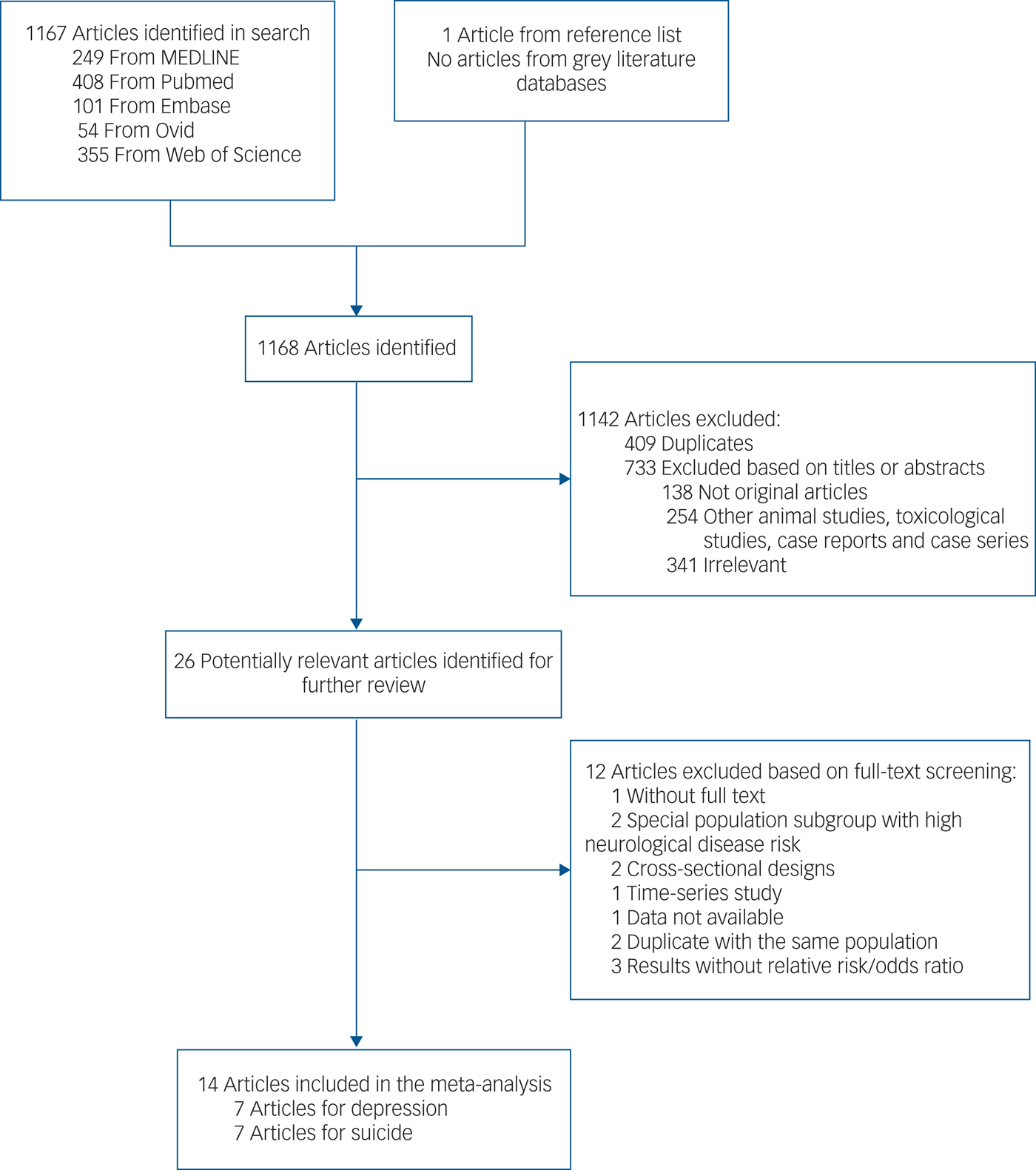
Fig. 1 Literature search for the meta-analysis.
Study characteristics
Characteristics of the 14 included articles are shown in Table 1. There were six time-stratified case-crossover studies and one cohort study for suicide; for depression there were three time-stratified case-crossover studies and four cohort studies. Study locations included North America, Europe and Asia. The total number of participants investigated in these studies was 684 859, including 429 678 (ranging from 1148 to 265 749) for suicide and 255 181 (ranging from 1367 to 118 602) for depression. The proportion of male participants ranged from 0 to 78.3% (excluding three studies which did not provide this information) and mean age ranged from 41.3 to 72.4 years (excluding two studies which lacked this information).
Table 1 Characteristics of studies included in the meta-analysis
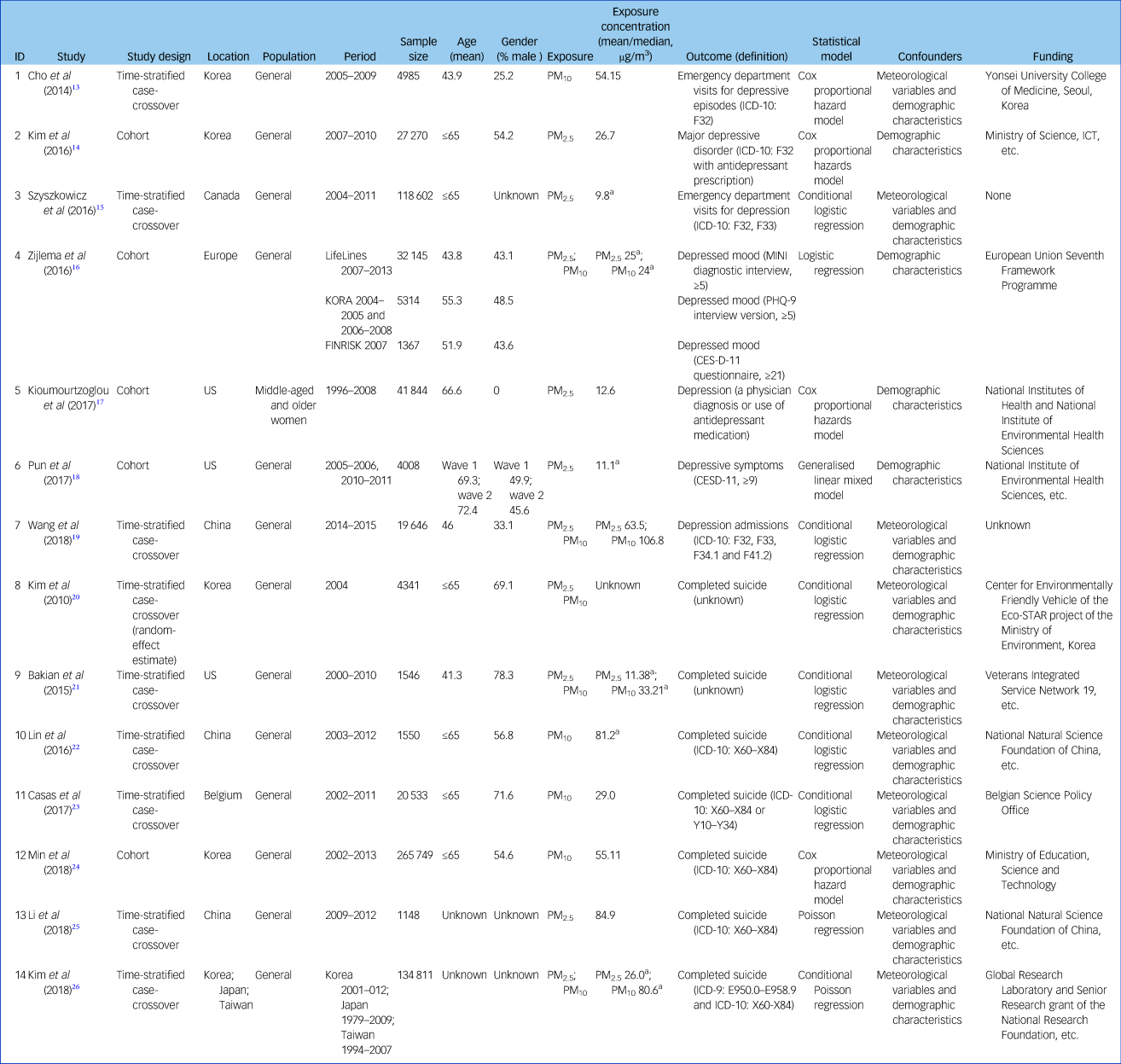
MINI, Mini-International Neuropsychiatric Interview; PHQ-9, Patient Health Questionnaire 9; CESD-11, Center for Epidemiological Studies Depression Scale 11.
a. If there were more than one mean value of pollutants stratified by case or control group, region, lag day, etc., the highest value was extracted.
Mean concentrations of PM exposure ranged from 9.8 to 84.9 µg/m3 for PM2.5 and 24 to 106.8 µg/m3 for PM10. Four studies used an exposure measure of PM10; five studies used PM2.5; four studies used both PM2.5 and PM10; one study did not report on the exposure concentration. Compared with World Health Organization (WHO) air quality guidelines,28 we found that PM2.5 concentrations in four of the included studies were close to the guideline (10 µg/m3), three studies were close to 20 µg/m3 and only two were higher than 60 µg/m3 (Fig. 2). In contrast, concentrations of PM10 were generally much higher than the guideline (20 µg/m3). In all studies investigating the impact of both PM2.5 and PM10, an increase in PM2.5 was associated with a higher risk of depression and suicide than a similar increase in PM10.

Fig. 2 Particulate matter exposure levels (mean ± SD or median, μg/m3) in studies included in the meta-analysis. PM, particulate matter; WHO, World Health Organization.
Outcome assessment was consistent for completed suicide as the studies used either the ICD code (ICD-9 [1978]: E950.0–E958.9 and ICD-10 [1992]: X60-X84 or Y10-Y34) or suicide records. In contrast, outcome assessment for depression varied across different studies: two studies ascertained depression mood/symptoms using depression scales or interviews, whereas five studies ascertained a diagnosis of depression mainly using the ICD code. With regard to the statistical analyses, most studies used Cox proportional hazard models or logistic regression models adjusted for meteorological variables and demographic characteristics.
Overall analyses
The overall meta-analysis showed that an increase of 10 µg/m3 in PM2.5 or PM10 was associated with increased risk of depression and suicide (Fig. 3).
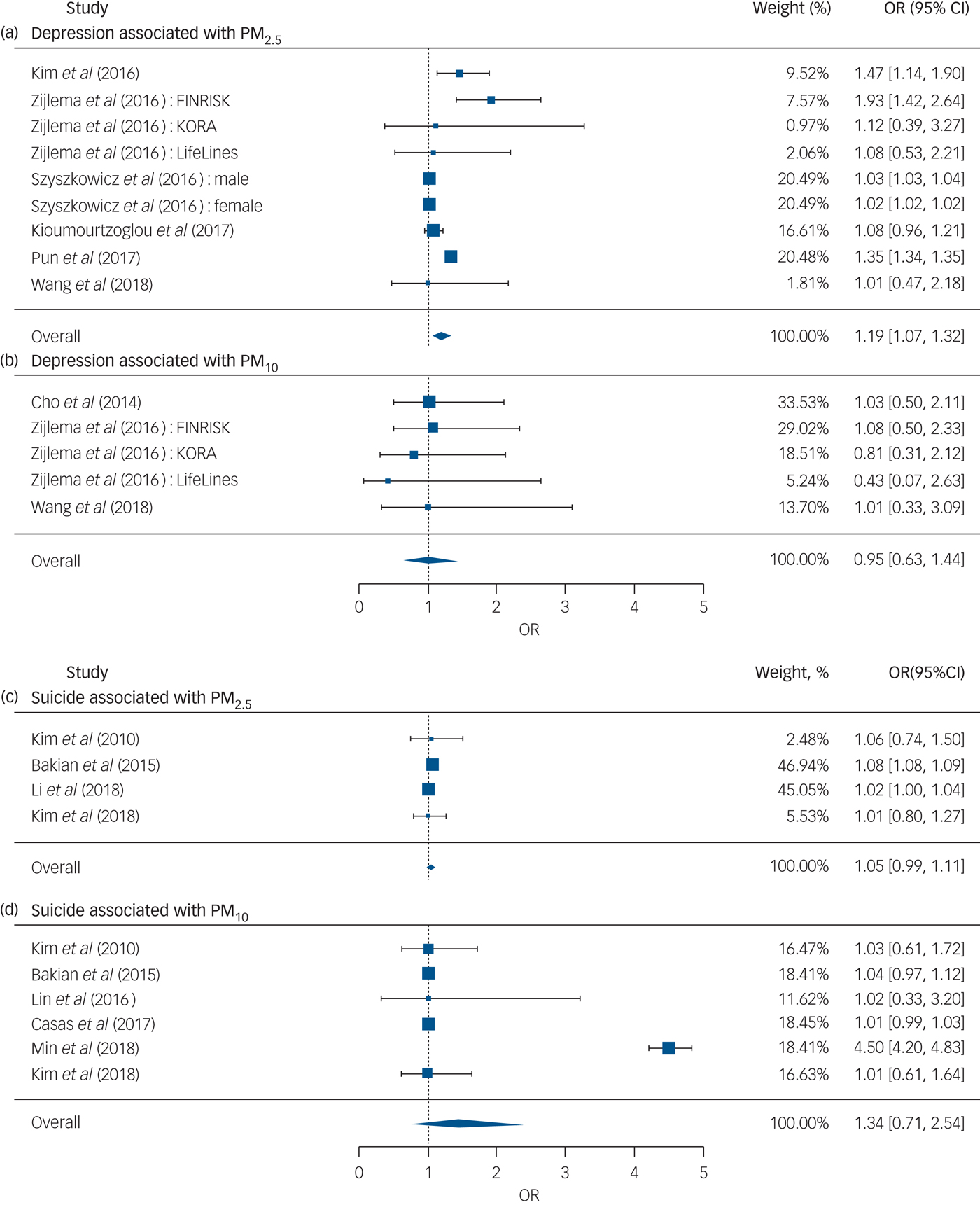
Fig. 3 Forest plots for overall analyses of (a–b) depression and (c–d) suicide risk associated with an increase of 10 µg/m3 in PM2.5 or PM10, respectively. OR, odds ratio; PM, particulate matter.
Depression outcome
We pooled nine effect estimates examining the association of PM2.5 with depression and found that an increase of 10 µg/m3 in PM2.5 was associated with a 19% higher risk of depression (odds ratio [95% CI] 1.19 [1.07, 1.33]), whereas the pooled effect estimate for PM10 was insignificant (Fig. 3). No evidence of publication bias was observed for the depression outcome with PM2.5 (P = 0.761) and PM10 (P = 0.233) (see funnel plot details in Supplementary Fig. 1).
According to the lag patterns shown in Table 2, exposure to PM2.5 was positively associated with depression risk for short-term, long-term, single-day and cumulative lag patterns, and the strongest pooled odds ratio appeared at the long-term lag pattern (odds ratio [95% CI] 1.25 [1.07, 1.45], P < 0.01) and cumulative lag pattern (odds ratio [95% CI] 1.26 [1.07, 1.48], P < 0.01). In contrast, pooled effect estimates for PM10 and depression were insignificant for all lag patterns. The association between PM2.5 and depression was positive at all specific lag times and significant at a lag of 3, 4 and 5 days as well as at the 1-year moving average, with the strongest association at the 1-year moving average (odds ratio [95% CI] 1.14 [1.04, 1.25]) (Fig. 4). Pooled effect estimates for risk of depression associated with exposure to PM10 were insignificant at all specific lag times (data not shown).
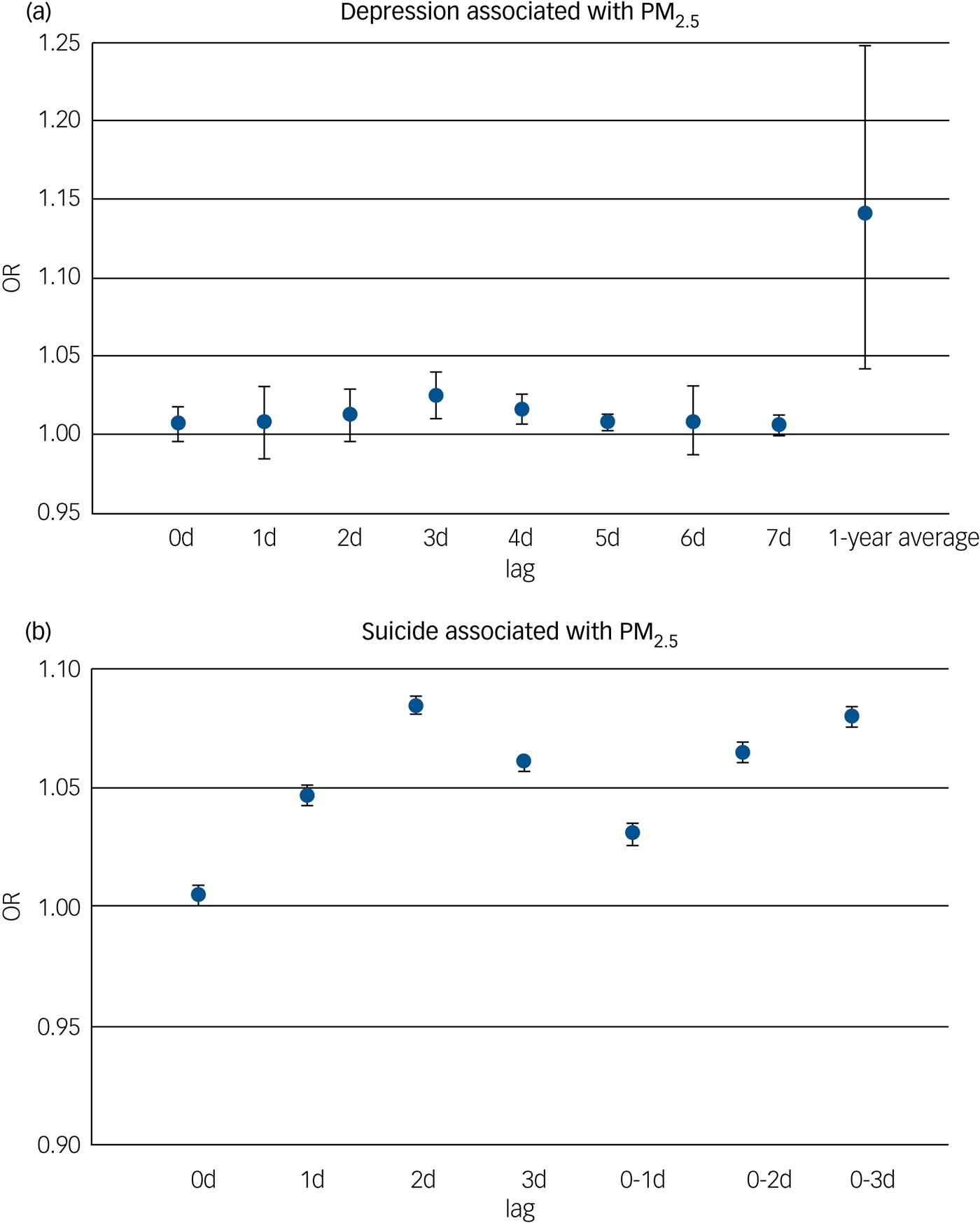
Fig. 4 Meta-analysis according to common lag times for (a) depression and (b) suicide risk associated with an increase of 10 µg/m3 in PM2.5. OR, odds ratio; PM, particulate matter.
Table 2 Overall combined effect estimates and analyses based on different lag patterns
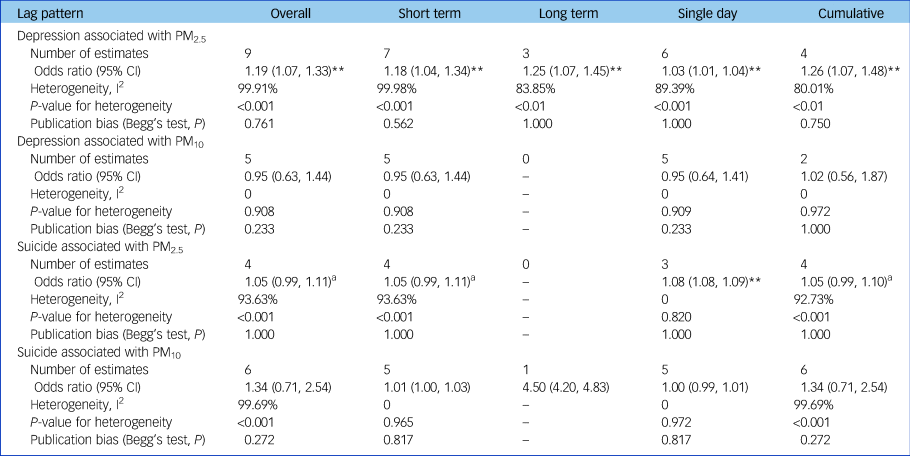
Odds ratios were shown per 10 µg/m3 increase in PM2.5 or PM10. Effect estimates in Zijlema et al (2016)Reference Bakian, Huber, Coon, Gray, Wilson and McMahon16 were reported in three cohort groups: LifeLines, KORA and FINRISK.
a. 0.05 < P < 0.10.
** P < 0.01.
Suicide outcome
We found that the overall result of four effect estimates examining the risk of suicide associated with PM2.5 was positive and marginally significant (odds ratio [95% CI] 1.05 [0.99, 1.11], P < 0.10), whereas the overall result of six effect estimates for PM10 was insignificant (Fig. 3). No evidence of publication bias was observed for the suicide outcome with PM2.5 (P = 1.000) and PM10 (P = 0.272) (see funnel plot details in Supplementary Fig. 1).
Exposure to PM2.5 was positively associated with suicide risk at a single-day lag pattern and marginally significant (P < 0.10) at short-term and cumulative lag patterns (Table 2). However, no lag pattern result was significant for PM10 (Table 2). For different lag times, the positive association between PM2.5 and suicide risk was found to be significant at all specific lag times including at a lag of 0, 1, 2 and 3 days and a lag of 0–1, 0–2 and 0–3 days, with the strongest association shown at a lag of 2 days (odds ratio [95% CI] 1.08 [1.08,1.09]) (Fig. 4). Pooled effect estimates for risk of suicide associated with exposure to PM10 were insignificant at all common lag times (data not shown).
Sensitivity and subgroup analyses
Depression outcome
Sensitivity analyses indicated that the significance of depression risk associated with PM2.5 and PM10 was robust. The study by Pun et al (2017)Reference Pun, Manjourides and Suh13 had the largest influence on the depression risk associated with PM2.5: the pooled odds ratio without this study decreased from 1.19 (95% CI 1.07–1.33) to 1.03 (95% CI 1.01–1.05) (Supplementary Table 3).
The pooled depression risk associated with PM2.5 stratified by gender ratio was significant (odds ratio [95% CI] 1.22 [1.01, 1.48]) in the subgroup with male proportion ≤50% but not in the subgroup with male proportion >50% (odds ratio [95% CI] 1.20 [0.85, 1.70]) (Table 3). Regarding study location, the depression risk associated with PM2.5 was significant in studies conducted in Europe (odds ratio [95% CI] 1.57 [1.04, 2.35]) and Asia (odds ratio [95% CI] 1.42 [1.11, 1.80]), but only marginally significant in North America (odds ratio [95% CI] 1.11 [0.99, 1.26]). The pooled depression risk was significant in both cohort studies and case-crossover studies, and was stronger in cohort studies (odds ratio [95% CI] 1.34 [1.14, 1.56]). In study quality assessment, five studies scored more than six and were classified as high-quality studies and two studies scored five were classified as medium-quality studies (Supplementary Table 2). The pooled odds ratio of depression associated with PM2.5 in high-quality studies was 1.03 (95% CI 1.01–1.04), which was lower than that for medium-quality studies (odds ratio [95% CI] 1.45 [1.14, 1.85]). In addition, subgroup results of the quality assessment categories demonstrated that the differences between high-quality and medium-quality studies were mainly due to variation in comparability and exposure/outcome scores, even though all combined odds ratios were significant. Specifically, medium-quality studies used statistical models only adjusted for demographic confounders but not meteorological confounders and the follow-up time was inadequate (Supplementary Table 2). The pooled risk for studies using depression mood or symptoms as the outcome definition (odds ratio [95% CI] 1.45 [1.14, 1.85]) was higher than that for studies using depression diagnoses as the outcome definition (odds ratio [95% CI] 1.03 [1.01, 1.04]). These subgroup results based on outcome assessment were consistent to the subgroup results based on study quality assessment. The depression risk associated with exposure to PM10 was insignificant in all subgroups (Table 3).
Table 3 Subgroup analyses based on study characteristics
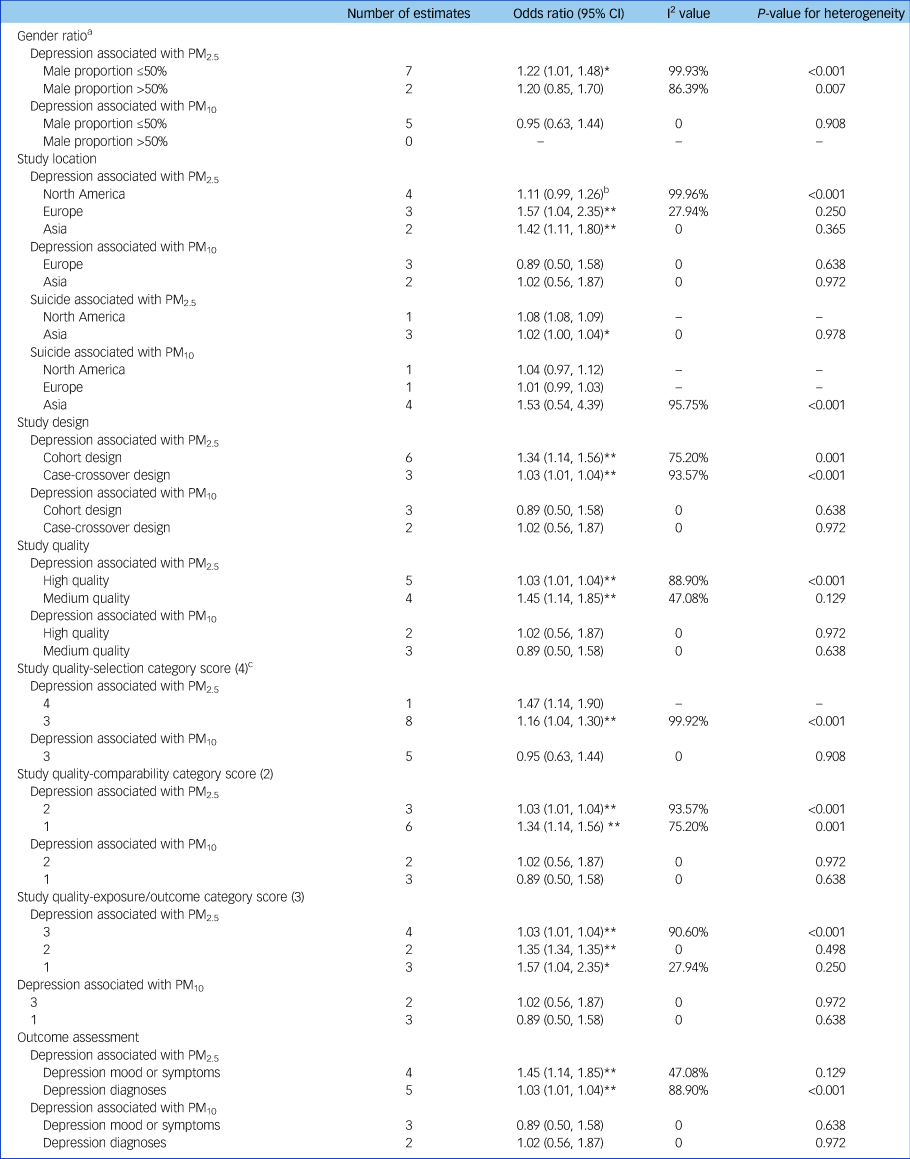
Odds ratios were shown per 10 µg/m3 increase in PM2.5 or PM10. Effect estimates in Zijlema et al (2016)Reference Bakian, Huber, Coon, Gray, Wilson and McMahon16 were reported in three cohort groups: LifeLines, KORA and FINRISK. PM, particulate matter.
a. Two studies did not report gender ratio.
b. 0.05 < P < 0.10.
c. The number included in the title of the subgroups indicate one category score number of the study quality assessment scale. For example, “Study quality-selection category score (4)” indicates that in the selection category of study quality assessment scale, the possible maximum total score is 4. Thus, these single-standing numbers in the left-hand column are referring to subgroups with different scores in relevant categories.
* P < 0.05, **P < 0.01.
Suicide outcome
Sensitivity analyses appreciably changed the significance of suicide risk associated with PM2.5 but not PM10. The study by Li et al (2018)Reference Li, Yan, Sun and Anderson20 had the largest influence on the suicide risk associated with PM2.5: the pooled odds ratio without this study was significant at 1.08 (95% CI 1.08–1.09), whereas the overall pooled odds ratio with this study was only 1.05 (95% CI 0.99–1.11) (Supplementary Table 3).
Studies for the suicide outcome were all determined to be of high quality by quality assessment (Supplementary Table 2). Because gender ratio, study quality and categories of quality assessment were consistent in studies for the suicide outcome, we did not perform subgroup analyses based on these variables. The pooled suicide risk associated with PM2.5 was 1.02 (95% CI 1.00, 1.04) for studies conducted in Asia whereas all subgroups were insignificant for PM10 based on study location (Table 3).
Discussion
After conducting our meta-analysis, we confirmed that exposure to PM2.5 had a significant positive association with risk of depression and a marginally significant positive association with risk of suicide. For an increase of 10 µg/m3 in PM2.5, we found a 19% increased risk of depression and a 5% increased risk of suicide in the meta-analysis. The pooled association between PM2.5 and depression risk was robust in subgroup and sensitivity analyses, and there was no substantial publication bias. We did not observe any significant association between exposure to PM10 and risk of depression or suicide.
To investigate the time course of pollutant exposure–response relationships, we performed meta-analyses for different lag patterns and specific lag times. Pooled effect estimates were all positive over these different lag patterns and specific lag times for depression and suicide risk associated with PM2.5. For the association between PM2.5 and suicide, we found significant effect estimates at single-day lag pattern (Table 2) and different lag times, and the strongest effect estimates appeared at a lag of 2 days and a lag of 0–3 days (Fig. 4), suggesting that risk of suicide was mainly associated with short-term exposure to PM2.5. In contrast, there was a 26% increased risk of depression at the cumulative lag pattern and a 25% increased risk of depression at the long-term lag pattern associated with exposure to PM2.5, suggesting that the potential exposure effect was stronger after accumulative exposure over a long time period. Results for common lag times confirmed that the association was much stronger at a 1-year moving average than at short-term lag times (Fig. 4).
However, exposure to PM10 was not found to be statistically significantly associated with risk of depression and suicide overall as well as at different lag patterns and common lag times.
Epidemiological and experimental evidence
Depression disorder is a major mental health condition which could cause suicide3 and is a common comorbidity for other illnesses such as cardiovascular disease,Reference Aben, Verhey, Strik, Lousberg, Lodder and Honig29, Reference Cully, Jimenez, Ledoux and Deswal30 stroke,Reference Aben, Verhey, Strik, Lousberg, Lodder and Honig29–Reference Dossa, Glickman and Berlowitz31 diabetes,Reference Rustad, Musselman and Nemeroff32 chronic obstructive pulmonary diseaseReference Maurer, Rebbapragada, Borson, Goldstein, Kunik and Yohannes33, Reference Yohannes and Alexopoulos34 and so on.
There is increasing epidemiological evidences for the association between air pollution exposure and mental health, not only depression and suicide, but also psychological stressReference Kim and Kim35–Reference Mehta, Kubzansky, Coull, Kloog, Koutrakis and Sparrow37 and anxiety.Reference Pun, Manjourides and Suh13, Reference Power, Kioumourtzoglou, Hart, Okereke, Laden and Weisskopf38 Moreover, recent studies have investigated the potential associations between air pollution and central nervous system outcomes including autism spectrum disorders,Reference Volk, Hertz-Picciotto, Delwiche, Lurmann and McConnell39 neurodevelopment in children,Reference Calderón-Garcidueñas, Engle, Mora-Tiscareño, Styner, Gómez-Garza and Zhu40–Reference Wang, Zhang, Zeng, Zeng, Wang and Chen42 neurodegenerative diseasesReference Kioumourtzoglou, Schwartz, Weisskopf, Melly, Wang and Dominici43–Reference Power, Adar, Yanosky and Weuve45 and stroke.Reference Mateen and Brook46 In support of the epidemiological studies, exposure to air pollutants has also been linked to cognitive function decline in animal models.Reference Calderón-Garcidueñas, Mora-Tiscareño, Ontiveros, Gómez-Garza, Barragán-Mejía and Broadway41
Although the exact mechanism has yet to be clarified, one hypothesis is that mental disorders occur because of oxidative stress and neuroinflammation pathways being induced by air pollutants. PM is a major air pollutant causing health damage and disease (such as cardiovascular disease,Reference Brook, Franklin, Cascio, Hong, Howard and Lipsett47, Reference Brook, Rajagopalan, Pope, Brook, Bhatnagar and Diez-Roux48) mainly by the inflammatory and oxidative stress pathways.Reference Schwartz49–Reference Brites and Fernandes51 Evidence from experimental investigation found that, as an essential stressor, PM is associated with increased depression-like responses in mice.Reference Davis, Bortolato, Godar, Sander, Iwata and Pakbin52 Mechanistic studies also showed that inflammation and oxidative stress are the two primary pathways for the neural system damage upon PM exposure.Reference Anisman and Hayley22–Reference Campbell, Oldham, Becaria, Bondy, Meacher and Sioutas25, Reference Schwartz49 The neural system is vulnerable to PM for its high-energy use, low endogenous scavenger levels and high metabolic demands. PM, as an inflammatory stimulus, activates common processes in which cytokines and inflammatory signalling molecules provoke the dysregulation of the development of depressive disorders and their comorbidities, such as heart disease, diabetes and autoimmune conditions.Reference Schwartz49
On the other hand, PM inhalation can also provoke increased expression of redox/glucocorticoid-sensitive genes in rats, suggesting the involvement of the hormonal pathway in the mental health disorders associated with PM.Reference Thomson, Vladisavljevic, Mohottalage, Kumarathasan and Vincent53 Chronic activation and inappropriate regulation of the hypothalamic–pituitary–adrenal axis have been associated with adverse neurobehavioural effects, including depression and anxiety disorders.Reference Thomson, Vladisavljevic, Mohottalage, Kumarathasan and Vincent53 Given the close relationship between air pollution exposure and mental health disorders, exposure to air pollutants could increase the risk of completed suicide.
We also found that PM2.5 may play a more important role than PM10 in all studies investigating the influence of both PM2.5 and PM10. Although PM10 has also been identified as a strong inflammatory stimulus, PM2.5 is thought to exert greater toxicity because of smaller physical sizes and higher concentrations of adsorbed or condensed toxicants on the surface. Compared with larger particles, PM2.5 could be inhaled into the lungs more easily, where it then enters the circulatory system and – by activating pro-inflammatory cytokines – subsequently causes systemic and neuronal inflammation and oxidative damage,Reference Sarnat, Chang and Weber54 possibly leading to mental disorders and other nervous system diseases.Reference Kioumourtzoglou, Schwartz, Weisskopf, Melly, Wang and Dominici43, Reference Kirrane, Bowman, Davis, Hoppin, Blair and Chen55
Subgroup analyses
We did subgroup analyses with a major focus on depression risk associated with PM, and found appreciable differences in effect estimates over subgroups for PM2.5 but not PM10. The pooled risk of depression associated with PM2.5 was significant in both cohort studies and case-crossover studies. The magnitude of the effect estimate was smaller for case-crossover studies than for cohort studies. This may be explained by the fact that cohort studies mainly investigated depression risk at a long-term lag pattern whereas case-crossover studies investigated depression risk at a short-term lag pattern, and long-term exposure to PM may increase the health risk more substantially than short-term exposure.
Moreover, the pooled association for PM2.5 and depression was still significant after excluding medium-quality and low-score studies in three quality categories, suggesting that the result was robust and reliable. Contributing factors for the relatively low scores in quality assessment were mainly due to a failure to adjust for all possible confounders, including demographic characteristics (such as age, gender, jobs and income), meteorological variables (such as temperature, humidity and sunshine duration) and physical conditions (such as underlying diseases), as well as insufficient follow-up time in cohort studies. Ideally, one would adjust for these contributing factors in the statistical models to guarantee the comparability of the groups. However, all included cohort studies did not control for meteorological variables. Additionally, we could not identify whether the differences between outcome assessments were due to the severity of assessed outcomes or quality differences because the included studies which ascertained depression mood or symptoms were all medium-quality studies. Therefore, we need more high-quality evidence from long-term investigations that adjust for meteorological variables, demographic characteristics and underlying diseases to confirm the association.
The depression risk associated with PM2.5 was significant in studies conducted in Europe and Asia but only marginally significant in North America, possibly because the PM2.5 pollution in studies conducted in the North America subgroupReference Szyszkowicz, Kousha, Kingsbury and Colman10, Reference Kioumourtzoglou, Power, Hart, Okereke, Coull and Laden12, Reference Pun, Manjourides and Suh13 were all at low levels close to WHO air quality guidelines. These results suggest that high PM2.5 levels may be associated with a greater risk of depression.
We also did subgroup analyses according to study characteristics. Because of the lack of individual data, we only divided studies into subgroups based on the proportion of males in the study population and did not find any obvious differences in risk of depression associated with PM2.5 in populations with male proportion ≤50% and those with >50% males.
Strengths and limitations
This study provides a comprehensive evaluation for the association between ambient PM and completed suicide and depression, two major disorders of mental health, in the general population and thus has fair representativeness. Meanwhile, all these included studies were published in the past decade, suggesting an increasing concern for the mental health disorders associated with air pollution. Our meta-analysis has several limitations. First, there is the issue of heterogeneity in the definition of depression among studies. The included studies for depression used two major tools for evaluation of depression: two studies ascertained depression mood/symptoms using different scales whereas five studies ascertained depression diagnosis mainly using the ICD codes. We were not able to perform a more detailed subgroup analysis due to the limited number of relevant articles in the literature. Moreover, we could not analyse the influence of depression severity on results because of the lack of relevant information in included studies. However, we used random-effect model to control for the heterogeneous effects of the depression criteria and disease severity and confirmed the association between PM and depression.Reference DerSimonian and Kacker56 Second, PM2.5 concentrations in most included studies of depression were relatively low and close to WHO air quality guidelines. Therefore, further studies in areas with high PM2.5 levels are needed to investigate the depression risk associated with higher PM2.5 exposure. Moreover, the elemental composition of PM2.5 in different countries would also contribute to the heterogeneity of associations.
Third, the number of studies included in this meta-analysis is relatively small and may have led to the marginal significance of suicide risk associated with PM2.5. More epidemiological evidence is needed to confirm the association of PM with suicide. Additionally, due to lack of individual characteristic data, we could not investigate the potential difference in exposure–response relationships in different population subgroups divided by individual characteristics. In particular, some evidence suggests that depressive symptoms are highly prevalent in the elderly.Reference Thielke, Diehr and Unutzer57
Clinical implications
Our findings suggest that individuals exposed to PM2.5 may be at a higher risk of depression or suicide. Because of the ubiquitous existence of PM pollution and the prevalence of depression and suicide, the associated disease burden of mental health would be substantial at the population level. Therefore, it is important to develop relevant public prevention methods for depression and suicide. Targeted strategies such as more stringent air pollution standards, air quality control measures and individual protection behaviours may be helpful to prevent the onset and exacerbation of depression or to decrease suicide cases due to air pollution, especially in heavily polluted regions and high-risk groups. In addition, several researchersReference Ji, Wang, Chen, Jin, Chen and Chai58–Reference Tan, Manchester, Qin and Reiter61 implied that melatonin might play a role in the prevention or treatment of depression associated with PM2.5. However, because the mechanism by which PM2.5 increases the risk of depression is unclear, the efficacy of the first melatonergic antidepressant agomelatineReference de Bodinat, Guardiola-Lemaitre, Mocaër, Renard, Muñoz and Millan62 is yet to be explored in the treatment of PM2.5-related depression. Thus, the positive association between PM pollution and depression/suicide offers a new insight and consideration for the treatment of depression and other mental disorders through reducing individual exposure to PM pollution and alleviating oxidative stress in the nervous system.
Future epidemiological studies are needed to confirm the association for PM and suicide and explore the association patterns of PM with depression and suicide in different population subgroups, and experimental studies are also encouraged to investigate the underlying mechanism for the reported associations.
Supplementary material
Supplementary material is available online at https://doi.org/10.1192/bjp.2018.295.
Funding
This work was supported by the National Key Research and Development Program of China (2017YFC0211600, 2017YFC0211601), National Young Thousand Talents Program of China and start-up funding from the Peking University Health Science Center (BMU2018QQ003, BMU20160549).










eLetters
No eLetters have been published for this article.