Globally, the number of colorectal cancer (CRC) survivors continues to rise, mainly due to population ageing, screening and improved treatments(Reference Bray, Ren and Masuyer1–Reference Parry, Kent and Mariotto3). CRC survivors frequently report decreased health-related quality of life (HRQoL) due to persisting problems related to the cancer and/or its treatment and the presence of co-morbidities(Reference Chambers, Meng and Youl4–Reference Marventano, Forjaz and Grosso6). Two commonly experienced problems are cancer-related fatigue and chemotherapy-induced peripheral neuropathy (CIPN). Cancer-related fatigue has estimated prevalence rates of 41 and 35 % among short-term (<5 years post-diagnosis) and long-term (>5 years post-diagnosis) CRC survivors, respectively(Reference Thong, Mols and Wang7). Approximately two-thirds of patients after chemotherapy cessation experience CIPN symptoms like tingling hands and feet, with falling rates to approximately one-third of patients at 6 months(Reference Seretny, Currie and Sena8). Both fatigue and CIPN can negatively affect physical functioning and daily living up to 10 years post-treatment(Reference Tofthagen9–Reference Mols, Beijers and Lemmens11). It is therefore of interest to identify modifiable factors, such as lifestyle factors, related to long-term HRQoL outcomes, fatigue and CIPN that provide input for recommendations or interventions aimed at improving the health and well-being of CRC survivors.
In 2018, the World Cancer Research Fund and the American Institute for Cancer Research (WCRF/AICR) updated their lifestyle recommendations based on extensive systematic reviews of evidence on diet, nutrition, physical activity, body weight and risk of cancer(12). As the evidence to formulate-specific recommendations for cancer survivors is insufficient to date, WCRF/AICR advises cancer survivors to follow the lifestyle recommendations for cancer prevention. Lifestyle behaviours such as higher levels of physical activity, a healthy body weight and consumption of diets low in red meat, high in vegetables and high in fibre are associated with less recurrence and increased survival in cancer survivors, including CRC survivors(Reference Romaguera, Ward and Wark13–Reference Van Blarigan and Meyerhardt16), but are also likely to be relevant for increasing HRQoL(Reference Breedveld-Peters, Koole and Muller-Schulte17–Reference Grimmett, Bridgewater and Steptoe20) and decreasing fatigue and neuropathy(Reference Greenlee, Hershman and Shi21). Most evidence on patient-reported outcomes has been derived from studies on physical activity and body weight. Studies have shown that lower levels of physical activity as well as not meeting physical activity guidelines were significantly associated with lower HRQoL in CRC survivors up to 10 years post-diagnosis(Reference Thraen-Borowski, Trentham-Dietz and Edwards22–Reference Lynch, van Roekel and Vallance24), whereas higher levels of moderate-to-vigorous physical activity (MVPA) were found to be associated with lower levels of fatigue and neuropathy(Reference Tofthagen9,Reference Van Roekel, Bours and Breedveld-Peters25,Reference Mols, Beijers and Vreugdenhil26) . Studies in CRC survivors have found that having a higher BMI was associated with lower HRQoL outcomes(Reference Schlesinger, Walter and Hampe19,Reference Adams, Ceballos and Newcomb27–Reference Trentham-Dietz, Remington and Moinpour34) .
Nutrition has a large contribution in the lifestyle recommendations, as five out of seven recommendations are focused on nutrition. The dietary recommendations comprise consuming a sufficient amount of fruit and vegetables and fibre, whilst limiting consumption of red and processed meat, fast foods, sugar-sweetened drinks and alcohol. However, only few studies have evaluated the potential role of dietary intake on HRQoL, fatigue and neuropathy in the specific population of CRC survivors, and results have been inconsistent. Two cross-sectional studies that used a single- or a two-item question to assess fruit and vegetables intake pointed towards a beneficial association of meeting the recommendation of five servings per d of fruit and vegetables with higher HRQoL(Reference Grimmett, Bridgewater and Steptoe20,Reference Blanchard, Courneya and Stein35) . Additionally, moderate alcohol drinkers (weekly alcoholic drinks 1–14 for women and 1–21 for men) reported higher physical, role and social functioning and lower fatigue compared with non-drinkers(Reference Grimmett, Bridgewater and Steptoe20,Reference Vissers, Thong and Pouwer36) , while no significant association was found between HRQoL and heavy drinking (weekly alcoholic drinks >14 for women and >21 for men)(Reference Grimmett, Bridgewater and Steptoe20). One cross-sectional study in survivors of different cancers, including 113 CRC survivors, determined diet quality from 24-h recalls and found that higher diet quality (Healthy Eating Index scores above 80) was positively associated with better physical HRQoL outcomes(Reference Mosher, Sloane and Morey31). A longitudinal study showed that CRC patients following a ‘Western’ diet characterised by high consumption of potatoes, red and processed meat, poultry and cakes had lower chances to improve in physical functioning 1 year post-treatment compared with patients following a diet rich in fruit and vegetables(Reference Gigic, Boeing and Toth37). To our knowledge no studies have assessed an association between fruit and vegetables and CIPN in CRC survivors; one study in breast cancer patients receiving taxane treatment, however, found no association(Reference Greenlee, Hershman and Shi21). Nevertheless, several small intervention studies in diabetic neuropathy showed that a low-fat, plant-based diet was associated with decreased pain of diabetic neuropathy in patients experiencing diabetic neuropathy complaints, increasing the plausibility that dietary factors may also be associated with CIPN complaints after CRC(Reference Smith, Russell and Feldman38,Reference Bunner, Wells and Gonzales39) .
Recently, we reported that higher adherence to the 2007 WCRF/AICR lifestyle recommendations (combined into an overall adherence score) was related with better physical functioning and with less fatigue in CRC survivors 2–10 years post-diagnosis(Reference Breedveld-Peters, Koole and Muller-Schulte17). As the majority of recommendations is focused on nutrition, but there is scarce evidence on how the individual dietary recommendations are related to HRQoL after CRC, we aimed to further analyse the role of the updated individual dietary recommendations from the 2018 WCRF/AICR guidelines in this paper. Making use of the food group classification system proposed in the 2018 WCRF/AICR Score(Reference Shams-White, Brockton and Mitrou40), we aimed to assess associations of the individual dietary recommendations regarding intake of fruit and vegetables, dietary fibre, fast foods, red and processed meat, sugar-sweetened drinks and alcohol consumption in relation to HRQoL, fatigue and CIPN in CRC survivors 2–10 years post-diagnosis.
Methods
Study design and population
Data from the cross-sectional part of the Energy for Life after ColoRectal cancer (EnCoRe) study were used. This part of the EnCoRe study was set up to evaluate the influence of lifestyle factors on HRQoL outcomes in long-term (2–10 years post-treatment) stage I–III CRC survivors(Reference van Roekel, Bours and de Brouwer41). Study measurements were conducted between May 2012 and December 2013. Patients diagnosed with and treated for stage I–III CRC at the Maastricht University Medical Center+ in the Netherlands between 2002 and 2010 were identified via the Netherlands Cancer Registry and invited to participate by mail. Reasons for exclusion are shown in Fig. 1. The study was approved by the Medical Ethics Committee of the University Hospital Maastricht and Maastricht University (Netherlands Trial Register no. NL6904) and patients provided written informed consent before participation.
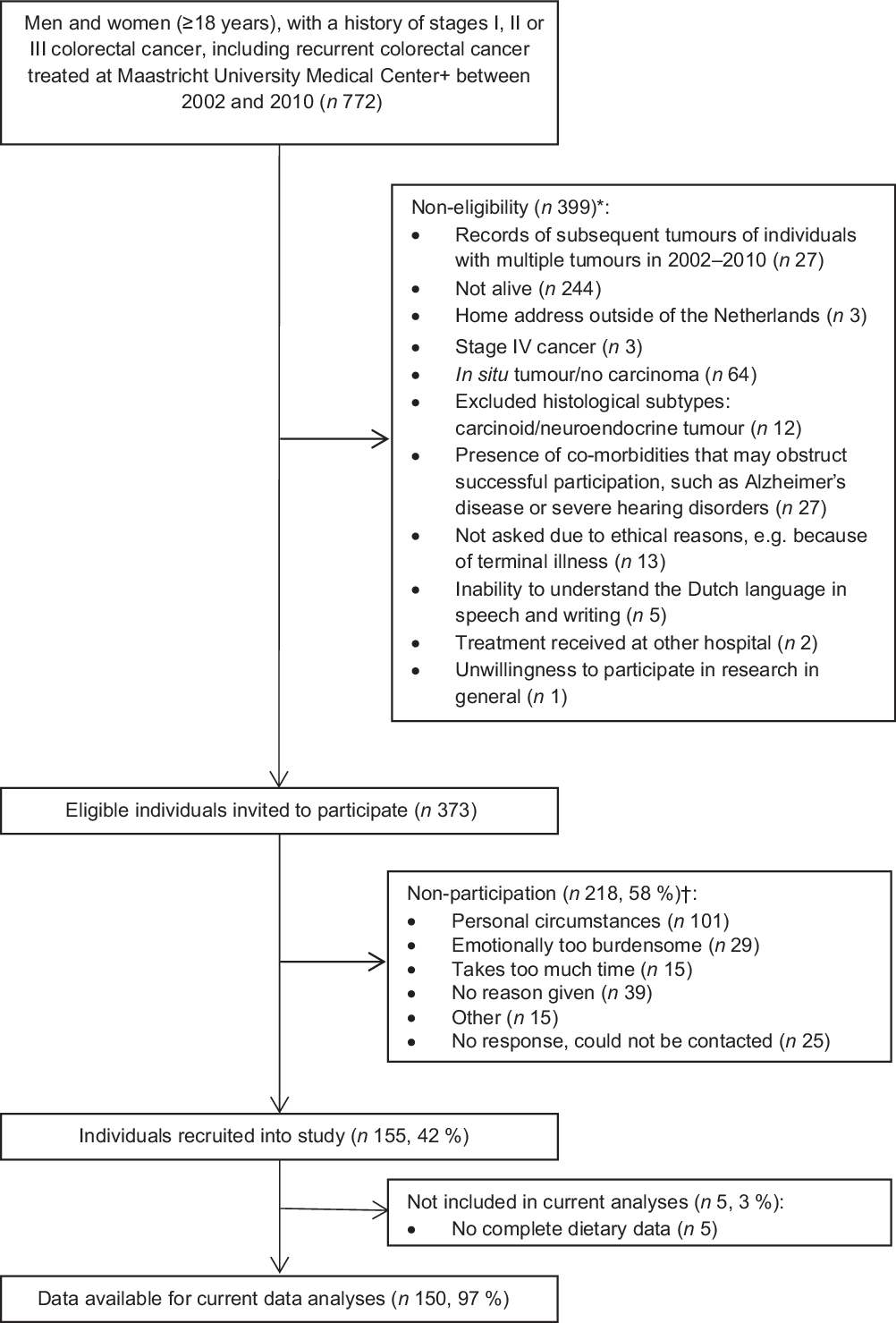
Fig. 1. Flow diagram of inclusion of colorectal cancer survivors. * Reasons for non-eligibility are given in order of exclusion. Totals do not add up because some exclusion criteria applied concurrently. † Totals do not add up because some individuals reported multiple reasons for non-participation.
Data collection
Data were collected by means of trained dietitians, who visited all patients at their homes to perform study measurements and leave behind questionnaires and a dietary record according to predefined standard operating procedures.
Dietary intake
Participants were asked to fill out a structured dietary record to obtain quantitative data on food and beverage consumption on seven consecutive days. In the dietary record, participants reported consumed meals, food and beverages with details on brand names, portion sizes and preparation. Participants received oral and written instructions on how to fill out the dietary record. Additionally, all completed dietary records were checked by the dietitians. When necessary, incomplete and/or inconsistent entries of the dietary record were clarified with the help of the participant.
Daily dietary intake was calculated utilising food calculation software (Compl-eat, Wageningen University) and based on the Dutch Food Composition database (NEVO-2011), using existing or specifically created dietary food groups in the software based on the 2018 WCRF/AICR dietary sub-recommendations (i.e. groups for fruit, vegetables, alcohol, fast foods, red meat, processed meat and sugar-sweetened drinks). Fruit and vegetable consumption (g/d) was calculated from the reported use of all fresh, frozen, dried and canned vegetables and fruit without added sugar. Calculation of total dietary fibre intake (g/d) and alcohol intake (i.e. ethanol in g/d) was based on the nutrient value from the food calculation table for the reported overall dietary intake.
The created food group for fast foods deserves particular attention. In agreement with the National Cancer Institute(Reference Shams-White, Brockton and Mitrou40), the fast food group was operationalised based on the group of ultra-processed foods (UPF) according to the NOVA classification system(Reference Monteiro, Cannon and Levy42). The NOVA classification system classifies food groups based on the extent of processing, and the UPF group contains food such as sweet or savoury packaged snacks and pre-prepared dishes. The UPF list from the NOVA classification was adapted by the National Cancer Institute to be in line with the WCRF/AICR report (i.e. food products made from white flour (e.g. white bread, rice and pasta))(12), national guidelines and to ensure that the adapted UPF group did not include food already accounted for in other dietary recommendations (i.e. sugar-sweetened drinks and red and processed meats). In the present study, the availability of extensive 7-d dietary records allowed us to make additional precise distinctions between products, compared with most previous studies based on FFQ. This led to additional food in our adapted UPF compared with the adapted UPF from National Cancer Institute (e.g. fruit yogurts). Our adapted UPF list is included in the online Supplementary material to show the food we included. For the fast food recommendation, we calculated the energy percentage of all food included in the UPF group based on participants’ self-reported intake.
Red meat consumption (g/d) was based on the intake of any kind of fresh raw red meat (including beef, pork, lamb and goat) that still needed to be prepared before consumption. Intake of processed meat (g/d) included intake of any meat that had been preserved by smoking, curing, salting or addition of chemical preservatives and was ready for consumption without preparation. This also included all processed meat in mixed food containing processed meats, such as soups or sausage rolls.
Sugar-sweetened drinks (g/d) included drinks with natural sugar, like fruit juices, as well as soft drinks. Drinks to which sugar was added by participants (e.g. coffee) were not included in the sugar-sweetened drink category.
Methods and procedures applied for the assessment and coding of dietary records are extensively explained in detail in Breedveld et al. (Reference Breedveld-Peters, Koole and Muller-Schulte17). Based on a random sample of dietary records (n 16, i.e. 10 % of all dietary records), agreement in coding between two dietitians of the EnCoRe study was evaluated and found to be high (ICC > 0·87).
Patient-reported outcomes
Patient-reported outcomes were measured comprehensively through several generic and cancer-specific validated questionnaires. Cancer-specific HRQoL was measured by the European Organization for the Research and Treatment of Cancer Quality of Life Questionnaire-Core 30 (EORTC QLQ-C30)(Reference Aaronson, Ahmedzai and Bergman43). The EORTC QLQ-C30 is a thirty-item cancer-specific questionnaire composed of five functioning scales (physical, role, cognitive, emotional and social functioning), three symptom scales (fatigue, pain, and nausea and vomiting), a global health/QoL scale and a number of items for specific cancer-/treatment-related symptoms. The scales have a 100-point score range, with higher scores on the global QoL and functioning scales reflecting better HRQoL or functioning, whereas higher symptom scale scores indicate more symptoms (i.e. worse fatigue). In addition, the recently established summary score (SumSc) developed by the EORTC Quality of Life Group was used. The SumSc was calculated from the mean of 13 of the 15 QLQ-C30 scores (excluding the financial difficulties and global QoL questions)(Reference Pompili, Koller and Velikova44).
In addition to the fatigue symptom scale from the EORTC QLQ-C30, fatigue was assessed in more detail by the Checklist Individual Strength (CIS)(Reference Vercoulen, Swanink and Fennis45). This questionnaire consists of twenty items with a seven-point Likert scale, a total score was derived by the summation of the items, ranging from 20 to 140 points with higher scores indicating worse fatigue. The CIS was originally developed and validated in patients with chronic fatigue syndrome(Reference Vercoulen, Hommes and Swanink46) but has also been applied in cancer survivors(Reference Servaes, van der Werf and Prins47).
To measure symptoms and complaints related to CIPN, the EORTC QLQ CIPN20 was used, consisting of sensory (nine items), motor (seven items) and autonomic subscales (two items) and a summary score (eighteen items) (excluding two conditional items)(Reference Postma, Aaronson and Heimans48). All scale scores were linearly converted to a 0–100 scale(Reference Lavoie Smith, Barton and Qin49). Higher scores on the CIPN20 scales indicate higher levels of neuropathy or more neuropathy-related complaints.
Lifestyle, clinical and sociodemographic factors
The Short QUestionnaire to ASsess Health-enhancing physical activity (SQUASH) was used to determine habitual activity level(Reference Wendel-Vos, Schuit and Saris50), assessing time spent on commuting, household, work and leisure time activities in the past week. Time spent on MVPA was calculated(51), and this was used to assess whether participants adhered to the physical activity recommendation of at least 150 min of MVPA per week. For objective measurement of sedentary time, the validated tri-axial MOX activity metre was used (Maastricht Instruments B.V.), as described previously by van Roekel et al. (Reference van Roekel, Winkler and Bours52). Briefly, the accelerometer was worn on the upper thigh for seven consecutive days (24 h/d) to assess average time spent per d in sedentary behaviour (i.e. sitting/lying with a low level of energy expenditure, during waking hours). Body composition measurements were performed by trained dietitians in a standardised way during home visits. Subjects’ weight was measured in light clothing without shoes on an electronic scale (Seco Ltd, type 861) and rounded to the nearest tenth of a kg. Height was measured in duplicate with the subject standing barefoot with heels together, arms at the side and head in the Frankfort horizontal plane with a portable stadiometer at the first home visit and measured to the nearest tenth of a cm. Measured weight and mean height were used to determine BMI in kg/m2. BMI was categorised according to the WHO guidelines as underweight (BMI < 18·5 kg/m2), normal weight (18·5 ≤ BMI < 25 kg/m2), overweight (25 ≤ BMI < 30 kg/m2) or obesity (BMI ≥ 30 kg/m2)(53). Waist circumference was measured midway between the lower rib margin and the iliac crest with a circumeter (type 05335, Premed) in cm; duplicate measurements were performed and the mean value was used. Clinical information (i.e. cancer stage, chemotherapy/radiotherapy treatment and tumour site) was collected through the Netherlands Cancer Registry. Self-reported data were collected on other factors, such as age, sex, education level, current smoking status and presence of co-morbidities. Co-morbidities were assessed using the Self-Administered Comorbidity Questionnaire(Reference Sangha, Stucki and Liang54).
Statistical analyses
Descriptive statistics (means and standard deviations or frequencies and percentages) were calculated to describe main sample characteristics, including intake of fruit and vegetables, dietary fibre, energy-dense food, fast foods, red and processed meat, sugar-sweetened drinks and alcoholic drinks.
Confounder-adjusted linear regression models were used to assess associations of each of the individual dietary recommendations in relation to the separate patient-reported outcomes. Six dietitians reached consensus on the relevant differences for each dietary recommendation, based on the recommended portion per d and on relevant differences in portion sizes (e.g. 100 g of fruit and vegetables is one portion and 10 g of ethanol is one alcoholic consumption). The individual dietary recommendations were modelled continuously per relevant difference for each recommendation, as defined a priori. Additionally, alcoholic consumption was also modelled as a categorical variable: no alcohol consumption; moderate alcohol consumption: 1–14 glasses per week (reference category) and heavy alcohol consumption: >14 glasses per week for both men and women. HRQoL outcomes included global QoL, physical functioning, role functioning, social functioning and summary score (EORTC QLQ-C30); fatigue (EORTC and CIS) and neuropathy scales (CIPN20). CIPN outcomes were only analysed for the subgroup of patients who received chemotherapy. A priori-defined confounders included in analyses were age (years), sex, BMI (kg/m2), MVPA (h/week), sedentary behaviour (h/d), number of co-morbidities (0, 1, ≥2), smoking (current, non-current), education level (low, medium and high), tumour stages (I, II and III), chemotherapy (yes, no), time since diagnosis (years) and total energy intake (kJ/week).
Two sensitivity analyses were performed. The first was a logistic regression analysis with dichotomised patient-reported outcomes using sex-specific median values as cut-off because some of the outcomes were not normally distributed(Reference Schlesinger, Walter and Hampe19,Reference van Roekel, Winkler and Bours52) . Another sensitivity analysis was a linear regression analysis excluding participants with outliers in dietary data. Outliers were identified by two methods. First, outliers in dietary data were based on the top and bottom 0·5 % of the distribution of the ratio of energy intake to the BMR, where BMR was estimated from body weight by using Schofield’s age- and sex-specific equations(Reference Black, Goldberg and Jebb55). Second, outliers were based on nutrient values exceeding three times the interquartile range from the upper quartile within the fifth quintile of the nutrient distribution(Reference Welch, Luben and Khaw56).
Statistical analyses were performed using IBM SPSS Statistics (version 23, IBM Corporation) with statistical significance set at P < 0·05 (two-tailed).
Results
Descriptives
In total, 373 eligible CRC survivors were invited to participate, of whom 155 (41·6 %) were included in the cross-sectional part of the EnCoRe study (Fig. 1). Five survivors did not provide dietary data and were therefore excluded from the current analyses. The remaining 150 CRC survivors recruited in the current analyses consisted of ninety-three males (62 %) and fifty-seven females (38 %) and were on average 69·7 (sd 8·7) years of age and diagnosed with CRC 5·7 (sd 1·8) years before study participation (Table 1). Based on BMI, 26·2 % of participants were underweight or normal weight (one participant was underweight), 45·6 % were overweight and 28·2 % were obese. A total of 136 (90·7 %) survivors adhered to the physical activity recommendation (≥150 min/week of MVPA) and 11 % were current smokers. Approximately half of the participants had colon cancer (53·3 %) and the other half had rectum cancer (46·7 %). Regarding self-reported co-morbidities, 24·8 % of the participants reported no co-morbidity, whereas half of the participants (50·3 %) reported two or more co-morbid conditions.
Table 1. Demographic, lifestyle and clinical characteristics of colorectal cancer survivors
(Mean values and standard deviations; numbers and percentages)
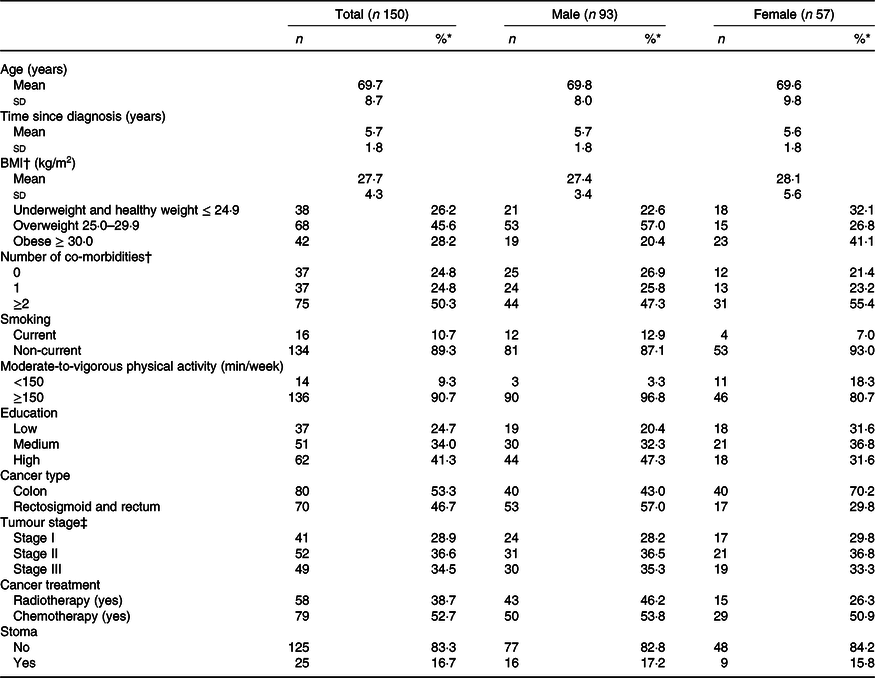
* Percentages may not add to 100 due to rounding.
† Data missing for one participant (female).
‡ Data missing for eight participants (male).
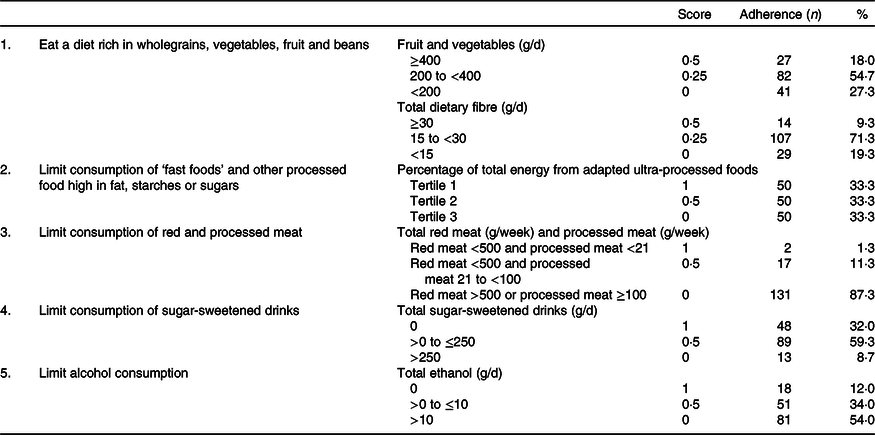
In Tables 2 and 3, an overview is given of the individual dietary recommendations and the adherence of included CRC survivors based on reported intake. Cut-off values are based on the National Cancer Institute operationalisation of the WCRF/AICR lifestyle recommendations(Reference Shams-White, Brockton and Mitrou40). Mean total energy intake was 9126 (sd 1753) kJ/d in men and 6697 (sd 1380) in women. Mean total intake of fruit and vegetables per d was 273 (sd 131) g, which was composed of 131 (sd 94) g of fruit and 142 (sd 70) g of vegetables. A total of 18·0 % of participants met the recommendation of eating 400 g/d of fruit and vegetables. Mean dietary fibre intake was 21·1 (sd 6·9) g/d, with 9·3 % meeting the recommendation of 30 g/d. Mean energy density of food was 701 (sd 112) kJ/100 g per d, and the mean energy percentage of daily fast food intake was 31·4 (sd 11·1) %. Mean intake of sugar-sweetened drinks was 97 (sd 150) g/d. For red meat, the mean intake was 592 (sd 295) g/week and for processed meat 45 (sd 32) g/d. Only two persons (1·3 %) met the recommendation of limiting processed meat (<3 g/d) and not exceeding 500 g of red meat per week. Total alcohol intake per d was 19·4 (sd 16·3) g/d in men and 7·1 (sd 8·3) g/d in women; 8·6 % of men and 17·5 % of women did not drink alcohol.
Table 2. Dietary World Cancer Research Fund and American Institute for Cancer Research (WCRF/AICR) recommendations and adherence in a colorectal cancer survivor population (n 150)*
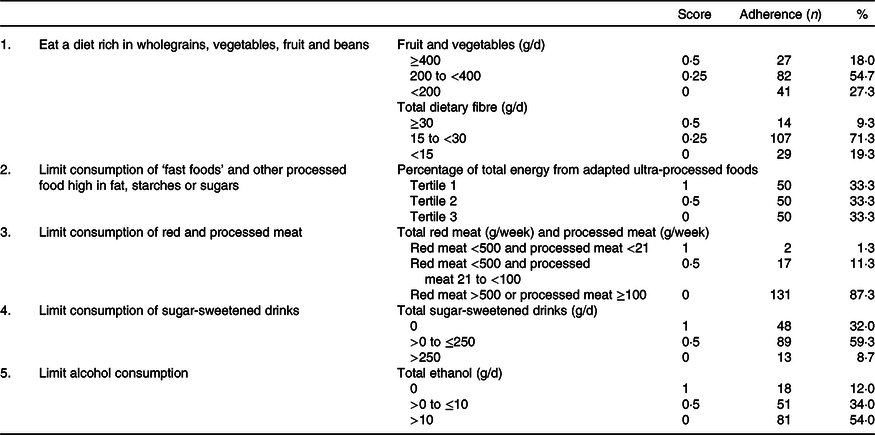
* Scoring system is based on the National Cancer Institute operationalisation of the 2018 WCRF/AICR recommendations.
Table 3. Mean dietary intake in colorectal cancer survivors (n 150)
(Mean values and standard deviations)
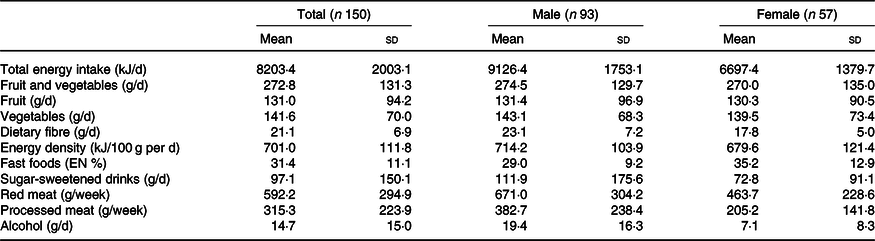
EN %, energy percentage.
Multi-variable analyses
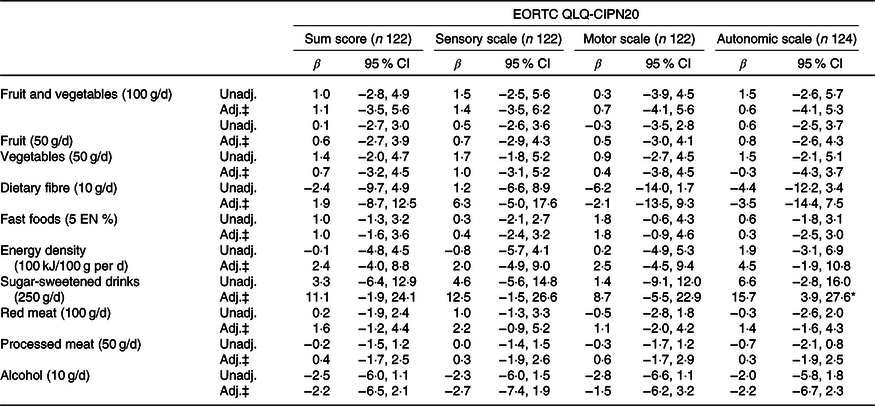
Tables 4 and 5 present the results of the unadjusted and confounder-adjusted linear regression analyses of dietary intake variables with HRQoL subscales, fatigue and CIPN. A higher intake of fruit and vegetables was associated with significantly better physical functioning (β/100 g 3·2; 95 % CI 0·8, 5·5). This association appeared to be mostly driven by vegetable intake, as higher vegetable intake was significantly associated with better physical functioning (β/50 g 3·3; 95 % CI 1·2, 5·5), whereas non-significant results were obtained for fruit intake (β/50 g 1·2; 95 % CI −0·5, 2·8). In addition, higher vegetable intake was associated with better global QoL (2·6; 95 % CI 0·6, 4·7) and lower levels of fatigue as measured by the CIS (−4·5; 95 % CI −7·6, −1·4). Higher intake of energy-dense food was associated with worse physical functioning (β/100 kJ per 100 g −4·2; 95 % CI −7·1, −1·2). When alcohol was analysed as a categorical variable, participants who did not drink alcohol had significantly lower levels on the physical functioning scale (β −10·2; 95 % CI −19·7, −0·8), role functioning scale (−13·1; 95 % CI −25·6, −0·6), social functioning scale (−12·6; 95 % CI −21·9, −3·3), summary score (−6·4; 95 % CI −12·3, −0·4) and higher levels of fatigue (10·8; 95 % CI 0·1, 21·5) compared with participants reporting moderate alcohol consumption. No statistically significant associations were found for heavy v. moderate drinkers and for alcohol analysed as a continuous variable.
Table 4. Associations between dietary recommendations and health-related quality of life outcomes†
(β-Coefficients and 95 % confidence intervals)
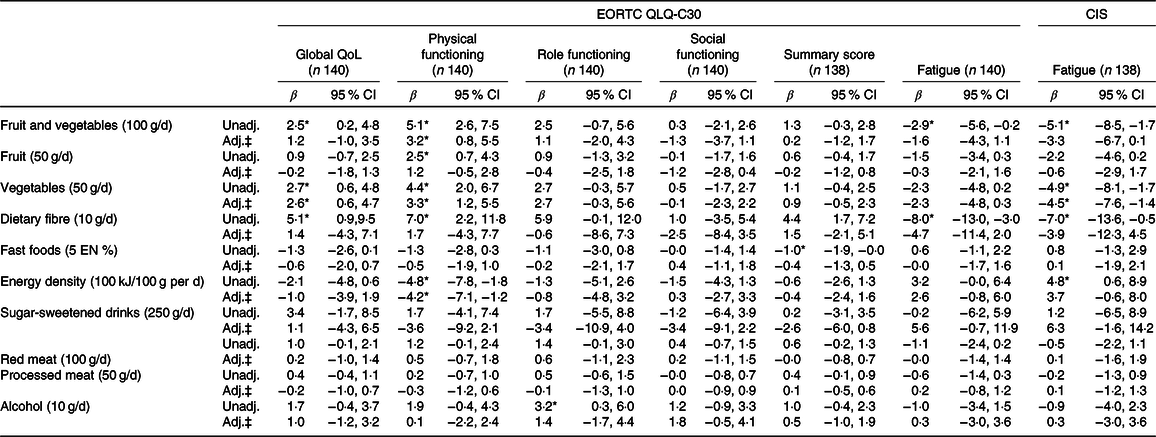
EORTC QLQ-C30, European Organization for the Research and Treatment of Cancer Quality of Life Questionnaire-core 30; CIS, Checklist Individual Strength; QoL, quality of life; Unadj., unadjusted; Adj., adjusted; EN%, energy percentage.
* Statistically significant (P < 0·05).
† Six dietitians reached consensus on the relevant differences for the dietary recommendations, based on the recommended portion per d and on relevant portion sizes.
‡ Adjusted for total energy intake (kJ/week), age (years), sex, BMI (kg/m2), moderate-to-vigorous physical activity (MVPA) (h/d), sedentary time (h/d), number of co-morbidities (0, 1 and ≥2), smoking (current, non-current), education level (low, medium and high), tumour stages (I, II and III), chemotherapy (yes, no) and time since diagnosis (years).
Table 5. Associations between dietary recommendations and neuropathy in chemotherapy patients†
(β-Coefficients and 95 % confidence intervals)
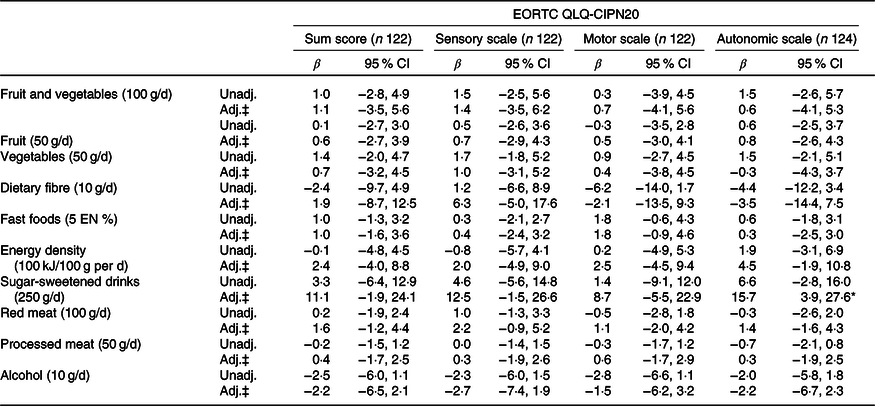
EORTC QLQ, European Organization for the Research and Treatment of Cancer Quality of Life Questionnaire; CIPN, chemotherapy-induced peripheral neuropathy; Unadj., unadjusted; Adj., adjusted; EN %, energy percentage.
* Statistically significant (P < 0·05).
† Six dietitians reached consensus on the relevant differences for the dietary recommendations, based on the recommended portion per d and on relevant portion sizes.
‡ Adjusted for total energy intake (kJ/week), age (years), BMI (kg/m2), moderate-to-vigorous physical activity (MVPA) (h/d), sedentary time (h/d), number of co-morbidities (0, 1, ≥2), smoking (current, non-current), education level (low, medium and high), tumour stages (I, II and III), chemotherapy (yes, no) and time since diagnosis (years).
Sensitivity analyses
When excluding participants with outliers for ratio of energy intake to BMR (n 1) and values exceeding three times the interquartile range from the upper quartile of the fifth nutrient quintile (n 5), we found mostly similar associations except for the association between sugar-sweetened drinks and HRQoL. After excluding outliers (n 6), higher intake of sugar-sweetened drinks was associated with lower HRQoL according to the EORTC summary score (β/250 g increase −4·6; 95 % CI −8·7, −0·5) and higher fatigue on both the EORTC fatigue subscale and fatigue measured by the CIS (9·1; 95 % CI 1·6, 16·6 and 12·6; 95 % CI 3·3, 22·0, respectively). Results from additional sensitivity analyses by logistic regression models with dichotomised patient-reported outcomes were comparable to the results of the linear regression analyses, which therefore appeared to be robust for non-normality of patient-reported outcomes (results not shown).
Discussion
In this cross-sectional study of 150 stage I–III CRC survivors on average 5·7 years post-diagnosis, we analysed how the individual dietary recommendations of the WCRF/AICR for cancer prevention were related to several patient-reported outcomes using quantitative data from 7-d dietary records. While there is consensus amongst researchers that dietary patterns, rather than individual components (specific nutrients or food) are closely associated with health, the potential role of food groups cannot be ignored. After adjusting for clinical, demographic and other lifestyle factors, we found increased fruit and vegetable consumption combined to be associated with better physical functioning and increased vegetable consumption alone to be associated with better physical functioning and global QoL and with less fatigue. By contrast, a higher intake of energy-dense food was found to be associated with worse physical functioning and more fatigue. Non-alcoholic drinkers had significantly lower levels of physical, role and social functioning, a lower EORTC summary score and higher levels of fatigue in comparison with participants who were moderate alcohol drinkers.
To the best of our knowledge, this is the first study to have assessed the relationship between all individual dietary recommendations and patient-reported outcomes in CRC survivors by quantitative data from 7-d dietary records. Two cross-sectional studies have previously examined how fruit and vegetable consumption are associated with HRQoL after CRC. Blanchard et al. found that participants meeting the recommendation of at least five servings of fruit and vegetables a day reported higher general HRQoL(Reference Blanchard, Courneya and Stein35). Similarly, Grimmett et al. found that meeting this recommendation was associated with higher global QoL and physical, role and cognitive functioning(Reference Grimmett, Bridgewater and Steptoe20). However, both cross-sectional studies assessed fruit and vegetable intake only by a single- or two-item question. The associations between fruit and vegetable intake and HRQoL found in our study appeared mostly driven by vegetable and not fruit intake. Previous studies have not investigated these separately, and our results need further replication. With regard to CIPN, no studies have been performed in CRC survivors, although a longitudinal study in breast cancer patients found no association between fruit and vegetable intake and CIPN(Reference Greenlee, Hershman and Shi21). These findings are in line with those presented here. The study of Grimmett et al. also considered alcohol intake. They found that moderate drinkers reported higher physical, role and social functioning and lower fatigue compared with non-drinkers and no significant association between heavy drinking (more than twenty-one and fourteen drinks per week in men and women, respectively) with HRQoL(Reference Grimmett, Bridgewater and Steptoe20). We also found that moderate drinkers had higher levels of physical functioning, role functioning, social functioning and an overall HRQoL score and lower levels of fatigue. However, we did not find a significant association between alcohol intake as a continuous variable and HRQoL. This is in line with research that shows that the relationship between alcohol consumption and health outcome is not a linear but may follow a ‘J’-shaped relationship. Non-drinkers and heavy drinkers were shown to have poorer health outcomes compared with moderate drinkers(Reference Myint, Smith and Luben57).
The 2018 WCRF/AICR lifestyle guidelines include a specific dietary recommendation focused on limiting ‘fast foods’, whereas the corresponding recommendation from the 2007 guidelines was focused on limiting energy-dense food. Based on the significantly changed description and content of this dietary recommendation, the operationalisation of this recommendation also needed to be adapted to a novel dietary assessment method for measuring fast foods via UPF consumption. It should be noted, though, that defining and operationalising the fast food group was of great difficulty(Reference Shams-White, Brockton and Mitrou40) and we therefore decided to also assess energy density of food as used in previous operationalisations. In our analyses, we observed no association between UPF intake and patient-reported outcomes. A higher percentage of UPF intake has been associated with overweight and obesity, a higher risk of developing hypertension, CVD and cancer (overall) and a higher likelihood of having asthma(Reference Monteiro, Cannon and Lawrence58,Reference Fiolet, Srour and Sellem59) . We could not identify any other studies on UPF and patient-reported outcomes with which to compare our results. However, we did observe an association between higher energy density and lower physical functioning. A possible explanation could be that by using UPF intake; we are trying to simplify a complex system. There are many different types of food processing and also the list of UPF varies a lot in terms of energy density and nutrient density (e.g. from white bread to pizza). Further research into optimising the operationalisation of the ‘fast foods’ recommendation of the WCRF/AICR guidelines is highly warranted.
The individual dietary WCRF/AICR recommendations form a package that, taken together, direct people towards healthy patterns of diet. The Mediterranean diet is a diet that encompasses many of the individual dietary WCRF/AICR recommendations. It is mainly a plant-based diet characterised by the consumption of large amounts of fruits, vegetables, legumes, nuts and seeds and whole grains. Intake of milk and dairy products, poultry and eggs is low to moderate, whereas red and processed meats, sweets and refined grains are consumed in low quantities. Finally, the Mediterranean diet is characterised by a moderate alcohol consumption, and in general, foods consumed in the Mediterranean region are minimally processed. The Mediterranean diet as a whole has been known for its protective role towards CRC incidence and other cancers(Reference Grosso, Buscemi and Galvano60–Reference Borzi, Biondi and Basile62). However, evidence for the relationship of this type of diet with patient-reported outcomes in CRC survivors is currently still lacking. In this sample of CRC survivors, the prevalence of overweight was high, the intake of fruit and vegetables was low and meat consumption was high when compared with the cut-off values mentioned in the 2018 WCRF/AICR recommendations. Most CRC survivors did not adhere to the recommendations. Men and women are advised to eat 400 g of fruit and vegetables per d. On average our participants did not meet this recommendation by far, with a mean intake of 141 g of vegetables and 131 g/d of fruit instead. The advice for dietary fibre intake changed to 30 g/d in the 2018 recommendations compared with 25 g/d in the 2007 recommendation. Intakes in our study population were mostly lower than recommended, and because of the changed advice regarding fibre intake, adherence to this recommendation decreased from 29·7 % adherence to the 2007 recommendation, to 9·3 % adherence to the 2018 recommendation(Reference Breedveld-Peters, Koole and Muller-Schulte17). UPF intake as a percentage of total daily energy consumed ranged in our participants from 4·4 to 63·4 % with a mean consumption of 31·4 % and energy density ranged from 443 to 1192 kJ/100 g with a mean consumption of 701 kJ/100 g. No comparison could be made between adherence to the energy density recommendation (4·1 %) and the new fast food recommendation (33·3 %). In addition, the WCRF/AICR advises limiting the intake of red meat to 350–500 g/week and to not consume processed meat at all. Red meat and processed meat intakes in our population were higher than recommended; only two participants met the combination of this recommendation. Finally, the WCRF/AICR recommends not consuming any sugar-sweetened or alcoholic drinks. In our study, 32·0 % of participants reported to not drink any sugar-sweetened drinks. As with dietary fibre, also a lower percentage of adherence for alcohol was seen between the 2018 recommendation compared with the 2007 recommendation. The recommendation for alcohol consumption changed from not drinking more than one glass of alcohol for women and two glasses of alcohol for men per d to not drinking alcohol at all. Consequently, adherence changed considerably from 64·1 % adherence to the 2007 recommendation to 12·0 % adherence to the 2018 recommendation regarding alcohol consumption. The intakes reported in our CRC survivor sample were comparable to the Dutch population aged 51–79 years in the Dutch National Food Consumption Survey, albeit the WCRF/AICR dietary recommendations differed slightly in definition from how the Dutch National Food Consumption Survey classifies dietary groups(Reference Ocké, Buurma-Rethans and Boer de63). This means that both for the CRC survivor population and for the Dutch population aged 51–79 years, there is still a lot to gain in order to meet adherence to the WCRF/AICR recommendations that is accompanied with risk reduction for cancer incidence(12), but also linked to many other health benefits such as increased survival and less recurrence in cancer survivors(Reference Romaguera, Ward and Wark13–Reference Van Blarigan and Meyerhardt16). Besides HRQoL benefits in the CRC survivors, adhering to the individual WCRF/AICR dietary recommendations could also have favourable effects on the well-being of the general population, as suggested by research on the Mediterranean diet in comparison with non-Mediterranean diet and increased fruit and vegetable consumption that was associated with better HRQoL(Reference Henriquez Sanchez, Ruano and de Irala64–Reference Chai, Nigg and Pagano67).
A major strength of this study was the use of 7-d dietary records, which enabled quantitative assessment of food and beverage consumption and absolute intake of individual food and nutrients, which is more accurate than commonly used FFQ data(Reference Willett68). The information on individual food products enables making food groups with great precision. Portion sizes were measured, details on food preparation were reported, instructions on how to fill out the records were provided by dietitians, and, when needed, additional information from participants was requested, which improved the validity of reported food and drink consumption(69,Reference Ortega, Perez-Rodrigo and Lopez-Sobaler70) . Additionally, assessment on multiple days makes the information gathered more reliable(Reference Willett68). Also, memory limitations are not expected to be a source of error, since intakes are asked to be recorded at the time of intake(Reference Willett68). Still, subjects may delay recording their intakes, alter their intake of food or not record their true intake due to social desirability or the relatively high burden of keeping a dietary record(Reference Willett68,Reference Ortega, Perez-Rodrigo and Lopez-Sobaler70) . Because of social desirability, self-reported intake of for example fruit and vegetables may thus have been overestimated, while intake of energy-density and alcohol may have been underestimated. Adherence to the recommendations therefore also might have been slightly overestimated.
The main limitation of this study is the cross-sectional design. Consequently, we cannot draw any firm conclusions regarding the direction of the observed associations. Diet may have affected patient-reported outcomes, or patient-reported outcomes may have altered diet. The relationship between diet and HRQoL is likely complex and bidirectional(Reference Jacka, Cherbuin and Anstey71). In addition, our results might suffer from limited statistical power, especially for the CIPN outcomes. CIPN outcomes were only relevant for the subgroup of patients who received chemotherapy (n 65), and analyses were thus only performed within this group. As a result, findings for CIPN can be considered to be exploratory. Finally, we cannot rule out that some of our results may be false positives because of the potential for multiple testing and that residual confounding could be present even though we corrected for many relevant confounders. Another limitation of our study is that survival bias might have occurred since participants included in our study were generally younger and perhaps differed in other (non-measured) characteristics from non-participants. People that passed away before they could participate or who had a worse health and prognosis were not part of our study population, which may have affected the generalisability of our results to only the healthier subpopulation of long-term CRC survivors.
In summary, our results suggest that already partly adhering to the WCRF/AICR guidelines for a healthy diet may have favourable effects on the HRQoL and complaints of fatigue in the years after the end of CRC treatment. Future studies are needed to further investigate the relationship between diet and health outcomes in CRC survivors. In particular, there is a need for prospective research with longitudinal analyses to gain insight into the direction of associations between the dietary recommendations and HRQoL and for studies with larger sample sizes to investigate the role of diet in CIPN among chemotherapy-treated CRC survivors. Ideally, intervention studies should be performed to investigate whether adhering to these individual dietary recommendations result in better quality of life and decreased fatigue and CIPN. More evidence on the relation between dietary recommendations and patient-reported outcomes could eventually lead to the improvement and fine tuning of evidence-based lifestyle recommendations for CRC survivors.
Acknowledgements
The authors would like to thank all participants of the EnCoRe study and the health professionals in the Maastricht University Medical Centre+ involved in the recruitment of study participants. The authors would also like to thank the MEMIC centre for data and information management for facilitating the logistic processes and data management of our study. Finally, the authors would like to thank the research dietitians and research assistant who were mainly responsible for patient inclusion and performing home visits, as well as data collection and processing.
M. K. is funded by the Wereld Kanker Onderzoek Fonds (WKOF), as part of the World Cancer Research Fund International grant programme (grant no. 2017/1619). J. J. L. B.-P. was funded and J. L. K. is currently funded by Kankeronderzoekfonds Limburg as part of Health Foundation Limburg (grant no. 00005739). E. H. van R. is funded by the Wereld Kanker Onderzoek Fonds (WKOF), as part of the World Cancer Research Fund International grant programme (grant no. 2016/1620). The EnCoRe study was also funded by grants from the Stichting Alpe d’HuZes within the research programme ‘Leven met kanker’ of the Dutch Cancer Society (grant no. UM-2010-4867 and UM-2012-5653).
The authors’ contributions are as follows: M. K., B. W. A. L. and M. J. L. B. contributed to analysing the data, interpretation of the findings and writing the original draft. M. P. W. and M. J. L. B. contributed to the study design, review and editing and supervision. J. J. L. B.-P., J. L. K., E. H. R., S. O. B. and F. M. reviewed and edited the manuscript. All authors read and approved the final version of the manuscript.
J. J. L. B.-P. has previously been employed in the health information department and is currently consultant for the healthy information programmes of Wereld Kanker Onderzoek Fonds (WCRF NL), the Netherlands. The authors declare that there are no conflicts of interest.
Supplementary material
For supplementary material referred to in this article, please visit https://doi.org/10.1017/S0007114520003487