CONTENTS
-
Introduction 3
-
Detection phase 5
-
Evaluation phase 11
-
Response phase 19
-
Operation phase 34
-
Operation objectives 35
-
Operation leadership and management team 36
-
Science input 36
-
Operation plan and achievements 37
-
Health and environmental implications 53
-
Documentation 54
-
Financial management 55
-
-
Monitoring phase 56
-
Review phase 64
-
Another satellite outbreak 65
-
What was learned 65
-
Conclusion 75
Introduction
As the volume of traffic related to the global trade of commodities continues to increase between continents, so too does the number of organisms that are transported to destinations outside of their native range (Mattson et al. Reference Mattson, Niemelä, Miller and Inguanzo1994; Liebhold et al. Reference Liebhold, MacDonald, Bergdahl and Mastro1995; Niemelä and Mattson Reference Niemelä and Mattson1996; Haack and Cavey Reference Haack and Cavey1997; Haack et al. Reference Haack, Law, Mastro, Ossenbruggen and Raimo1997; Haack Reference Haack2006; Eyre and Haack Reference Eyre, Haack and Wang2017; Meurisse et al. Reference Meurisse, Rassati, Hurley, Brockerhoff and Haack2019). Depending on the pathway followed, a number of these organisms will be intercepted by national plant protection organisations during routine inspections of cargo before the organisms escape into novel environments; however, other organisms will escape detection and successfully establish breeding populations that may spread into and throughout ecosystems over time (Liebhold and Tobin Reference Liebhold and Tobin2008). Although many of these organisms will have little apparent impact, in the past, others have caused substantial environmental and economic damage (Pimentel et al. Reference Pimentel, Lach, Zuniga, Morrison, Hallman and Schwalbe2002; Wagner and van Driesche Reference Wagner and van Driesche2010), with consequent and permanent effects on the composition, structure, and function of the invaded landscapes (Gandhi and Herms Reference Gandhi and Herms2010). Some notable examples of insects that have invaded and altered the composition and structure of forested landscapes in North America include Lymantria dispar (Linnaeus) (Lepidoptera: Lymantriidae), the winter moth, Operophtera brumata (Linnaeus) (Lepidoptera: Geometridae), the balsam woolly adelgid, Adelges piceae (Ratzeburg) (Hemiptera: Adelgidae), the hemlock woolly adelgid, Adelges tsugae (Annand) (Hemiptera: Adelgidae), and more recently, the emerald ash borer, Agrilus planipennis Fairmaire (Coleoptera: Buprestidae). Some of these pests have caused gradual and near-complete disappearance of their invaded hosts (Brockerhoff et al. Reference Brockerhoff, Liebhold, Richardson and Suckling2010b).
Another organism with the presumed potential to drastically alter deciduous forests is the Asian longhorned beetle, Anoplophora glabripennis (Motschulsky) (Coleoptera: Cerambycidae), an insect originating from China and the Korean Peninsula (Lingafelter and Hoebeke Reference Lingafelter and Hoebeke2002) that has recently invaded urban and suburban landscapes in North America and Europe (Hu et al. Reference Hu, Angeli, Schuetz, Luo and Hajek2009; Haack et al. Reference Haack, Hérard, Sun and Turgeon2010). In 2003, a breeding population of this insect, which is considered a pest of quarantine significance and one of the world’s top 100 worst invasive alien species (Global Invasive Species Database 2020), was discovered on the border between the cities of Toronto and Vaughan (hereafter “Toronto/Vaughan”), Ontario, Canada (Hopkin et al. Reference Hopkin, de Groot and Turgeon2004).
Around the world, plant protection agencies have responded, and continue to respond (Coyle et al. Reference Coyle, Trotter, Bean and Pfister2021), to the detection of this insect outside its native range by attempting to eradicate it. Myers et al. (Reference Myers, Savoie and van Randen1998) and Myers and Hosking (Reference Myers, Hosking, Hallman and Schwalbe2002) appealed for documentation of both successful (e.g., Hosking et al. Reference Hosking, Clearwater, Handiside, Kay, Ray and Simmons2003) and unsuccessful eradication programmes, pointing out that reviews and analyses of such programmes tend to be plagued by a lack of readily available and published information. They argued that much can be learned from these single experiments and that such information is essential not only for openly weighing the cost and benefits of eradication programmes but also for comparing various responses to invasions. Such information has led to the creation of the Global Eradication and Response Database, which summarises incursion responses and eradication programmes from around the world against terrestrial arthropod pests and plant pathogens (Kean et al. Reference Kean, Suckling, Sullivan, Tobin, Stringer and Smith2021).
Herein, the primary objective is to document the events, issues, challenges, and considerations associated with the various phases of the emergency response to the invasion of Toronto/Vaughan, Ontario by A. glabripennis. The response was implemented by the Canadian Food Inspection Agency (CFIA), which under Canada’s Plant Protection Act and Regulations (Canada Department of Justice 1990) is typically the lead agency when dealing with alien species introduced into Canada’s forests. Documenting the response process from start to finish is essential for effective knowledge transfer, critical review of the programme, and the betterment of future programmes (Myers et al. Reference Myers, Savoie and van Randen1998; Brockerhoff et al. Reference Brockerhoff, Liebhold, Richardson and Suckling2010b; Tobin et al. Reference Tobin, Kean, Suckling, McCullough, Herms and Stringer2014; Porth et al. Reference Porth, Dandy and Marzano2015; Kean et al. Reference Kean, Suckling, Sullivan, Tobin, Stringer and Smith2021). Another objective of the present paper is to broadly describe the research opportunities that arose while implementing this response and how treatment dovetailed with a research programme to generate knowledge and information on the beetle’s origin, host selection, within-tree colonisation, and dispersal. This information was used to help improve the emergency response and provides the foundation for more detailed analyses to follow on how A. glabripennis colonised and spread through the urban landscape of Toronto/Vaughan over time.
Hosking (Reference Hosking2001) developed a guide that subdivided incursion response plans into six phases: detection, evaluation (or delimitation), response decision, operation, monitoring, and review. The guide further presented and examined strategies and mechanisms that could be considered within each phase, the structures and inputs necessary to effect them, and the accountabilities and documentation that should emerge from them. The work was updated by Brockerhoff et al. (Reference Brockerhoff, Liebhold, Richardson and Suckling2010b) to include as many as 14 steps; however, our documentation of the CFIA’s emergency response followed the six phases identified by Hosking (Reference Hosking2001) because that was the only framework available in 2003.
Detection phase
Hosking (Reference Hosking2001) defined the detection phase as the period between the initial discovery of the invader and the completion of a preliminary technical evaluation. The five key elements of this phase included detection preparedness and facilitation, the detection event, interim actions, project leadership, and a preliminary technical evaluation.
Detection preparedness and facilitation
Canada and the United States of America were the first two countries to report interceptions of A. glabripennis outside of its native range (Haack et al. Reference Haack, Hérard, Sun and Turgeon2010). The first interception in Canada occurred in 1992 during routine inspections of imports (Table 1). By the end of 2019, Anoplophora spp. had been intercepted on 39 occasions by Canadian inspectors. Twenty-nine of these interceptions were made at ports of entry by the CFIA (before 2005) or the Canada Border Services Agency (after 2005); the remainder were found in warehouses and were reported to the CFIA. Interceptions of A. glabripennis have also been reported in Australia, New Zealand, and, as of June 2018, in at least 13 European countries (European and Mediterranean Plant Protection Organisation 2001, 2009, 2010a, 2010b, 2012a, 2012b, 2012c; Maspero et al. Reference Maspero, Jucker and Colombo2007; Haack et al. Reference Haack, Hérard, Sun and Turgeon2010; Pajović et al. Reference Pajović, Petrić, Bellini, Pajović and Quarrie2017). The congener Anoplophora chinensis (Forster), which is presumed to be as devastating as A. glabripennis, was also intercepted once in Canada (Table 1) and at many ports of entry around the world (Haack et al. Reference Haack, Hérard, Sun and Turgeon2010).
Table 1. Historical records of Anoplophora spp. interceptions either at one of Canada’s ports of entry or after entry in a warehouse, together with the country of origin and the commodity infested † . BC, British Columbia; ON, Ontario; AB, Alberta; WPM, wood packaging materials
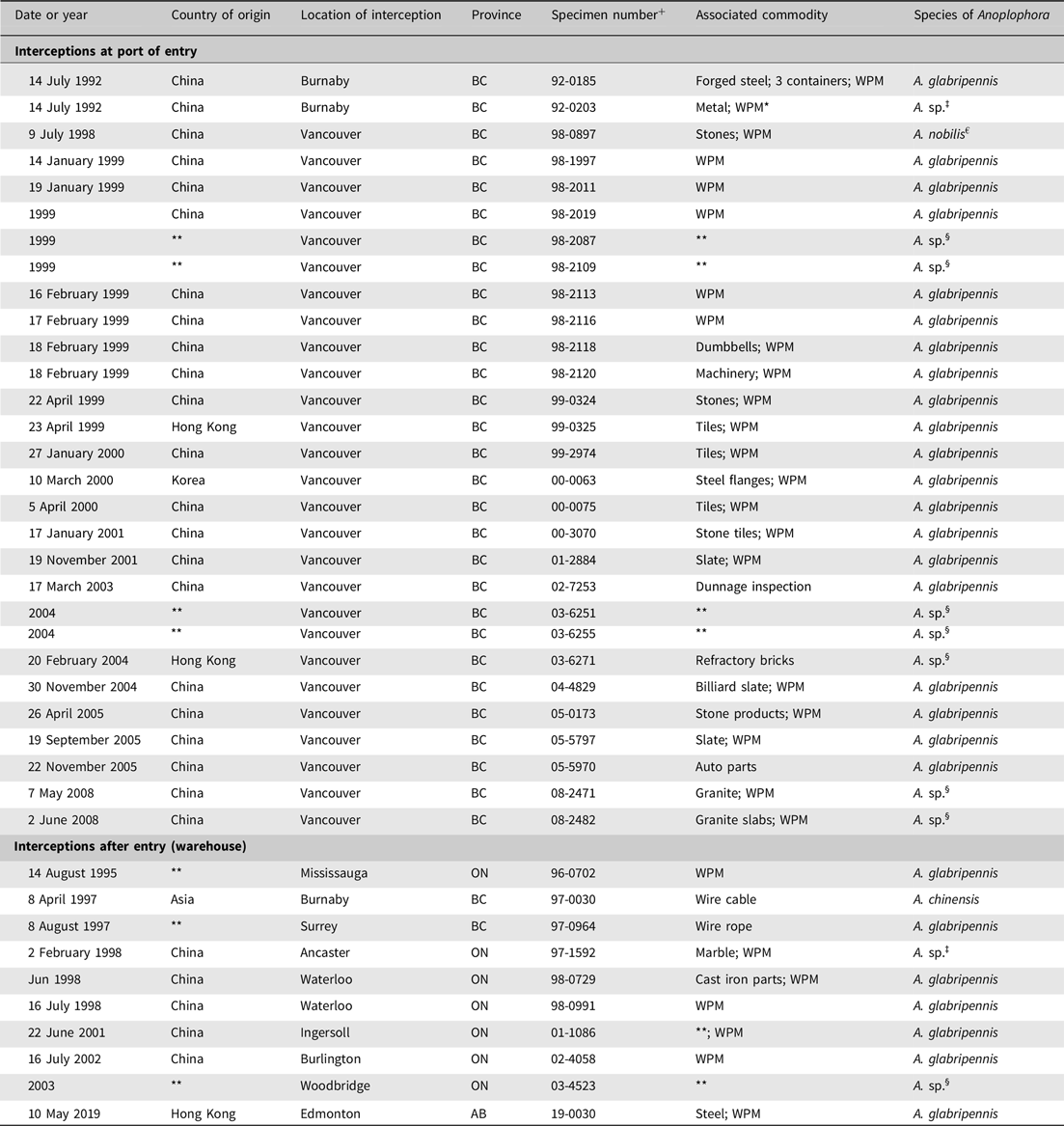
+ Specimen number assigned per fiscal year (1 April to 31 March) rather than calendar year.
* Wood packaging material (WPM) includes materials such as pallets, crates, supports, wood crates, wooden boxes, wood, wood crating, or packing crates.
‡ Probably Anoplophora glabripennis.
§ Larva.
€ Synonym of A. glabripennis.
† Source: B.D. Gill, M. Marcotte, G. Thurston, and E. Bullas-Appleton, Canadian Food Inspection Agency, personal communication.
** Incomplete record.
At Canada’s ports of entry, inspections of dunnage and wood packaging material used to ship commodities such as wire cable, cast iron, glass, marble, and cut stones have led to discoveries of adult A. glabripennis specimens and of Anoplophora spp. larvae (Table 1) within the packaging materials. All port-of-entry interceptions of A. glabripennis adults were made in British Columbia, whereas post-entry detections occurred predominantly in Ontario. New treatment and fumigation standards for wood packaging material used in international trade, known as the International Standards for Phytosanitary Measures No. 15 (ISPM 15; Food and Agriculture Organisation of the United Nations 2018b), which prescribe the use of heat treatment or methyl bromide to kill all life stages of insects and pathogens associated with wood packaging material (Barak et al. Reference Barak, Wang, Xu, Rong, Hang and Zhan2005, Reference Barak, Wang, Yuan, Jin, Liu, Lou and Hamilton2006a, Reference Barak, Wang, Zhan, Wu, Xu and Huang2006b), were adopted in 2002 and implemented in 2006 in North America (Food and Agriculture Organisation of the United Nations 2018b; Haack et al. Reference Haack, Hérard, Sun and Turgeon2010). These standards, which were revised in 2009 to set maximum limits on the amount of residual bark allowed on wood packaging material, were expected to block this pathway and to prevent new invasions, one of the key requirements for successful eradications (Myers et al. Reference Myers, Simberloff, Kuris and Carey2000). However, a few interceptions of A. glabripennis have been reported since these standards came into effect in Canada (Haack et al. Reference Haack, Hérard, Sun and Turgeon2010) and around the world (European and Mediterranean Plant Protection Organisation 2012b, 2012c; Haack et al. Reference Haack, Britton, Brockerhoff, Cavey, Garrett and Kimberley2014; Eyre and Haack Reference Eyre, Haack and Wang2017). It has not yet been determined whether the lower frequency of these interceptions since 2006 (Table 1) is a direct result of the implementation of these standards – which would indicate the standards have been effective in closing this pathway – or if the reduced frequency is coincidental. An interception in 2019 in Alberta, Canada highlighted the need for continued vigilance. Whether the regulatory measure requires further improvements, such as those proposed by Brockerhoff et al. (Reference Brockerhoff, Aukema, Cavey, Garrett, Haack and Kimberley2010a), also remains to be determined.
Soon after the discovery of A. glabripennis in Chicago, Illinois, United States of America in 1998, the CFIA performed a pest risk analysis (CFIA, unpublished) to evaluate the threat this species could represent to Canada. In addition, the CFIA expanded its surveillance beyond ports of entry by initiating formal field surveys that specifically targeted this beetle to increase the likelihood of its detection. Between 1998 and 2000, the CFIA surveyed nearly 1700 sites across Canada, looking for characteristic signs (physical damage caused to a tree by this insect) and symptoms (a tree’s response to insect attack) of injury on trees surrounding facilities importing high-risk commodities (R. Favrin and M. Marcotte, CFIA, personal communications). In April 1999, the CFIA prepared a draft emergency response plan, with input from cooperating agencies (CFIA, unpublished), and in July 1999, it conducted emergency simulations across Canada (CFIA, unpublished). In 2000, the CFIA postponed these surveys to focus on the emergency management and eradication of the plum pox virus (Potyviridae), one of the most serious diseases of stone fruit plants, Prunus spp. (Rosaceae), which had been detected in Ontario and Nova Scotia, Canada (North American Plant Protection Organization 2007), as well as the 1999 discovery of the brown spruce longhorn beetle, Tetropium fuscum (Fabricius) (Coleoptera: Cerambycidae), in Halifax, Nova Scotia (Natural Resources Canada 2020). In September 2002, the CFIA once again redirected human and financial resources away from A. glabripennis surveys to deal with the discovery of another alien invasive beetle in Ontario, the emerald ash borer, Agrilus planipennis Fairmaire (Coleoptera: Buprestidae) (North American Plant Protection Organization 2003).
Detection event
The presence of a breeding population of A. glabripennis in Canada was first detected by a member of the public in 2003. An employee of a business on Hanlan Road, Vaughan, Ontario found a live adult beetle on his vehicle. The adult beetles are 17–39 mm long and glossy black, with 15–20 distinct and irregularly shaped white patches on the elytra (Lingafelter and Hoebeke Reference Lingafelter and Hoebeke2002; Hajek et al. Reference Hajek, Curtiss and Liebherr2004). The individual brought the beetle to the attention of a supervisor, who took it home, thinking it could be used by his child for an upcoming science project. A quick Internet search by the family led them to a United States Department of Agriculture website that had a simple message: “If you find this beetle, alert your local Plant Protection Office.” The supervisor brought the specimen to the CFIA’s Toronto office on Thursday, 4 September 2003. Later that day, the specimen was sent to the CFIA’s Centre of Plant Quarantine Pests in Ottawa, Ontario for expert identification. Late on Monday, 8 September 2003, the CFIA’s Toronto office received confirmation that the specimen was A. glabripennis.
Interim actions
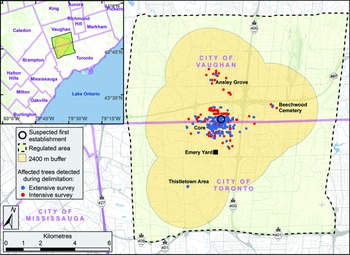
For the next two days, managers at the CFIA’s Toronto office contacted their programme network team to inform them that a live specimen of A. glabripennis had been brought in by the public. Briefing notes were written for senior managers to explain the significance of the discovery and to recommend actions that were based on the CFIA’s draft emergency response plan. On 10 September 2003, a team of inspectors from the CFIA’s Toronto Plant Health Division was dispatched to the area where the specimen had been found to perform a more detailed site evaluation. The discovery site was on the north side of Steeles Avenue, which is the southern border of the City of Vaughan and the northern border of the City of Toronto (Fig. 1, inset). During their evaluation, the inspectors surveyed the area, assessed the international trade pathways of local businesses, identified whether additional interim actions such as the establishment of a local quarantine or trace-back surveys were necessary, and ensured that the site’s integrity would not be compromised further.
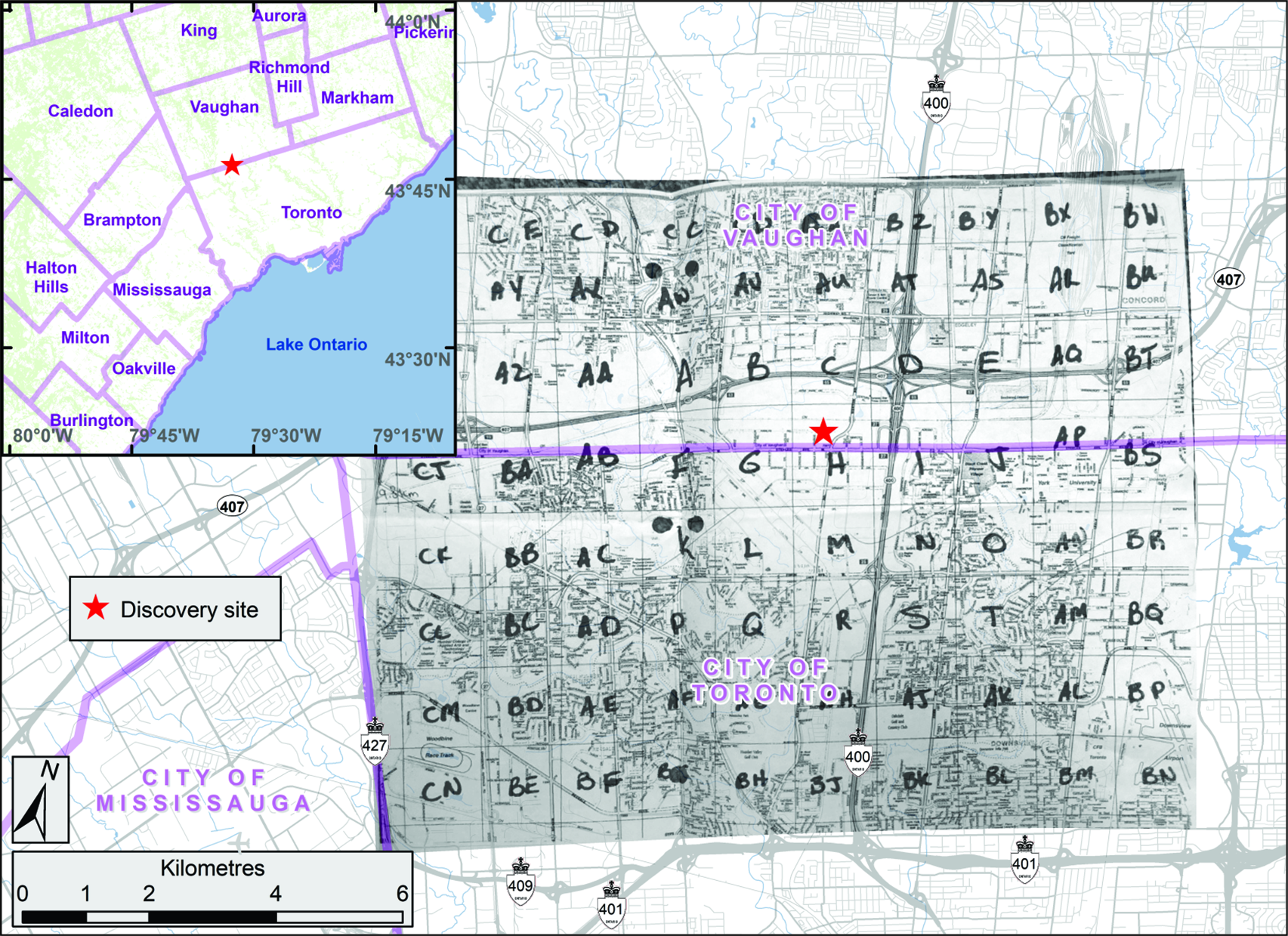
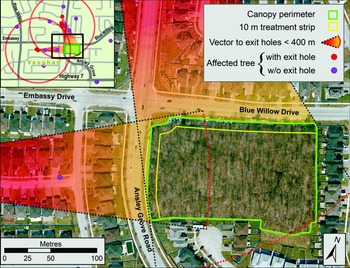
Fig. 1. Discovery site of Anoplophora glabripennis and the grid of cells (A, B, C, D… AA, BB…), each approximately 1.25 × 1.25 km, established around it to guide the extensive delimitation survey. The inset shows the discovery site in relation to part of the Greater Toronto Area. (Map adapted from CFIA 2003.)
Observations made during this preliminary evaluation revealed the presence of wooden crates and pallets and of about half a dozen tree stumps. A subsequent search of the data from the Canada Border Services Agency’s import retrieval system showed that the property with pallets had a history of glass imports from China, suggesting that wood packaging material might have been how A. glabripennis arrived in Ontario. During their visit, the inspectors also found a live beetle and observed the presence of at least 50 trees with numerous signs of A. glabripennis damage, including emergence holes (Hopkin et al. Reference Hopkin, de Groot and Turgeon2004) – a clear indication the population had become established. The inspectors also noted that some tree stumps had oval feeding tunnels in the heartwood that appeared characteristic of injury by older A. glabripennis instars. The presence of these holes in stumps suggested that some injured trees had been removed from the property and sent to waste disposal sites before the outbreak was discovered. Follow-up interviews with local property owners revealed that none was aware that tree-removal activities had taken place on their properties, predominantly because such activities were typically contracted out to landscaping companies. None of these yard-maintenance companies had detailed records on whether the trees taken from the site had been shredded or sent for disposal. Managers at the CFIA Toronto office held further discussions with their programme network team about the impact these findings would have on actions to be taken and prepared new briefing documents for upper management.
Meanwhile, an area response team was assembled, a command centre was established, and the equipment to implement an emergency response plan was acquired. In addition, the CFIA contacted and apprised the North American Plant Protection Organization and stakeholders of the situation (North American Plant Protection Organization 2003) and activated all partnerships identified in the CFIA (1999) draft emergency response plan (see section, Project leadership, below, for a list of partners). The agency also assigned a CFIA spokesperson with experience in responding to arrivals of nonnative plant pests to assist with the implementation of its preliminary emergency response plan. As part of this, news releases were sent out to inform the public of the discovery, and a toll-free telephone number (hereafter “hotline”) was dedicated to receiving calls from the public regarding possible sightings of the beetle or its damage and was publicised via the media.
Project leadership
Another requirement for a successful response to the establishment of an alien invasive species is that lines of authority are clear and allow the agency or an individual to take all necessary actions (Myers and Hosking Reference Myers, Hosking, Hallman and Schwalbe2002). In this instance, and as pointed out earlier, the CFIA was the only agency with full legal authority to deal with the emergency response to an alien plant pest. Depending on the pest, however, the lead agency might not have the staffing or the operational tools required to fully implement all responses deemed necessary.
The CFIA’s original emergency response plan contained three main management activities: surveys to delimit the core infestation and to detect satellites, if any; containment to prevent the spread; and control to eliminate plants that are infested or are suspected of being infested. To address issues that might arise from the response plan’s implementation, the CFIA established consultative subcommittees to handle communications, operations, and science. Each of these three subcommittees was assigned specific and clearly defined roles and responsibilities, and all reported to the CFIA-designated area response team leader who would bring the committees’ recommendations to a CFIA-led committee of senior managers for final decision-making.
The communication subcommittee’s responsibilities included developing a strategy to coordinate press releases and community meetings, identifying communication needs, opportunities, and messages, identifying volunteer groups and presentation venues, and coordinating information distribution. The operation subcommittee was responsible for coordinating delimitation survey and treatment activities, identifying areas at risk, coordinating survey and tree-removal training, coordinating tree-disposal sites, and developing operational guidelines for mitigation activities on various landscapes. The science subcommittee was responsible for providing expert advice, reviewing scientific information, and identifying research opportunities.
Within days of the discovery of A. glabripennis in the region, the CFIA invited experts and stakeholders from various municipal, provincial, and federal agencies in both Canada and the United States of America to participate on the subcommittees and to fill the expertise gaps needed to implement the emergency response plan. The partnership included experts and stakeholders from the municipalities of Toronto and Vaughan, the Regional Municipality of York, the Toronto and Region Conservation Authority, the Ontario Ministry of Natural Resources, three agencies of the United States Department of Agriculture (the Forest Service, the Agricultural Research Service, and the Animal and Plant Health Inspection Service), and Natural Resources Canada’s Canadian Forest Service.
Preliminary technical evaluation
Based on its preliminary assessment of the discovery site, the CFIA confirmed the existence of a breeding population of A. glabripennis. The abundance of injured trees, the advanced decline – including the death of some trees – together with the evidence that some trees had been removed from the discovery site and the possibility that the beetle had a multiyear life cycle, suggested the arrival of the beetle in Ontario had occurred several years earlier. At first glance, the outbreak appeared to be confined to a small area of Toronto and Vaughan, not widespread across Greater Toronto, a region that consists of the City of Toronto and 25 surrounding cities, including Vaughan (Fig. 1, inset).
The presence of wood packaging material at the site of discovery, combined with the existence of a business with a history of importing glass from China, suggested that A. glabripennis had likely arrived in Ontario through a known pathway. Carter et al. (Reference Carter, Smith, Turgeon and Harrison2009b) later confirmed that the beetle’s population in the Toronto/Vaughan outbreak had limited genetic variability and had likely originated from China, although the origin could not be pinpointed to a specific Chinese province or region.
Evaluation phase
The evaluation phase extends from the moment when pest identification is confirmed to the time when all necessary information for formulating a response decision is assembled (Hosking Reference Hosking2001). The primary goal of this phase is to gather information to identify potential response options. Key elements of this phase include a delimitation survey, a preliminary impact assessment, potential response options, and consultation with stakeholders.
Delimitation survey
The relevant information required to carry out an efficient and reliable delimiting survey includes, among many other considerations, familiarity with or knowledge of the following: the general appearance of the pest’s different life stages; the common signs and symptoms of injury caused by the pest; the key host plants; a relevant survey design (e.g., what, when, and how to survey); and the specifications and tools to collect, record, access, and analyse information gathered during the various phases of the emergency response plan. Knowledge of A. glabripennis’s appearance and signs of the injuries it causes was already available at the time of the discovery in Toronto/Vaughan. As a result, delimitation focused on the following elements: survey design, hosts, data collection, and data interpretation.
On 11 September 2003, teams from the CFIA’s plant protection programme returned to the discovery site to continue site assessment and to initiate the extensive survey that would establish the coarse geographic extent of this outbreak. This information was essential to quickly establish preliminary boundaries of the infested area, to estimate the size of the containment area to regulate, and to develop an adequate response strategy.
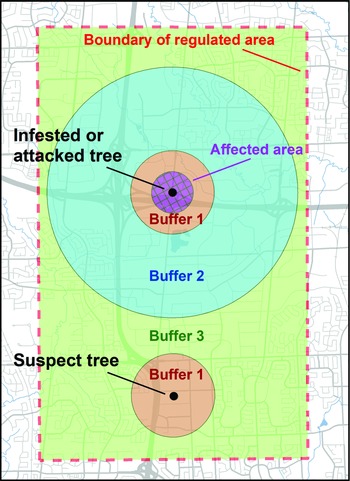
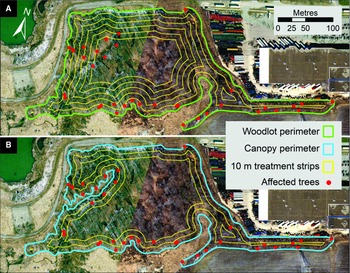
The preliminary evaluation of properties surrounding the discovery site revealed the presence of more than 200 trees with signs of injury by A. glabripennis (Fig. 2).
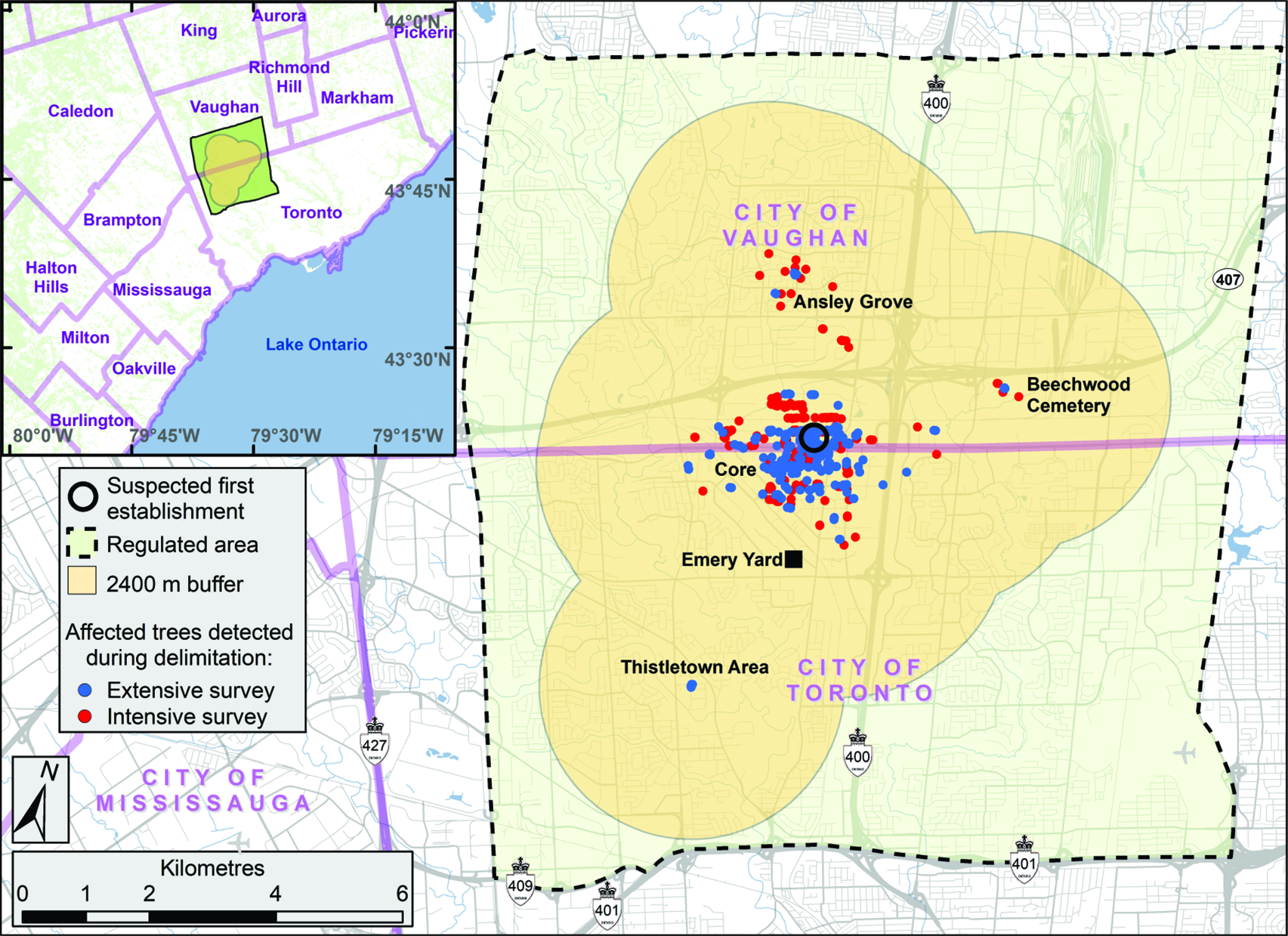
Fig. 2. Location of trees with Anoplophora glabripennis injury known at the end of the extensive delimitation survey in early October 2003 (blue dots) and known in January 2004 at the end of the intensive delimitation survey (red dots). Also indicated are the core infestation area and the three satellite infestations – Ansley Grove, Beechwood Cemetery, and Thistletown – of the Toronto/Vaughan outbreak. The outer boundary of buffer 2 (2400 m from an affected tree) and the extent of the regulated area established as part of the regulatory measures implemented by the Canadian Food Inspection Agency to eradicate the Toronto/Vaughan outbreak are shown. Emery Parks Yard, a gated and fenced city maintenance facility where yard waste and all wood products removed during this programme were stored and decontaminated and where infested wood samples were subsequently assessed, is also shown (black square). The inset shows the extent of buffer 2 and of the regulated area in relation to part of the Greater Toronto Area.
Survey design and specifications
In 2003, no long-range attractant or trapping technique that exclusively targeted A. glabripennis was known. For this reason, the only available approach to carry out extensive delimitation was to perform visual inspections of tree boles and branches to detect signs or symptoms of A. glabripennis injury (Ric et al. Reference Ric, de Groot, Gasman, Orr, Doyle and Smith2007; Turgeon et al. Reference Turgeon, Ric, de Groot, Gasman, Orr and Doyle2007).
The inspectors used several methods to detect signs of injury. Some performed visual inspections of trees from the ground, sometimes aided by binoculars, searching for signs of injury. Other inspections were performed by trained professionals using bucket trucks or by climbing trees to examine the upper crown when trees or branches could not be inspected from the ground. Using trained arborist tree climbers was assumed to be the most effective method to survey trees to detect A. glabripennis, especially for larger trees and in forested landscapes, but it was also a slower and more costly inspection method (Hu et al. Reference Hu, Angeli, Schuetz, Luo and Hajek2009). Nonetheless, it was known from the onset of the evaluation phase that the detection of all injured trees was highly unlikely, especially in the early stages of the programme. It was assumed that signs and symptoms of injury by the beetle would be missed for a variety of reasons, including the differences in training, experience, and observational skills among the inspectors; the low number of signs of injury on some trees reducing the probability of detection; the location of some signs on parts of trees that would make the signs difficult or impossible to see from the ground or to distinguish from other marks on the bark or from injury by other pests; and the changing appearance of some signs over time that would make the signs less obvious or cause them to vary among tree species (Ric et al. Reference Ric, de Groot, Gasman, Orr, Doyle and Smith2007). In addition, preliminary results from the earlier eradication programme in Chicago had shown that survey techniques and timing influenced the detectability of injured trees (J. McCarthy, City of Chicago, personal communication). For example, ground surveys offered a much lower probability of detecting trees that had signs of injury than did surveys conducted from a bucket truck, which in turn were slightly less effective than those performed by tree climbers. The influence of survey techniques was observed whether surveys were carried out in winter, when snow might be present, or in summer, when leaves were present, but was more pronounced in winter.
Immediately after the initial meeting of potential partners, many employees from various organisations (e.g., City of Toronto, City of Vaughan, the Regional Municipality of York, and Toronto and Region Conservation Authority) were reassigned temporarily from their regular duties to assist the CFIA in completing its extensive survey and in beginning its intensive survey. Shortly thereafter, one important issue arose: only CFIA staff employed within the agency’s plant protection programme had the legal authority to inspect any property in search of injured trees. Neither CFIA staff not employed within the plant protection programme nor staff employed by partners assisting with the surveys had that authority. After consulting with its legal advisors, the CFIA temporarily designated those other workers as inspectors under the Plant Protection Act and Regulations (Canada Department of Justice 1990), effectively giving them powers and duties similar to those of its own plant protection staff. The City of Toronto was the only partner that, in the aftermath of the Chicago outbreak, had already passed legislation to enable its survey staff to enter and inspect all properties within city limits for signs of A. glabripennis.
The extensive survey was ground-based (no tree climbers or hoists) and was conducted by several teams of two inspectors who had been brought to the discovery site for training on what to look for and where on trees. A grid of cells (A, B, C, D… AA, BB…), each approximately 1.25 × 1.25 km in area, was established around the discovery site (Fig. 1), and each team was instructed to examine trees in front yards and along streets within each cell. When an injured tree was found, inspectors stopped surveying and moved to the next street of the cell or to the next cell, depending on the street’s location within a cell. By early October 2003, less than a month after the beetle’s discovery, the CFIA’s extensive survey covered an area of approximately 10.0 × 12.5 km around the discovery site (Fig. 1). At that time, the size of the infested area, which had to include buffers (see section, Affected zones, areas, and buffers, below) around injured trees (Food and Agriculture Organisation of the United Nations 2018a), was still being assessed. Because of this, the CFIA decided after consulting with its stakeholders and legal advisors that major transportation corridors, which happened to match the grid of the survey cells, would be used to define the initial boundaries of the area to regulate rather than the exact buffer area dictated by the confirmed locations of injured trees. Days later, the CFIA expanded the area covered by the extensive delimitation survey to 12.5 × 12.5 km and proceeded to complete the extensive survey. This action was assumed to ensure the incorporation of sufficient space for buffers of at least 2400 m (Fig. 2).
Soon after the coarse delimitation survey had begun and a picture of the extent of the infested area began to emerge, the CFIA reassigned some survey staff from extensive to intensive survey activities. The intensive survey was performed predominantly from the ground, assisted by a limited number of tree climbers. The objectives of the intensive survey were to confirm and expand the findings of the extensive survey, to identify the exact location of all trees that could be confirmed as injured, and to provide more detailed insight into the scale and type of management response needed. At that time, treatment activities were expected to take place primarily during winter, when foliage on broadleaf trees would be absent, and the CFIA wanted to minimise the number of nontarget trees that would be surveyed or mistakenly treated.
To achieve that goal, the CFIA trained the inspectors to improve their search effectiveness for signs and symptoms of injury and to perfect their skills at identifying tree species without foliage. The CFIA also tested the inspectors on tree genus identification and required a passing mark of 90% to be approved as inspectors. Given the legal implications of the emergency response and to ensure some measure of consistency in detectability, it was decided that volunteers, although desirable (Fitzpatrick et al. Reference Fitzpatrick, Preisser, Ellison and Elkinton2009), would not be used to conduct official intensive surveys. Instead, the public would be encouraged to report sightings of the beetle or possible beetle-caused damage using the hotline. A team of inspectors would be dispatched to follow up on the information provided.
This decision also helped the CFIA deal with the sudden appearance of a rogue independent tree climbing company during the early stages of the intensive surveys. It was later revealed that this company, unbeknownst to the CFIA, was charging private citizens within the affected area a fee to climb and inspect trees on their property. When these climbers could not find any sign of attack, they “certified” the trees as A. glabripennis-free. The CFIA acted swiftly to stop this practice before it could undermine the integrity of the regulatory programme.
Hosts
Anoplophora glabripennis was a known polyphagous pest that had been reported to feed on and complete development in several genera of broadleaf trees from several families (Lingafelter and Hoebeke Reference Lingafelter and Hoebeke2002; Morewood et al. Reference Morewood, Neiner, McNeil, Sellmer and Hoover2003, Reference Morewood, Hoover, Neiner, McNeil and Sellmer2004, Reference Morewood, Hoover, Neiner and Sellmer2005; Sawyer Reference Sawyer2003; Wang et al. Reference Wang, Mastro and Gao2005a; Hérard et al. Reference Hérard, Ciampitti, Maspero, Krehan, Benker and Boegel2006, Reference Hérard, Maspero, Ramualde, Jucker, Colombo, Ciampitti and Cavagna2009; Ric et al. Reference Ric, de Groot, Gasman, Orr, Doyle and Smith2007; Smith and Wu Reference Smith and Wu2008; Hu et al. Reference Hu, Angeli, Schuetz, Luo and Hajek2009). Some of the key host genera in its native range, in order of importance, are Populus Linnaeus (Salicaceae) and Salix Linnaeus (Salicaceae), but they also include Ulmus Linnaeus (Ulmaceae), Acer Linnaeus (Sapindaceae) (Xiao Reference Xiao1992; Luo et al. Reference Luo, Wen and Xu2003; Pan Reference Pan2005; Yin and Lu Reference Yin and Lu2005), and other genera. In addition, the beetle was found to attack healthy and stressed trees of almost any size (Haack et al. Reference Haack, Law, Mastro, Ossenbruggen and Raimo1997, Reference Haack, Hérard, Sun and Turgeon2010; Haack Reference Haack2006; Hérard et al. Reference Hérard, Ciampitti, Maspero, Krehan, Benker and Boegel2006).
An annotated categorisation of A. glabripennis hosts in the three known outbreaks in the United States of America, which had occurred in New York and Chicago, and in Jersey City, New Jersey was already available (Sawyer Reference Sawyer2003). In that list, tree genera observed in these outbreaks had been assigned to one of six categories: (1) very good hosts – i.e., Acer, Aesculus Linnaeus (Sapindaceae), Salix, and Ulmus; (2) good hosts – i.e., Betula Linnaeus (Betulaceae) and Platanus Linnaeus (Platanaceae); (3) occasional or rare hosts in the United States of America – i.e., Albizia Durazzini (Fabaceae), Celtis Linnaeus (Cannabaceae), Fraxinus Linnaeus (Oleaceae), Populus, and Sorbus Linnaeus (Rosaceae); (4) questionable hosts in United States of America – i.e., Hibiscus Linnaeus (Malvaceae), Malus Miller (Rosaceae), Morus Linnaeus (Moraceae), Prunus Linnaeus (Rosaceae), Pyrus Linnaeus (Rosaceae), Quercus Linnaeus (Fagaceae), Robinia Linnaeus (Fabaceae), and Tilia Linnaeus (Malvaceae); (5) no records in the United States of America – i.e., Alnus Miller (Betulaceae), Elaeagnus Hill (Elaeagnaceae), and Melia Linnaeus (Meliaceae); and (6) nonhosts – i.e., Ailanthus Desfontaines (Simaroubaceae). Until that information could be assessed critically in Canada, all trees belonging to genera in the first three categories, except Fraxinus (see section, Targeted hosts, below, for justification), would be targeted for treatment.
Data collection
During the intensive delimitation surveys, the inspectors inventoried and inspected all trees on each property, irrespective of their category, but affixed a uniquely numbered tag only to trees of the first three host categories located within 800 m of a tree with injuries. The rationale for this distance is provided in the subsection, Affected zones, areas and buffers.
The CFIA needed a case-specific information management system that would track property ownership within the area to be treated, the abundance and diversity of host trees on each property, the results of the delimitation surveys, control operations, and monitoring surveys, and the legitimacy of compensation claims, should compensation be approved at a later date. To manage this information, the CFIA needed a customised and reliable database. It developed its own information management system, called the “Outbreak Control Monitoring System,” or “OCMS.” At the time, the complete list of information requirements had not been finalised because the CFIA had not yet decided which management strategy it would use. Despite this constraint, some of the basic requirements were easily identified, and steps were taken immediately to obtain them (Supplementary material, Appendix 1). For example, the CFIA obtained data on ownership for all public and private properties located within the regulated area covered by the extensive delimitation survey. This information was essential because the regulations required that each owner of a property targeted for control activities receive a “notice to dispose” before the onset of control activities in affected areas (Supplementary material, Appendix 2).
Data interpretation
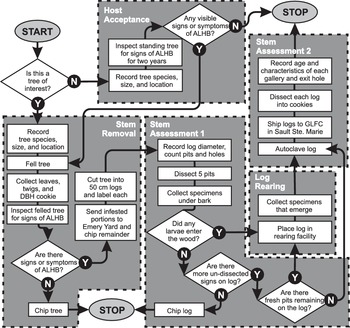
The International Plant Protection Convention and the international standards for phytosanitary measures define an infestation (of a commodity) as the presence in a commodity of a living pest of the plant or plant product concerned (Food and Agriculture Organisation of the United Nations 2018a). At this early stage of the emergency response, it had not always been possible to determine if all trees with signs or symptoms of A. glabripennis injury contained live specimens. The CFIA therefore standardised the terminology used by referring to standing trees with signs and symptoms of injury that resembled those of A. glabripennis as “trees of interest.” When such a tree was found, the inspector contacted the supervisor, who re-examined a sample (see section, Stem assessment: infestation status and injury description, below, for details) to confirm or reject the tree’s field injury status. Samples were re-examined in greater detail later within a containment facility to determine their infestation status. After comparison with voucher specimens, trees containing a live specimen of A. glabripennis were deemed “infested.” Those with only dead specimens were classified as “attacked.” Those with only signs or symptoms characteristic of A. glabripennis injury were labelled “suspect.” Trees in any of these three status groups were referred to as “affected.” All other trees were deemed “unaffected.” This terminology differed slightly from that used in the United States of America, where a host tree was considered infested when it presented any sign of A. glabripennis activity or damage (United States Department of Agriculture 2014).
To compensate for the predicted low survey accuracy, the science subcommittee recommended that unaffected trees not be referred to or considered as “healthy” trees. Instead, the term “unaffected” should indicate only that signs were not detected, not that the tree was healthy, particularly when an infested or attacked tree was located nearby. This distinction in interpretation of the survey results served as a constant reminder to both the public and programme staff that all actions had to allow for the likelihood that not all affected trees would be found – a highly likely outcome that underscored the need for quality control programmes for the various management activities. The terminology also spoke to the challenge of finding affected trees that exhibited few signs of injury outside the core infestation area (Fig. 2). The advantages and disadvantages of this nuance in interpreting the outcome of inspections were discussed with partners to ensure consistent messaging when meeting with the public. This interpretation was also presented when meeting with public audiences so that they would understand the necessity of treating more than just trees known to be affected. From the onset, the public was made aware of the challenges of detecting affected trees and the need for buffers around them.
Preliminary impact assessment
The information that is needed to prepare a rigorous preliminary assessment of a pest’s impact typically includes knowledge of the pest’s biology and of the resource at risk in general – and affected trees in particular – a spread analysis of the outbreak, and cost–benefit analyses. As has been the case for many adventive species, biological information on A. glabripennis was limited when it was found in Ontario in 2003, knowledge on the resource at risk was also limited, and the analyses were unavailable.
Biology
A major gap in knowledge at the time of the Toronto/Vaughan discovery was the exact duration of A. glabripennis’s life cycle in Ontario. Because of the province’s climate, it was assumed that the larvae spend from 9 to 18 months inside a tree. It was already known that adult beetles live for about one month (Smith et al. Reference Smith, Bancroft and Tropp2002), during which time they feed on twigs and foliage, which could result in twig and leaf mortality; however, that impact seemed to be of minor importance. The impact of larval feeding appeared to be more detrimental to tree health. It was known that the beetles’ early instars create surface galleries at the interface of a host tree’s inner bark and current-year sapwood, whereas older instars tunnel into the sapwood and heartwood (Haack et al. Reference Haack, Law, Mastro, Ossenbruggen and Raimo1997; Cocquempot and Hérard Reference Cocquempot and Hérard2003). Larval galleries contribute to the build-up of callus tissue and the formation of cracks in the bark, directly affecting nutrient and water transport, and tunnels cause structural weakness (Pan Reference Pan2005). Years of repeated attacks typically result in tree death.
Resource at risk
By 2003, attacks by A. glabripennis outside of its native range had resulted in limited tree mortality (Haack et al. Reference Haack, Law, Mastro, Ossenbruggen and Raimo1997; Haack Reference Haack2006; Hérard et al. Reference Hérard, Ciampitti, Maspero, Krehan, Benker and Boegel2006; Ric et al. Reference Ric, de Groot, Gasman, Orr, Doyle and Smith2007). It was known however that, in China, this beetle had killed millions of trees planted during a massive reforestation programme begun in 1978 that was aimed at establishing over 35 million hectares of plantations and shelterbelts (Pan Reference Pan2005; Yin and Lu Reference Yin and Lu2005). Because of the beetle’s affinity for Acer spp. outside of its native range and the abundance of this host in eastern North America in general and in Canada in particular, the question to ask became: Would Acer spp. in North America suffer the same fate as Salix spp. and Populus spp. in China? Also at risk were the then-unquantified losses related to the suite of values associated with maples and other broadleaf species, including runoff control, shade provision, carbon sequestration, timber products, habitat provision, and many others (Turner-Skoff and Cavender Reference Turner-Skoff and Cavender2019).
Spread analysis
Coarse delimitation of the area containing injured trees in the Toronto/Vaughan outbreak was completed within four weeks of the discovery event. The distribution and prevalence of trees with signs of injury suggested that the infestation consisted of a core and what at the time were considered three satellite areas. The first two satellites, Ansley Grove and Beechwood Cemetery (hereafter “Beechwood”), were in Vaughan, and the third satellite, in the Thistletown area (hereafter “Thistletown”), was in Toronto (Fig. 2).
Based on circumstantial evidence (i.e., abundance of emergence holes – also commonly referred to as “exit holes”), the subcommittee believed the original tree (Trotter and Hull-Sanders Reference Trotter and Hull-Sanders2015) of the outbreak was or had been located at the rear of two properties in Vaughan in the city block delineated by Steeles Avenue West to the south, Pearce Road to the east, Hanlan Road to the north, and Scholes Road to the west. The evidence can be summarised as follows: this is where the single-stem tree with the greatest number of emergence holes (227) was found; a nearby tree with four stems had 272 holes; and the site also had two trees, with signs of injury, that broke when strong winds hit the region in the aftermath of Hurricane Isabel in September 2003. Because no protocol on handling and disposal of infested material was available at that time, both trees were chipped and buried before data could be collected. However, the stumps of these two windfall trees showed emergence holes, suggesting that both trees had been structurally compromised by a high density of galleries and tunnels.
The initial discovery site was in Vaughan, but the coarse delineation of the core infestation in early October 2003 extended roughly equally between Toronto and Vaughan (Fig. 2). Indeed, the core appeared to extend about 2 km from the presumed original trees that were located in an industrial area. At that time, the farthest injured trees to the north, east, west, and south of the original tree were about 849, 2096, 1937, and 1675 m away, respectively.
A cursory examination of the injured trees in the satellites revealed that only one of the areas, Ansley Grove, presented signs of adult emergence. The other two satellites had signs only of oviposition and young larvae, suggesting the attack likely had occurred during the year of discovery – or during the previous year, if the beetle’s life cycle lasted more than one year. Whether the satellite infestations were the result of natural dispersion, human-assisted movements, or a combination of both could not be ascertained. The Beechwood satellite was located in a cemetery, whereas the other two satellites were in residential neighbourhoods. Most of the injured trees at Beechwood were along roadways. It was later found that a limousine service operating from the discovery site often booked travel to the cemetery. Injured trees in the other two satellite infestations were located in front yards, next to driveways, or between the street and the sidewalk. While investigating the origin of these two satellites, inspectors from the CFIA confirmed that in both cases, someone who lived near these injured trees worked at the outbreak’s epicentre. Despite the lack of conclusive proof, this suggested that human-mediated modes of transportation may have been a factor in the spread of A. glabripennis adults and the establishment of satellites, as has since been documented for other nonnative insect species (Buck and Marshall Reference Buck and Marshall2009; Short et al. Reference Short, Chase, Feeley, Kees, Wittman and Aukema2020).
The intensive delimitation survey was completed in mid-January 2004. All injured trees discovered during the survey were located at least 2400 m inside the boundaries of the proposed regulated area that had been identified in early October 2003, when the extensive survey had been completed (Fig. 2). This meant buffers could easily be accommodated within the proposed regulated area and that no modifications to its boundaries were needed.
Cost–benefit analyses
A detailed assessment of A. glabripennis’s potential overall economic impact in Canada was not available at the time of the beetle’s discovery in Ontario (Colautti et al. Reference Colautti, Bailey, van Overdijk, Amundsen and MacIsaac2006). For this reason, the criteria used to determine a specific response to the beetle’s invasion had to be based on its feeding habits and host range. That Toronto and Vaughan’s urban forests were directly linked to natural forest stands outside the proposed regulated area via ravines was also important. Nowak et al. (Reference Nowak, Pasek, Sequeira, Crane and Mastro2001) had estimated that in addition to natural forests, 30% of urban trees (or 35% of tree canopy or per cent leaf area) in the United States of America was considered at risk for infestation by A. glabripennis.
Ecological services provided by urban forests and other green spaces foster economic growth in urban environments and include reduced stormwater runoff and erosion, increased energy conservation, climate control, improved water and air quality, and improved property values. The potential impact of A. glabripennis on Canada’s natural hardwood forests would be more difficult to estimate. Such an assessment would have to consider how the resulting change in forest structure and composition would negatively affect the country’s forest industry, which generates more than $11 billion in hardwood forest products annually (Toronto Parks, Forestry & Recreation 2004), maple syrup production, with Canada producing about 27 million litres of maple syrup (2008), worth an annual value of about $273 million dollars (Halferty Reference Halferty2011), fall-foliage tourism, recreation, wildlife habitat (Lovett and Mitchell Reference Lovett and Mitchell2004), ecological processes or services (Kenis et al. Reference Kenis, Auger-Rozenberg, Roques, Timms, Péré and Cock2009), endangered species (Wagner and van Driesche Reference Wagner and van Driesche2010), and other environmental benefits. Lovett and Mitchell (Reference Lovett and Mitchell2004) predicted that a substantial change in abundance of sugar maple (Acer saccharum Marshall (Sapindaceae)) would produce a significant shift in nitrogen cycling in the forests of eastern North America: a decline in sugar maple abundance would result in less leaching and therefore less acidification of forest soils if the replacement tree species were to reduce nitrification in those soils.
In addition to the potential losses and costs identified above, the impacts of A. glabripennis becoming established would also include those associated with beetle management and control actions, with the trade restrictions imposed on Canada as a result of the discovery of the beetle in Ontario – Taiwan, Canada’s third largest importer of hardwood after the United States of America and China, did impose trade restrictions on Canada – and with the accrued expenditures linked to compliance with new export standards. As Myers et al. (Reference Myers, Savoie and van Randen1998, Reference Myers, Simberloff, Kuris and Carey2000) noted, predicting whether all or any of these costs or impacts would have materialised, should this invasive population have been allowed to establish for a longer period, would have been a highly speculative and biased endeavour.
Potential response options
The response strategy for a particular alien pest is selected based on the characteristics of the host species, the size of the outbreak at the time of discovery, the invader’s potential impact (fact-based or perceived), and whether the long-term costs of damage and control exceed the short-term costs of successful, permanent elimination (Myers and Hosking Reference Myers, Hosking, Hallman and Schwalbe2002; Pedigo Reference Pedigo2002). In most cases, not all of this information is known when an alien pest is found to have been introduced to a novel territory, which is why responses to pest invasions can be controversial (Myers et al. Reference Myers, Savoie and van Randen1998).
The response to the establishment of alien invasive pests could fall into one of four categories: do nothing, eradication, suppression, or containment.
Do nothing
The arrival of adventive species in new ecosystems can be deliberate or inadvertent (Ewel et al. Reference Ewel, O’Dowd, Bergelson, Daehler, D’Antonia and Gómex1999; Simberloff Reference Simberloff, Resh and Cardé2009). Most inadvertent arrivals result in no or little deleterious impact to the invaded ecosystems and in no trade restrictions (Brockerhoff et al. Reference Brockerhoff, Aukema, Cavey, Garrett, Haack and Kimberley2010a) and thus trigger no response, or a “do nothing” response, by plant protection agencies. In the case of A. glabripennis in Canada, this response strategy did not appear to be a realistic option because of the great damage this beetle had caused in some areas of its native range (Pan Reference Pan2005) and the perceived impact it could have on the structure and function of the urban and natural forests of North America and Europe (Nowak et al. Reference Nowak, Pasek, Sequeira, Crane and Mastro2001). Furthermore, this beetle had already been categorised as a regulated pest (i.e., a pest of potential economic importance to the area affected and being officially controlled; Food and Agriculture Organisation of the United Nations 2018a) by plant protection agencies (MacLeod et al. Reference MacLeod, Evans and Baker2002) and the CFIA’s own plant health risk analysis (CFIA, unpublished). For these reasons, any management option that led to no active enforcement of mandatory phytosanitary regulations and related procedures to prevent further natural spread was deemed unacceptable and untenable. Indeed, a response to do nothing would have prompted Canada’s trading partners to consider the entire country as being infested by A. glabripennis and to regulate the export of all the beetle’s host species.
Eradication
Eradication is the application of phytosanitary measures to eliminate a pest from an area (Food and Agriculture Organisation of the United Nations 2018a). This option had been the response selected following the discovery of all A. glabripennis outbreaks found outside its native range before 2003 (Haack et al. Reference Haack, Hérard, Sun and Turgeon2010). The CFIA’s pest risk analysis, prepared soon after the 1998 discovery of A. glabripennis in Chicago, also proposed eradication as a response if this beetle were to be found in Canada (CFIA, unpublished) and if conditions were conducive to this response. Conduciveness would be based mainly on the number of known affected trees in the outbreak, as determined during the delimitation survey. If these numbers were sufficiently low and if most of the remaining eight factors that positively influence the success of eradication programmes (Myers et al. Reference Myers, Savoie and van Randen1998) were present, eradication within a reasonable period would be deemed feasible. Under this option, the CFIA would consider A. glabripennis a quarantine pest, establish a regulated area, implement quarantine phytosanitary restrictions, and carry out a treatment schedule (i.e., implement treatment methods to kill, inactivate, or remove the pest at a stated efficacy).
Suppression
Suppression, which might appear to be a more realistic goal than trying to remove every specimen of a pest (Myers et al. Reference Myers, Savoie and van Randen1998), is the application of phytosanitary measures to reduce, rather than eliminate, pest populations (Food and Agriculture Organisation of the United Nations 2018a). This strategy would be considered if the size of an outbreak was so large that eradication was highly unlikely to succeed or would be extremely challenging to undertake. This option could lead to a reduced impact of the pest on the hosts or the ecosystem (Myers et al. Reference Myers, Savoie and van Randen1998). Under this scenario, the CFIA would declare A. glabripennis a quarantine pest, establish a regulated area, and implement quarantine restrictions. In addition, the CFIA would develop and implement a treatment schedule consistent with the stated objective (Food and Agriculture Organisation of the United Nations 2018a). For this strategy to be effective, it was assumed that only affected trees would be targeted for treatment, especially in the early years. One expectation of this option was that the efficacy of this treatment would be assessed annually to determine if the objectives were achieved and to reassess whether adjustments were desirable or required.
Containment
This strategy has been identified as a potential alternative to eradication when the exotic pest in question is well and widely distributed at the time of discovery (Myers and Hosking Reference Myers, Hosking, Hallman and Schwalbe2002). The objective of this strategy is to apply phytosanitary measures in and around affected areas to prevent the pest’s reintroduction and to slow its further spread (Food and Agriculture Organisation of the United Nations 2018a). This management strategy would be considered the sole option only if the pest’s distribution was much broader than originally suggested by the initial extensive survey, making eradication impossible. Under this option, the CFIA would identify, create, and implement any legislation, regulation, or official procedure needed to achieve this objective and to meet or satisfy export restrictions.
Consultation with stakeholders
The ultimate goal of the evaluation phase during the Toronto/Vaughan outbreak was to identify a response to the establishment of A. glabripennis. To achieve this, the area response team leader consulted with the various subcommittees. In the absence of rapid participatory methods (Mackenzie and Larson Reference Mackenzie and Larson2010) and in response to the perceived time constraints to prevent further spread, the area response team assembled a communications team and developed a communications plan to educate the public, to keep the public informed of the situation, and to present the range of response options being considered and their potential impact on or significance to property owners during a series of open house meetings held within the regulated area. There was great interest shown in residential neighbourhoods regarding the selection of a response option, but there was limited interest in the industrial and commercial areas.
Response phase
The goals of the response decision phase are to determine the action to be taken and the scientific and strategic rationale on which it is based and to consider all potential response options (Hosking Reference Hosking2001). Key elements of this phase include the response recommendation and decision groups, the response options, and the recommended actions.
Response recommendation and decision groups
The area response team leader asked the science subcommittee to consider available evidence on the current outbreak and the potential costs, benefits, and risks associated with each response strategy to the arrival and establishment of A. glabripennis in Toronto/Vaughan and to use that information to recommend a response strategy.
The subcommittee consisted of 11 scientists from academia and government agencies from Canada and the United States of America, most of whom had experience in dealing either with this beetle or with other invasive species. The narrow composition of this group helped to ensure that discussions and recommendations would avoid becoming mired in policy and operational details; those aspects were left to the CFIA to identify and address. The subcommittee’s roles were to answer specific entomological questions formulated by the area response team leader, to provide scientific and expert advice, to liaise with leaders of other response programmes around the world, to review the scientific information available on A. glabripennis, to provide recommendations on survey activities and directions, mitigation options, and strategies, and to identify and coordinate scientific research opportunities.
The main four tasks of the subcommittee consisted of gathering and critically reviewing all published and unpublished scientific knowledge available on this pest, broadly assessing the pest’s long-term ecological and economic impacts, identifying and recommending the most appropriate management options and monitoring survey methods available to confine the beetle to its current distribution and to prevent its spread to unaffected areas of the country and continent, and identifying knowledge gaps and research needs to support, improve, or complement the selected management strategy.
To perform these duties, the subcommittee met in person to discuss the programme’s entomological implications. Operational updates were provided regularly via conference calls. At all meetings, the area response team leader served as a resource person. This allowed the team leader to be present to explain the basis for his questions and to better understand the rationales behind the recommendations formulated by the subcommittee so that they could be taken into consideration when the recommendations were being translated into operational or regulatory directives. Most but not all of the recommendations made by the subcommittee were based on consensus. Their recommendations were transmitted to the CFIA in the form of special reports. The area response team leader would then discuss the recommendations with decision-makers within the CFIA’s Programs Branch and decide which recommendations would be accepted as is, which would be modified to comply with existing policies, regulations, or operational requirements before being passed on to the operation subcommittee for implementation, and which would be rejected.
Response options
On 15 October 2003, the science subcommittee officially recommended that the A. glabripennis infestation in Toronto/Vaughan be eradicated. The response recommendation was based on the following factors: preliminary assessments of the outbreak’s extent showed that it appeared to be confined to the putative regulated area; about 500 trees were known to be affected; the size of the area potentially affected appeared manageable; affected trees seemed predominantly limited to industrial and commercial properties; the potential ecological and economic impact this insect could have on ecosystems in Canada and eastern North America was presumed to be devastating; and several factors and conditions that positively influence the success of an eradication programme were present (Myers et al. Reference Myers, Savoie and van Randen1998).
According to Myers et al. (Reference Myers, Simberloff, Kuris and Carey2000), Myers and Hosking (Reference Myers, Hosking, Hallman and Schwalbe2002), and Brockerhoff et al. (Reference Brockerhoff, Liebhold, Richardson and Suckling2010b), successful eradication programmes share several operational requirements. There must be a commitment to finance this programme until it is completed, especially in cases where eradication cannot be achieved rapidly or within a few years of its start. Clear lines of authority must be established to allow individuals or agencies to take all management actions. The biology of the target pest must be susceptible (e.g., the pest must have a low reproductive rate, limited host range, etc.) to the mitigation activities available for control. The pathways of invasion must be blocked to prevent reinvasion. The pest must be detectable at very low densities to facilitate early detection of residual populations and to prevent its spread to other areas. At the onset of the Toronto/Vaughan eradication programme, the first four of these five requirements were present.
The science subcommittee members also looked at the factors that could influence the programme’s outcome (Myers et al. Reference Myers, Savoie and van Randen1998; Brockerhoff et al. Reference Brockerhoff, Liebhold, Richardson and Suckling2010b) and the results from the eradication programmes conducted in the United States of America (Haack et al. Reference Haack, Hérard, Sun and Turgeon2010). Early evidence suggested that it would be difficult to detect this insect at low densities and during summer when foliage was present (J. McCarthy, City of Chicago, personal communication). The vast host range was also expected to be a serious impediment to the programme’s success.
Nonetheless, the CFIA did not have to start from scratch to develop an eradication plan. The agency had already prepared an emergency response plan in anticipation of the arrival of A. glabripennis in the Greater Toronto Area several years before the beetle’s 2003 discovery there. That plan was similar in several aspects to the one developed and implemented by the United States Department of Agriculture, Animal and Plant Health Inspection Service, to deal with the earlier A. glabripennis outbreaks in New York, Chicago, and Jersey City. By 2003, the plan originally developed in 1996 to eradicate A. glabripennis in New York and Chicago had evolved because of increased knowledge about the pest and had therefore been modified when the beetle was found in Jersey City. The CFIA further modified that plan to account for conditions unique to Toronto/Vaughan.
The United States Department of Agriculture, Animal and Plant Health Inspection Service plan included the following elements: organisational structure, regulatory activities, survey procedures for detection and delimitation of infested trees, control techniques and tools, restoration activities, public outreach, and media relations (Haack et al. Reference Haack, Hérard, Sun and Turgeon2010). The CFIA needed to develop and implement a response plan that addressed those same elements.
The CFIA eradication programme for the Toronto/Vaughan outbreak, how it addressed these various elements, and how it evolved as new information became available are described below.
Organisational structure
The CFIA is the only agency with the legal authority to respond to the introduction and management of an alien plant pest in Canada, but in 2003, it lacked the people with the expertise, skills, experience, and familiarity with some of the tools needed to fully implement an eradication response in an urban forest. For example, CFIA inspectors had no formal experience or expertise in applying insecticides or in felling and climbing trees, and such activities were likely to be essential while performing control and monitoring activities in the A. glabripennis eradication programme. The City of Toronto’s Parks, Forestry & Recreation Division, however, had access to such expertise and equipment. The ability to use contractors already approved by a tender process with the City of Toronto was expected to simplify the programme’s operation phase.
In addition, the City of Toronto provided space within the regulated area to serve as headquarters for the implementation of control activities and monitoring surveys for the programme’s duration. Other partners provided short- or long-term (multiyear) assistance with the initial delimitation survey and experienced forest health monitoring officers during removal, survey, and research activities. Contributions also included inventories of public trees, ownership data for properties, and staff with scientific and operational backgrounds to serve on committees, help lead teams of inspectors, and develop training programmes.
Finally, collaborating stakeholders had established links to councillors, politicians, and senior managers and could keep them informed about the latest news, as well as keep them aware of and on board with decisions.
Regulatory measures
Regulatory measures were needed because the beetle represented a threat of great potential economic importance to Canada. For this reason, the CFIA established a quarantine area (hereafter “regulated area”) within which the beetle would be officially controlled. This area included locations where the beetle was known to be present and any area that constituted a buffer around a known infestation. A “regulated area” was defined as an area into, within, and from which plants, plant products, and other regulated articles were subject to phytosanitary regulations or procedures to prevent the introduction and spread of quarantine pests or to limit the economic impact of regulated nonquarantine pests (Food and Agriculture Organisation of the United Nations 2018a). Some of these regulatory tools could include but were not limited to: issuance of written prohibition of movement certificates to prevent regulated material from leaving the regulated area, quarantine notices, government ministerial orders, and movement certificates for regulated material treated according to a CFIA-approved process to mitigate the risk of A. glabripennis dispersal. In 2003, the list of regulated plants and plant products targeted by the phytosanitary measures was expected to mirror the host list being prepared. An approved process to certify that the regulated material was phytosanitarily safe or pest-free also had to be identified and described.
In addition to these regulatory measures, the CFIA needed to carry out surveys on an annual basis around the regulated area to assess the efficacy of regulatory measures in preventing further spread of the pest, to gather information on the extent of potential range expansion, and to adjust the boundaries should infested trees or their buffers fall outside of the original regulated area. Potentially high-risk sites in other urban centres across Canada also had to be surveyed to satisfy international trading partners that areas outside the regulated area were also pest-free.
Upon completion of the intensive delimitation survey in early January 2004, a federal government ministerial order was issued, identifying the regulated area (15 217 ha) and the regulated plants and plant products (CFIA 2003; Fig. 2). The implementation and enforcement of these regulatory measures were expected to be essential to the beetle’s eradication; however, the measures would not directly affect pest densities within the affected area.
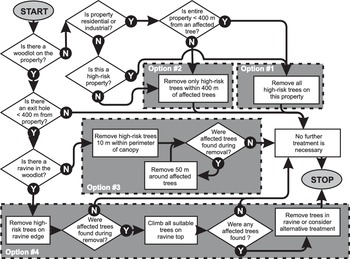
Mitigation
The proposed response option – eradication – required a plan, tools, and techniques to directly affect A. glabripennis population densities. The first step in developing such a mitigation plan was to create a list of tree species in Toronto/Vaughan and, based on existing knowledge and uncertainties, to assess their suitability as potential or targeted hosts. The list would be used to help identify which species to target for treatments during the operation phase and in surveys during the monitoring phase. The second step was to define the size of affected and buffer areas. These areas would be useful in determining the type, intensity, and frequency of management actions to take. The last step was to select a mitigation strategy and the techniques and tools that would lead to eradication.
Targeted hosts
The hosts being targeted in outbreaks within and outside the beetle’s native range had a number of things in common but also important differences (Lingafelter and Hoebeke Reference Lingafelter and Hoebeke2002; MacLeod et al. Reference MacLeod, Evans and Baker2002; Morewood et al. Reference Morewood, Neiner, McNeil, Sellmer and Hoover2003, Reference Morewood, Hoover, Neiner, McNeil and Sellmer2004, Reference Morewood, Hoover, Neiner and Sellmer2005; Sawyer Reference Sawyer2003; Wang et al. Reference Wang, Mastro and Gao2005a; Hérard et al. Reference Hérard, Ciampitti, Maspero, Krehan, Benker and Boegel2006, Reference Hérard, Maspero, Ramualde, Jucker, Colombo, Ciampitti and Cavagna2009; Smith and Wu Reference Smith and Wu2008; Hu et al. Reference Hu, Angeli, Schuetz, Luo and Hajek2009). It was therefore critical to create an all-inclusive list of the forest resources available to the beetle in the regulated area and, based on available knowledge, identify the species that would be targeted for treatment and surveillance. To create a list of broadleaf trees present, the science subcommittee used a two-step process.
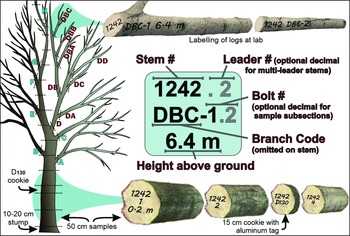
First, it obtained local information, including lists of genera and species of broadleaf trees known to occur within the two affected cities, as well as a report commissioned by the Regional Municipality of York that summarised the tree inventory inside and outside of woodlots in the presumed regulated area. The report also provided estimates of tree richness (genera and species), abundance, size (by classes of diameter at 130 cm above ground, hereafter referred to as “D130,” which is a more precise term for the more commonly used “diameter at breast height”; Brokaw and Thompson Reference Brokaw and Thompson2000), and potential suitability (by host category; see next paragraphs) for a range of buffer zone sizes around injured trees (Puttock and Gynan Reference Puttock and Gynan2003).
The second step consisted of reviewing all scientific literature available in 2003 on reported hosts for A. glabripennis and the annotated host list for the New York, Chicago, and Jersey City outbreaks. The science subcommittee used this information to assign each broadleaf genus in the regulated area to one of three categories of hosts (instead of the six categories used in the United States of America). These categories were “suitable,” “questionable,” and “unknown” (Ric et al. Reference Ric, de Groot, Gasman, Orr, Doyle and Smith2007). A genus would be considered “suitable” if evidence suggested A. glabripennis had completed its entire development in tree species within that genus under field conditions in the United States of America or in its native range (Turgeon et al. Reference Turgeon, Ric, de Groot, Gasman, Orr and Doyle2007, Reference Turgeon, Jones, Smith, Orr, Scar and Gasman2016). A genus would be considered of “questionable” suitability if laboratory or field evidence suggested that A. glabripennis oviposition and larval development were possible within tree species of that genus but no verifiable evidence yet existed indicating that the insect had completed development in any of those species under field conditions. For example, Morewood et al. (Reference Morewood, Hoover, Neiner and Sellmer2005) demonstrated that A. glabripennis could complete development in red oak (Quercus rubra) under laboratory conditions, but no records existed of Q. rubra being injured under field conditions. Genera of broadleaf trees present in the Toronto/Vaughan outbreak without evidence in the scientific literature of A. glabripennis injuries were considered of “unknown” suitability (Ric et al. Reference Ric, de Groot, Gasman, Orr, Doyle and Smith2007).
The original all-inclusive list of broadleaf trees within Toronto/Vaughan contained at least 46 genera. Because of the limited information available in 2003 on tree species at risk from A. glabripennis, especially outside of the beetle’s native range, members of the science subcommittee agreed that decisions concerning the need for treatment and surveillance of host trees would be made at the genus level. Based on a review of the above information, the subcommittee recommended that 10 genera, namely Acer, Aesculus, Albizia, Betula, Celtis, Platanus, Populus, Salix, Sorbus, and Ulmus (Smith et al. Reference Smith, Turgeon, de Groot and Gasman2009; Haack et al. Reference Haack, Hérard, Sun and Turgeon2010), be categorised as suitable hosts and thus at high risk of being injured by A. glabripennis in Ontario (hereafter referred to as “suitable high-risk genera”). The subcommittee also recommended that only suitable high-risk genera be targeted in control operations.
Genera of questionable suitability included Alnus, Elaeagnus, Fraxinus, Hibiscus, Malus, Morus, Prunus, Pyrus, Quercus, Robinia, and Tilia. The remaining genera – Ailanthus, Amelanchier Medikus (Rosaceae), Carpinus Linnaeus (Betulaceae), Carya Nuttall (Juglandaceae), Catalpa Scopoli (Bignoniaceae), Cercidiphyllum Siebold and Zuccarini (Cercidiphyllaceae), Cercis Linnaeus (Fabaceae), Cornus Linnaeus (Cornaceae), Corylus Linnaeus (Betulaceae), Crataegus Linnaeus (Rosaceae), Euonymus Linnaeus (Celastraceae), Fagus Linnaeus (Fagaceae), Gleditsia Clayton (Fabaceae), Gymnocladus Lamarck (Fabaceae), Hamamelis Linnaeus (Hamamelidaceae), Juglans Linnaeus (Juglandaceae), Koelreuteria Laxmann (Sapindaceae), Liriodendron Linnaeus (Magnoliaceae), Magnolia Linnaeus (Magnoliaceae), Ostrya Scopoli (Betulaceae), Rhamnus Linnaeus (Rhamnaceae), Sambucus Linnaeus (Adoxaceae), Syringa Linnaeus (Oleaceae), Viburnum Linnaeus (Adoxaceae), and Zelkova Spach (Ulmaceae) – were determined to be of unknown suitability.
One additional key difference between the recommended host list for the 2003 Toronto/Vaughan outbreak and that used in the United States of America was the absence of the genus Fraxinus from the Ontario list. This discrepancy was a result of inconclusive information available on this genus. For example, Luo et al. (Reference Luo, Wen and Xu2003) had reported that Fraxinus chinensis Roxburgh was not a host of A. glabripennis. There had been records of injured Fraxinus trees in 1998, the first year of tree removal in Chicago, but no further such records were reported in the years since, and no such records were reported from the New York and New Jersey outbreaks. Morewood et al. (Reference Morewood, Neiner, McNeil, Sellmer and Hoover2003, Reference Morewood, Hoover, Neiner, McNeil and Sellmer2004) reported that in greenhouse tests, oviposition and larval development had occurred on Fraxinus pennsylvanica Marshall but with high mortality, suggesting that complete larval development was possible but unlikely despite recent reports to the contrary (Meng et al. Reference Meng, Hoover and Keena2015).
Similar doubts existed for the genus Tilia. Anoplophora glabripennis was known to oviposit on Tilia mongolica Maximovich, and first- and second-instar larvae were observed successfully mining galleries at the cambium–bark interface of several Tilia species (Turgeon et al. Reference Turgeon, Jones, Smith, Orr, Scar and Gasman2016), but no evidence could be found of larvae tunnelling into the xylem or developing beyond the third instar. Similar observations were reported by Morewood et al. (Reference Morewood, Neiner, McNeil, Sellmer and Hoover2003, Reference Morewood, Hoover, Neiner, McNeil and Sellmer2004). According to the American annotated host list, Tilia spp. were common in all outbreaks, but only two records of oviposition on that genus in the United States of America existed (Haack et al. Reference Haack, Bauer, Gao, McCarthy, Miller, Petrice and Poland2006).
One important similarity between the Toronto/Vaughan and the American host lists was that all fruit trees were considered to be of questionable suitability, despite the existence of questionable records of oviposition reported on the annotated American list and reports that fruit trees of the genera Malus, Prunus, Pyrus, and so on were hosts for the beetle (Yang et al. Reference Yang, Zhou, Wang and Cui1995; Nowak et al. Reference Nowak, Pasek, Sequeira, Crane and Mastro2001; Lingafelter and Hoebeke Reference Lingafelter and Hoebeke2002; MacLeod et al. Reference MacLeod, Evans and Baker2002). It was therefore deemed unnecessary to treat these genera in Canada – at least until additional evidence suggesting otherwise became available. It is possible that some of the confusion surrounding the suitability of these hosts was due to misidentifications (e.g., A. chinensis is now known to feed on fruit trees; Haack et al. Reference Haack, Hérard, Sun and Turgeon2010).
The status of fruit trees as hosts became a hotly discussed topic during meetings with the public. Some 2003 press releases published after the discovery of the outbreak in the Greater Toronto Area (Mittelstaedt Reference Mittelstaedt2003; Strauss Reference Strauss2003) listed fruit trees as hosts of the beetle. Owners of residential properties within the area that was likely to be regulated opposed the idea that these trees might require removal, especially if there was no sign of injury detected.
Affected zones, areas, and buffers
The terminology used to describe affected trees and affected zones, areas, and buffers evolved during the course of the eradication programme. Changes to the names and functions associated with these boundaries were finalised during the development of a spatial decision support system at the beginning of the monitoring phase (Fournier and Turgeon Reference Fournier and Turgeon2017). Rather than describing this evolution, we present only the final terminology to avoid confusion.
As previously discussed (see section, Data interpretation), the definition of an infested tree took into account international standards that stated that an infested plant was one with a live specimen of the pest (Food and Agriculture Organisation of the United Nations 2018a). Indeed, relying primarily on visible signs and symptoms of injury to determine the infestation status of a tree was deemed to lack rigour and could lead to mistaken interpretations or conclusions. The science subcommittee therefore recommended that information be collected on gallery or tunnel contents (i.e., live, dead, or no specimen at any stage of development) when trees of interest found during treatment or monitoring surveys were processed. The subcommittee further determined that several of the characteristic signs or symptoms of A. glabripennis injury were required to be present in a tree before the suspect tree could be labelled as “affected” (Ric et al. Reference Ric, de Groot, Gasman, Orr, Doyle and Smith2007). For example, three trees with a single sign of what looked like an oviposition pit but with no oviposition stain visible under the bark were considered “unaffected” trees.
The subcommittee also recommended the establishment of buffers within the regulated area, comparable to those used in eradication programmes in the United States of America. The buffers reflected the perceived risks associated with natural A. glabripennis dispersal and made it possible to tailor different types of management activities that were consistent with available human and financial resources.
Buffer sizes were based on both recapture rates of A. glabripennis at various distances from a release point observed during field studies carried out in China by Smith et al. (Reference Smith, Bancroft, Li, Gao and Teale2001, Reference Smith, Tobin, Bancroft, Li and Gao2004) and a cumulative distribution function of the distance of trees with signs of oviposition to the nearest tree with at least one sign of adult emergence established in Chicago (Sawyer et al. Reference Sawyer, Panagakos, Horner and Freeman2010).
Based on this information, the CFIA defined the “affected area of an infested or attacked tree” as that tree plus the area within a 400 m radius of that tree, whereas the “affected area of a suspect tree” was defined as only that individual tree. Each affected area was surrounded by at least two buffer zones (Fig. 3). Buffer 1 comprised the area between the outer edge of the affected area and an 800 m radius from the affected tree. Buffer 2 comprised the area between 800 and 2400 m from an infested or attacked tree. Suspect trees were assigned no buffer 2. A third buffer zone in Toronto/Vaughan included the area between 2400 m from an infested or attacked tree (or 800 m in the case of a suspect tree) and the boundary of the regulated area that was established in January 2004. This third buffer was necessary because, once the boundaries of the regulated area had been publicised, it became imperative to demonstrate that the entire regulated area, not just buffers 1 and 2, was pest-free. Buffers were adjusted whenever additional infested, attacked, or suspect trees were found. In terms of dispersal risk, buffers 1 and 2 were presumed to represent areas with a high and moderate risk for natural dispersal, respectively (Fournier and Turgeon Reference Fournier and Turgeon2017), and buffer 3 was presumed to represent the lowest risk of natural dispersal within the regulated area.
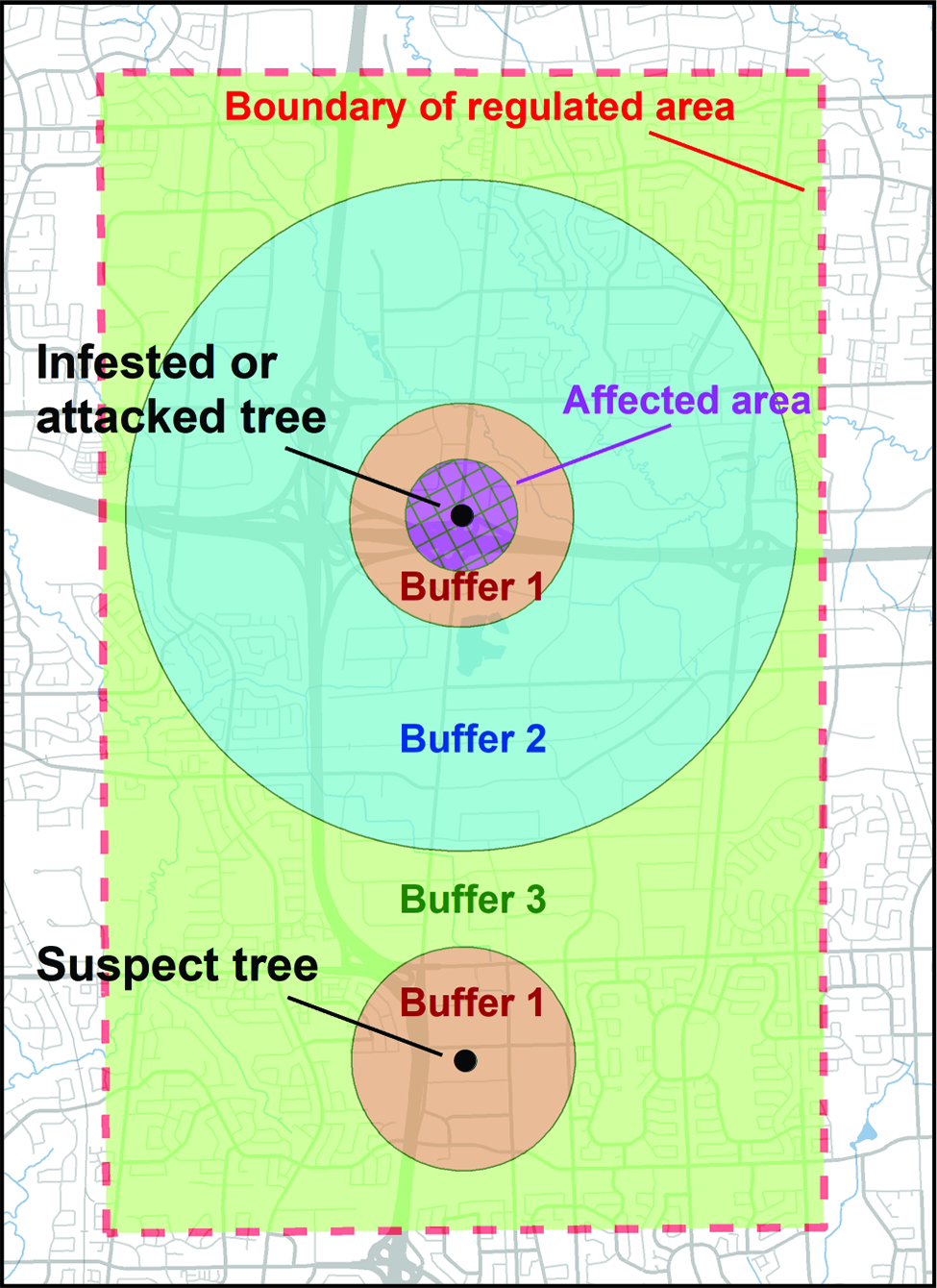
Fig. 3. Diagram illustrating the concepts of affected areas and buffers for trees that were infested, attacked, and suspected of being injured by Anoplophora glabripennis. See text for definitions of infestation status (i.e., “infested,” “attacked,” and “suspect”).
By creating these three buffers, the CFIA could then implement different detection, treatment, and monitoring strategies and tools to address the varying levels of presumed risk of dispersal within each.
Strategies, techniques, tools, and processes
In addition to the regulatory measures described above, the strategy to eradicate A. glabripennis from the regulated area required tools, techniques, and processes to control the beetle either directly or indirectly, to treat infested material to make it phytosanitarily safe and prevent further spread, to implement measures that would increase confidence in the programme’s outcome, and to learn as much as possible about the pest while these processes were being implemented. Several tactics and tools can be used either alone or in combination with several others, via integrated pest eradication, to achieve eradication (Brockerhoff et al. Reference Brockerhoff, Aukema, Cavey, Garrett, Haack and Kimberley2010a).
In 2003, a limited number of options and tools were available to control A. glabripennis and other wood borers (e.g., Wang et al. Reference Wang, Gao, Mastro and Reardon2005b; Hajek et al. Reference Hajek, Huang, Dubois, Smith and Li2006; Poland et al. Reference Poland, Haack, Petrice, Miller and Bauer2006a, Reference Poland, Haack, Petrice, Miller, Bauer and Gao2006b). Options being developed in North America included research on pathogens (Hajek et al. Reference Hajek, Huang, Dubois, Smith and Li2006) and natural enemies (Turgeon and Smith Reference Turgeon, Smith, Mason and Gillespie2013; Brabbs et al. Reference Brabbs, Collins, Hérard, Maspero and Eyre2015). No research had yet been done on how to manage A. glabripennis by modifying its development or behaviour or with sterile insects. Extensive research conducted in China had revealed the existence of differences in tree susceptibility among clades of poplars and willows (Pan Reference Pan2005; Yin and Lu Reference Yin and Lu2005; Hu et al. Reference Hu, Angeli, Schuetz, Luo and Hajek2009), but this research appeared to be of limited value in North America, where maples were the dominant hosts needing immediate protection. No assessment of the susceptibility of maples had been completed. Experimental field trials and laboratory tests with pesticides using a variety of application methods were underway (Smith et al. Reference Smith, Turgeon, de Groot and Gasman2009). Since 2003, Hu et al. (Reference Hu, Angeli, Schuetz, Luo and Hajek2009) and Haack et al. (Reference Haack, Hérard, Sun and Turgeon2010) have reviewed the biological, chemical, physical, and silvicultural measures being developed or that had been tested to control A. glabripennis in urban areas of China.
As a basis for its discussions, the science subcommittee looked at the strategy developed and used by the United States Department of Agriculture to eradicate A. glabripennis populations in the states of New York, Illinois, and New Jersey. This strategy had evolved over the years. The original strategy, used in 1996 in New York, consisted of removing all infested trees (Haack et al. Reference Haack, Hérard, Sun and Turgeon2010). It was modified slightly in 2000 in Chicago: in addition to removing infested trees, very good (i.e., Acer, Aesculus, Salix, and Ulmus) and good (i.e., Betula and Platanus; Sawyer Reference Sawyer2003) hosts within 200 m of a tree with signs of injury were treated with systemic insecticides using different application methods (e.g., soil injection, soil drench, or stem injection) in case not all injured trees had been detected. In 2002, treatment with insecticides was extended to hosts within 400 m of an injured tree and, in 2003, to within 800 m (Haack et al. Reference Haack, Hérard, Sun and Turgeon2010). In 2003, in Jersey City, hosts within 400 m of an injured tree were cut, chipped, and often burned instead of being treated with pesticides (Haack et al. Reference Haack, Hérard, Sun and Turgeon2010). The main question therefore was: which strategy would be used in Toronto/Vaughan? More specifically, would treatment be limited solely to affected trees, or would suitable high-risk trees (see the host list in section, Targeted hosts, above) located near affected trees also be treated?
Ultimately, the strategy’s selection was dictated by the science subcommittee’s knowledge that the detection of all affected trees, especially those with few signs of injury, was highly unlikely during the detection, delimitation, or monitoring surveys. (See section, Survey design and specifications, above, for a detailed list of reasons why signs and symptoms of injury can be missed.) Indeed, field observations in Toronto/Vaughan and in other outbreaks outside of Canada had clearly shown that the density, diversity, and conspicuousness of signs of injury, and their location on trees, varied greatly among affected trees, making detection of all affected trees challenging and unlikely, thereby compromising the success of the programme. For these reasons, the subcommittee recommended that the CFIA use a strategy similar to that implemented in New Jersey, whereby infested trees and those considered at high risk of injury within a fixed distance would be treated. Canada’s Plant Protection Act and Regulations allow for the treatment of infested plants and of those suspected of being infested (Canada Department of Justice 1990). Thus, the subcommittee’s recommendation was to treat all infested, attacked, and suspect trees and all trees belonging to genera considered to be at high risk of injury located within 800 m of an infested or attacked tree.
A few additional questions needed to be addressed because they had the potential to affect the number of trees that would be treated and how. The first question was: should the core and all three satellites of the Toronto/Vaughan infestation be treated similarly? The underlying issue was whether infested trees with only oviposition pits should be treated similarly to those with emergence holes, given the assumption that they did not represent a similar risk of dispersal. The rationale behind this question was that the Beechwood and Thistletown satellite infestation areas had only trees with oviposition pits despite having been surveyed intensively multiple times with the same outcome: no exit holes were found. Because the survey accuracy was known to be limited, the subcommittee eventually recommended that all satellite infestations be treated similarly to the core area, whether emergence holes were present or not.
Other questions were asked of the subcommittee. Should suspect and infested trees be treated similarly (Food and Agriculture Organisation of the United Nations 2018a)? Would treatment of uninfested trees in buffer zones be similar to that of infested trees? If so, which treatment would that be? And if not, which treatments should they receive? Finally, what type of treatment could be used in woodlots and ravines if infested trees were discovered there?
Answers to most of these questions depended on the availability of treatment tools and techniques. For that reason, the subcommittee developed treatment options to address the questions.
Treatment tools and techniques: insecticide
The science subcommittee contacted all colleagues involved in pesticide research trials for A. glabripennis control to determine whether insecticides should be included in Canada’s eradication strategy and in what role. All those contacted agreed to confidentially share their unpublished data and results, making it possible for the subcommittee to review all pertinent information then available on insecticides tested against A. glabripennis and the methods of application. In addition, the subcommittee reviewed the strategy that was being successfully used in the United States of America. All this information proved essential and valuable in assessing treatment response options and in identifying the advantages and disadvantages of each.
Since 2003, there has been no change in the insecticide delivery options used in the United States of America to protect trees against A. glabripennis. These options include injections into the trunk, injections into the soil surrounding each tree, and soil drenches for containerised plantings (United States Department of Agriculture 2014). The insecticide used was imidacloprid, a neonicotinoid; the amount and formulation varied according to delivery method. Applications were repeated annually over a three-year period to ensure that the insecticide concentration within treated trees was at a level sufficient to kill any A. glabripennis already present in the tree and to prevent new attacks. What was unknown, however, was whether any of these options would be effective and approved for repeated use in Ontario wherever A. glabripennis–infested trees were found.
Experimental field trials carried out in China (Wang et al. Reference Wang, Gao, Mastro and Reardon2005b; Poland et al. Reference Poland, Haack, Petrice, Miller, Bauer and Gao2006b; Smith et al. Reference Smith, Turgeon, de Groot and Gasman2009) and in the laboratory (Poland et al. Reference Poland, Haack, Petrice, Miller and Bauer2006a) revealed that none of the insecticides tested were 100% effective at killing beetles already present in infested trees, mainly because of variability in treatments, weather conditions, and tree health. The results also suggested that insecticide translocation and efficacy in heavily injured trees could be negatively affected by the presence of larval galleries created by the beetles feeding in the sapwood and inner bark and thus result in nonuniform distribution of imidacloprid within a tree (Kreutzweiser et al. Reference Kreutzweiser, Good, Chartrand, Scarr, Holmes and Thompson2008a, Reference Kreutzweiser, Good, Chartrand, Scarr and Thompson2008b). In addition, insecticides did not seem as effective against large larvae already in a tree’s heartwood at the time of treatment (United States Department of Agriculture 2014).
Based on these results, the usefulness of insecticides appeared limited mostly to that of a prophylactic treatment (i.e., to protect or guard against new attacks). In that role, insecticides could be used effectively to protect trees in the buffer 1 areas surrounding affected trees considered at high risk of being injured but not trees that were already affected. For an eradication strategy to be successful, a tool or technique that would reliably provide the greatest level of control possible was needed.
If insecticides were to be used only on healthy or apparently healthy trees surrounding infested trees, other challenges needed to be addressed. These included a recent municipal restriction on the application of pesticides within the Greater Toronto Area, uncertainty regarding whether insecticides could be used in ravines and riparian habitats, and a need to mitigate pesticide side effects on other organisms.
In May 2003, Toronto’s city council passed a pesticide bylaw (By-law 456-2003, Municipal Code, Chapter 612; found at https://www.toronto.ca/legdocs/bylaws/lawlists.htm?1641609224409) that was seen as a balanced approach to support pesticide reduction efforts while allowing their use where valuable green assets were threatened by pests. Restriction on the application of pesticides within the city’s boundaries did not apply when pesticides were to be used for injection into tree, stumps, or wooden poles. Nonetheless, the CFIA’s regulatory unit contacted Toronto’s medical officer of health and preemptively received a letter supporting the injection of trees with a pesticide to control A. glabripennis. The formulation of imidacloprid, the neonicotinoid pesticide that was used in A. glabripennis–eradication programmes in the United States of America (Imicide for trunk injections – Mauget, Arcadia, California, United States of America; Merit 75WP for soil injections – Bayer CropScience, Leverkusen, Germany), was not registered for use in Canada in 2003. Therefore, the CFIA and the Ontario Ministry of Natural Resources cosponsored an application for an emergency label expansion for Merit and A. glabripennis. An “emergency” is generally deemed to exist when a pest outbreak or pest situation occurs that can cause significant economic, environmental, or health problems and when no effective product or application method is registered in Canada for the control of the pest or no effective alternative control method is available (Health Canada 2016).
It was also unknown at the time whether treating trees with insecticides prophylactically in ravines and riparian habitats within the regulated area would be acceptable, feasible, and effective. It was assumed that approval for soil injection in such habitats was unlikely because of restrictions on application methods in Toronto and the potential for leaching (Doccola and Wild Reference Doccola, Wild, Soloneski and Larramendy2012).
In addition, many homeowners in the infested areas had multiple fruit trees in their backyards. This raised other red flags, such as the effect of neonicotinoid insecticides on pollinators – a topic much in the news at that time (Blacquière et al. Reference Blacquière, Smagghe, van Gestel and Mommaerts2012).
Furthermore, it appeared unlikely that the insecticide would be injected in trees that were being tapped for maple syrup production. Although not confirmed at the time, it was assumed that some pesticide residue would contaminate an injected tree’s sap, rendering the product no longer pure. Later research by Cowles et al. (Reference Cowles, Lagalante and Lombard2014) confirmed that maple sap is contaminated with imidacloprid and its metabolites following soil or trunk injection.
A similar assumption was made for foliage. Subsequent research showed that foliage from trees located in or near riparian areas that had been stem injected with imidacloprid could impact some nontarget organisms such as worms and other detritivores (Kreutzweiser et al. Reference Kreutzweiser, Good, Chartrand, Scarr, Holmes and Thompson2008a, Reference Kreutzweiser, Good, Chartrand, Scarr and Thompson2008b). Sawyer (Reference Sawyer2007b) reported that the overall survival rate of A. glabripennis in infested trees following soil injections in the American eradication programmes was extremely low; however, no efficacy data were available for the use of Confidor (Bayer CropScience, Leverkusen, Germany), the imidacloprid product that would have to be used to inject the stems of trees in Canada.
In summary, it was concluded that insecticides should not be used to treat infested trees but could be considered for treatment of trees at high risk of being attacked. Treating only high-risk trees, however, increased the likelihood that some low level of emergence would occur from trees on which no signs of attack had been detected during surveys. This strategy would in turn require that all treated trees be retreated and resurveyed multiple times for several years after the first treatment was applied to complete or confirm treatment efficacy and that the remainder of the regulated area also be resurveyed multiple times in the same time frame. Such an approach would require significant human resources.
Treatment tools and techniques: host removal
During the science subcommittee’s discussions, tree removal was another treatment technique considered. Tree removal seemed to have a few advantages over treatment with insecticides. First, this option theoretically provided 100% control of the beetle population in infested, attacked, and high-risk trees, regardless of tree health and other factors that affected insecticide efficacy. Second, this treatment could be applied at any time after detection of newly affected trees, regardless of the time of year. Third, the outcome of this treatment option could be achieved immediately after removal rather than years later (e.g., after treating trees with insecticides for three years). Another important advantage of host removal over insecticide application was that removing trees eliminated the need for additional treatments and surveys in subsequent years. This treatment would allow teams to focus their monitoring surveys in the outer buffers of the regulated area.
One disadvantage to removing trees to eradicate A. glabripennis included the many operational challenges associated with access to and evaluation and removal of all host trees. Field crews would need to overcome harsh weather, problematic access, and safety hazards while also minimising damage to private property (Fig. 4).

Fig. 4. Operational challenges to the successful eradication of Anoplophora glabripennis in Ontario, Canada included harsh weather conditions such as A, snow and ice and B, rain and mud; problematic access to trees because of C, flooded landscapes, D, waterways, and proximity to E, industrial scrap, E, J, fences, and J, concrete slabs; safety hazards when working with heavy equipment near F, railways and G, highways and power lines; and efforts to minimise property damage in sensitive areas such as H, parks, golf courses, and cemeteries, and I, home landscaping, and J, near commercial fencing.
In addition, for tree removal to be completely effective, the trees’ stumps must be entirely beetle free to prevent reinvasion. To ensure the beetle-free status of stumps, inspection and treatment of the stumps themselves would be needed, as would treatments to prevent any stump sprouting, which could provide host material for reinfestation or reinvasion. Effective stump treatment could be mechanical (e.g., using stump grinders) or chemical (i.e., using herbicide). Although herbicide treatment would kill the stump, it was unknown whether larvae could survive in herbicide-treated stumps, given that larvae already in heartwood can complete their development in dead wood (e.g., in wood packaging material).
It was also unlikely that all stumps would be accessible, especially in urban landscapes. For example, removal would not be an option in all cases (e.g., in cases where a tree was growing at the base of concrete walls or along chainlink fences; Fig. 4). Selection of the most appropriate stump treatment would therefore be based on stump accessibility and operational feasibility. The preferred treatment for removed trees would be to grind the stump to a depth of 45 cm below ground level. In cases where access to stumps with stump-grinding equipment was not possible, they would be treated with an herbicide to prevent sprouting.
In choosing to remove trees that were affected or at high risk of being affected, the CFIA needed a process to sanitise all host material removed from the regulated area.
Processing and disposal of host material
The CFIA investigated and assessed the feasibility and costs of various options to dispose of host material from affected and unaffected trees removed during treatment. The options considered included burning the wood in a biofuel production facility to generate electricity or heat greenhouses, using the logs in the production of asphalt shingles (National Institute of Standards and Technology 2018), or using the wood chips in a water treatment facility. Each of these options had specific requirements that were unacceptable to the CFIA. For example, all logs had to be 2.4 m long, processed chips had to be softwood (A. glabripennis hosts are hardwood species), and chips had to be transported outside the regulated area.
Because the science subcommittee was considering the option of not using insecticides in Ontario, the decontamination process of host material had to meet or exceed the standards in place in the United States of America, where chips no larger than 15 mm in at least one dimension were deemed phytosanitarily safe (Wang et al. Reference Wang, Mastro and McLane2000) and, therefore, were no longer subject to federal or state regulations. In Canada, the subcommittee recommended that the final size of the treated material be chips no bigger than 15 mm in two dimensions. Such a size requirement provided a higher degree of confidence that the chipped material would prevent survival of A. glabripennis’s immature stages, which were much larger than 15 mm (Peng and Liu Reference Peng and Liu1992). An advantage of selecting this chip size was that the resulting chips would become unregulated material and, as such, could be removed from the regulated area.
At least two processes were needed regarding the handling of host material classified as either infested or at risk of being infested. The first process had to address how to treat three distinct types of host material removed from properties. These host-material types were the small- and medium-sized stems and branches of trees removed during treatment operations that could be manipulated by hand and potentially treated onsite, the very large stems and branches of trees removed during treatment that could be manipulated only by boom trucks, and the material generated from within the regulated area by yard-maintenance companies on private properties or by programmes such as municipal yard waste collections during spring and fall.
The CFIA would need a large marshalling site with restricted access at which to stockpile host material until it could be decontaminated, and the site would have to be located within the regulated area so as to eliminate the need for movement certificates when handling the host material. The City of Toronto had a maintenance facility with a gated and fenced yard at Emery Parks Yard (hereafter “Emery Yard”; https://opengovca.com/toronto-address/27_Toryork_Dr,_North_York; Julian Jacobs Architects 2017). Part of the yard and the attached facility were made available to the CFIA, which ended up using it as its centre of operations for the programme’s duration (Figs. 2 and 5). To further ensure the physical security of potentially contaminated materials that could be seen as free firewood, the CFIA placed the yard under surveillance.

Fig. 5. Gated and fenced section of Emery Yard where A, yard waste from the entire regulated area and B, C, unaffected stems removed from areas affected by Anoplophora glabripennis were collected and stored until they could be processed through a tub grinder.
The CFIA decided that wood chippers would be used at the site of removal (hereafter “curbside chipping”) to preprocess stems and branches, thereby immediately reducing the risk of reinvasion, and that the resulting waste material would be stored at the marshalling yard within the core affected area. Very large stems and branches that could not be processed via curbside chipping, as well as all municipal yard waste collected in the regulated area, would be forwarded directly to the marshalling yard. At the marshalling yard, the chips from curbside chipping would be screened, and those that did not meet the 15-mm-size requirements, along with large stems and branches, would be passed through a tub grinder. Screening again would separate chips bigger than 15 mm, and these would be passed through the grinder a second time to reach the compliant size. Yard waste would also be passed though the tub grinder twice (Fig. 6).

Fig. 6. Wood chips from A, curbside chipping that met the minimum 15-mm size requirement and B, wood material processed twice through a tub grinder and shredder; examples of the piles of wood chips created from C, processing yard waste and D, high-risk stems removed during the eradication programme against Anoplophora glabripennis in Ontario, Canada.
Processing thousands of logs at a single site resulted in a pile of chips that was several storeys high and produced a significant amount of heat (Fig. 6). According to the Ontario Office of the Fire Marshall (Ontario Ministry of the Solicitor General 1998), temperatures in woodchip piles can reach and exceed 66 °C, exceeding the 40 °C temperature at which Keena and Moore (Reference Keena and Moore2010) found all A. glabripennis larval instars fail to develop.
The second process regarding the handling of host material addressed how infested material could be moved to a research facility outside the regulated area, where A. glabripennis colonisation behaviour and patterns could be assessed and studied. The CFIA would issue a movement certificate to transport infested logs in sealed metal canisters from Emery Yard to a walk-in oven at the Faculty of Forestry, University of Toronto, where logs would be heated at 56 °C for about 20 hours. Heat probes inserted into several logs undergoing the treatment would monitor temperature and confirm that the heartwood of the largest logs had been heated to at least 56 °C for at least one hour. Once treated, these logs would be deemed phytosanitarily safe and transported to the Great Lakes Forestry Centre in Sault Ste. Marie, Ontario for the second step of the assessment process.
Post-treatment activities
The science subcommittee determined that, following treatment of affected areas, processes would be needed to verify that areas had been treated as prescribed (see section, Evaluation of operation efficacy, below). These processes were deemed essential to ensure cohesiveness among elements of the strategy to create a pest-free regulated area. The lack of an effective attractant to attract and detect residual populations of A. glabripennis meant that visual monitoring surveys were the only method available to the CFIA to detect these residual populations. This process is described in the section, Monitoring phase, below.
Public outreach and media relations
The communication subcommittee comprised professionals representing key stakeholder organisations – the CFIA, the City of Toronto, the City of Vaughan, the Regional Municipality of York, the Toronto and Region Conservation Authority, the Ontario Ministry of Natural Resources, the Ontario Ministry of Agriculture and Food, and Natural Resources Canada. The subcommittee met biweekly by conference call from September 2003 to May 2004 and monthly thereafter.
The original (2003) communication plan outlined four key objectives: to enlist the support of the public in reporting sightings by providing information to help them identify the beetle; to communicate to the public the importance of not moving tree materials (including trees, nursery stock, firewood, and tree pruning waste) from inside or near infested areas; to encourage partnerships with stakeholders and to encourage the public to help the CFIA find the beetle and prevent its spread; and to publicise the role of the CFIA as the lead agency with respect to developing and enforcing policies to control or eradicate invasive alien species such as A. glabripennis.
The CFIA revised these objectives in May 2004 and added the following seven objectives: to provide information to the public about the programme, policies, and services that was accurate, timely, relevant, and understandable; to ensure the CFIA was visible and accessible to the public it served; to designate primary spokespersons to communicate with the public and media; to foster open dialogue with the public on issues involving risk and to build a climate of trust; to provide ongoing situation analysis to assist in risk assessment and decision-making processes; to educate and inform the public and industry about the Asian Longhorned Beetle Infested Place Order, 2004 (federal government ministerial order) – what it was, what it meant, why it was necessary, and how to comply; and to educate and inform the public and industry about the Introduced Forest Pest Compensation Regulations programme and why participation was important. Overall, the subcommittee considered the communications priorities to be educating the public on what was at stake and what to look for, enlisting their support, and encouraging them to report suspect signs or insect specimens via the hotline.
Recommended actions
The science subcommittee’s recommended eradication response to treat the regulated area consisted of 11 actions and was designed to be implemented in the core infestation area and in all three satellite areas:
-
1. Human-assisted spread beyond the current regulated area should be prevented using regulatory means;
-
2. All visibly affected trees identified during delimitation surveys should be removed;
-
3. All suitable trees considered at high risk of being attacked that were within an 800 m radius of an infested or attacked tree (see section, Targeted hosts, above) in the core infestation and in all three satellites should be removed;
-
4. All removed tree material should be brought to a marshalling yard within the regulated area for processing;
-
5. All stems and branches from trees removed within the regulated area, including yard waste, should be chipped or tub-ground to a maximum size of 1.5 cm in two dimensions;
-
6. The stumps of removed trees should be ground to a depth of 45 cm to eliminate any beetles or larvae possibly remaining in the stump or, if the stumps were inaccessible, they should be sprayed with an herbicide such as Triclopyr to prevent sprouting;
-
7. Treated yards and landscape settings or damaged areas should be restored by grading, bare soil should be seeded with grass, and fences damaged during tree removal or removed because a tree was growing through it should be replaced;
-
8. Multiple systematic targeted surveys (hereafter referred to as “sweeps”) of areas or properties treated should be performed to ensure all infested and suitable high-risk trees had been identified and treated;
-
9. Monitoring surveys throughout the regulated area and around it should be performed to detect newly infested trees, to gather data on the extent of potential range expansion, and to adjust the boundaries of the regulated area, if necessary;
-
10. A tree planting programme aimed at reforesting the affected areas with tree species considered unsuitable for A. glabripennis should be initiated; and,
-
11. Five years of consistently negative survey results of the entire regulated area should be required before it could be declared pest-free.
At the programme’s start, no affected tree had yet been found in woodlots and ravines; therefore, the recommendation was that woodlots with or without ravines were to be treated on a case-by-case basis due to the lack of available guidelines on how to treat such areas. Some of the factors that would be considered in determining the extent of tree removal in woodlots and ravine landscapes were the distance from an infested tree with at least one exit hole, the location of high-risk tree genera within woodlots and ravines, the taxonomic richness of suitable host genera, stand density, whether other infested trees within the woodlot or ravine had been detected, and the amount of time since the infestation had first been present in the wooded area.
One final recommendation made by the science subcommittee was that descriptive information be gathered during the operation phase. This action would help support the CFIA in fulfilling its legal requirements and allow the area response team to address knowledge gaps and any needs necessary for implementing subsequent phases of the A. glabripennis–eradication programme and to assist in the development of site-specific management options, as needed.
Operation phase
The operation and monitoring phases of invasive pest–eradication programmes are typically intertwined in an iterative cycle that begins with an initial operation. The operation phase consists of implementing a response option, and it lasts until the desired outcome or response is achieved – in the case of the Ontario outbreak, until the removal of all known trees affected by or at high risk for being injured by A. glabripennis. The monitoring phase, on the other hand, includes activities designed to determine the success of the operation and to measure progress towards eradication, and it lasts until the regulated area is declared pest-free (Hosking Reference Hosking2001). When surveys carried out during the monitoring phase reveal the presence of previously undetected residual populations, additional control activities must be performed, consistent with the operation phase, but without completely stopping the monitoring phase. Once additional treatment is completed, the programme reverts completely to monitoring activities. This back-and-forth process continues until the regulated area is declared pest-free.
Key elements of the CFIA’s operation phase for the A. glabripennis–eradication programme in the Toronto/Vaughan area included operation objectives, an operation management team, science input, an operation plan and achievements, assessment and mitigation of health and environmental implications, documentation, and financial management. Hosking (Reference Hosking2001) had incorporated two additional elements in this phase that the operation subcommittee did not include because they were addressed by other committees.
The initial operation phase for the Toronto/Vaughan outbreak officially began 20 November 2003 with the removal of an affected tree during a media event, and it ended on 31 March 2004, when the removal of all the trees known at the time to be injured and all surrounding high-risk trees within the regulated area had been completed. The CFIA completed supplementary treatment operations around the last affected tree on 7 March 2008, before the expected and predicted date of beetle emergence for that year.
Operation objectives
The objective of the CFIA response plan against A. glabripennis was to implement phytosanitary measures and management protocols to eradicate this pest from Ontario. To achieve this objective, the CFIA designed an eradication programme that used a combination of regulatory and mitigation actions. (See sections, Regulatory measures and Recommended actions, above.)
Operation leadership and management team
The programme’s area response team leader remained in charge of the operation phase but received assistance from a certified arborist and a researcher. The arborist’s role was to coordinate and facilitate access to the certified personnel and specialised equipment needed to plan and implement host-removal and -disposal activities. The researcher’s role was to assemble a team that would determine how to organise the acquisition of data to meet operational needs and to fill knowledge gaps.
These three individuals worked to clearly identify and integrate the programme’s informational and operational needs and to develop standard operating procedures and protocols for all aspects of the operation.
Science input
The area response team leader recognised several knowledge gaps from the programme’s start and deemed it important to integrate research and operations to fill those gaps. The purpose of this integration was to improve what, when, and where control and survey activities should take place and to develop site-specific management options as needed. For example, ravines and other forested areas existed throughout the regulated area, but at that time, most outbreaks of A. glabripennis in other countries had occurred in residential areas: this gap led to a lack of guidelines on how to treat wooded areas for this pest. Because of this and other knowledge gaps, the researcher’s first task was to assemble a team dedicated solely to identifying research objectives based on those gaps and requirements and how to meet them. Ensuring that all participants and stakeholders understood the value and benefits that could be derived from the integration was equally important. By necessity, the approach required the researcher and the arborist to cooperate closely in designing the standard operating procedures and protocols and in coordinating their execution. Once a protocol for tree removal was completed, a flowchart detailing each step was created (Fig. 7). The steps then were discussed with the operational coordinator to identify the best approach to integrate them with all other activities associated with tree removal (e.g., fence repair, stump removal or treatment, grass seeding, sweeps, etc.).
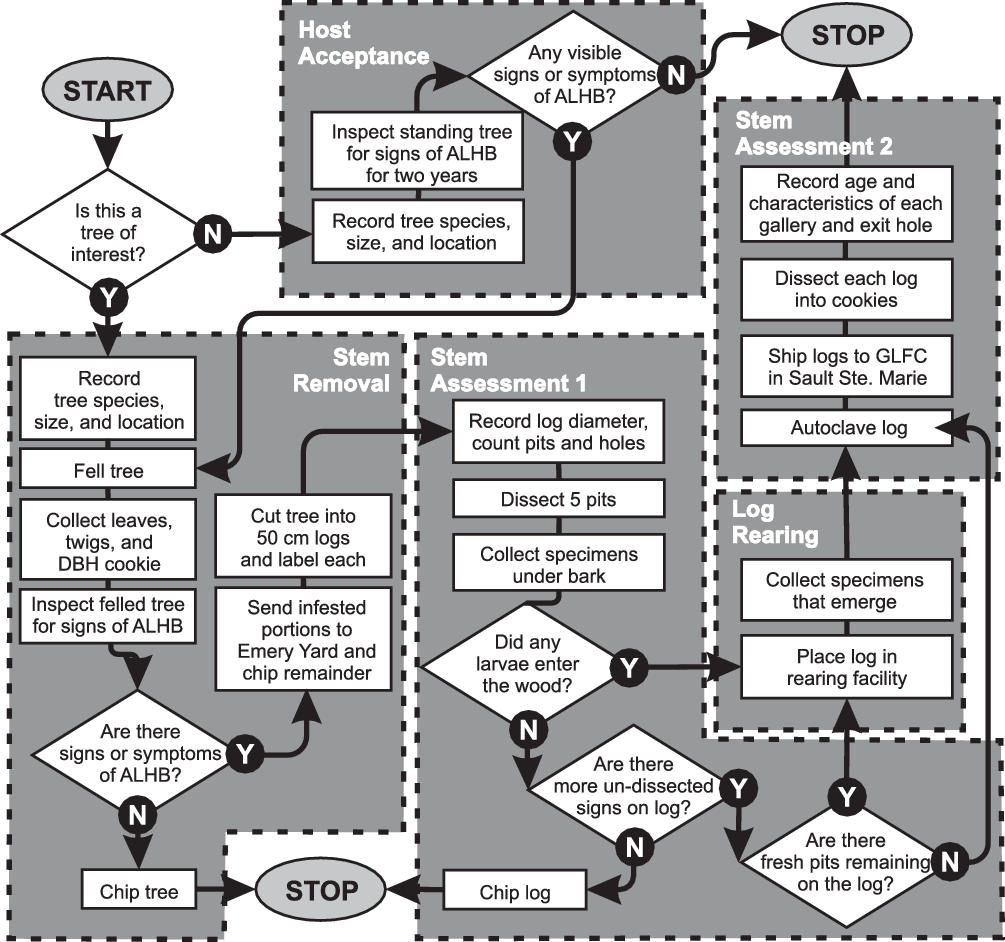
Fig. 7. Flowchart illustrating the process developed to integrate mitigation and research activities during the eradication programme targeting Anoplophora glabripennis in Ontario, Canada. Stems were examined to establish their injury status, removed, identified to determine host acceptance, and assessed to determine the infestation status and injury descriptions and level (stem assessment 1) and the colonisation behaviour and patterns (stem assessment 2). Infested logs with more than five signs of injury were stored under containment conditions in the laboratory to allow for emergence of adult A. glabripennis.
The research team had seven main science objectives to address to support the programme. These included identifying the site of establishment or origin of the infestation, improving the ability to detect and diagnose injured trees by identifying all signs and symptoms of injury associated with each life stage, and acquiring knowledge on host selection and suitability. Objectives that focused on increasing understanding of the beetles’ behaviour and biology dealt with characterising A. glabripennis galleries and tunnels so that they could be distinguished from those of native woodboring-beetle species, identifying the beetle’s within-tree colonisation processes and patterns that would be necessary to develop adequate rapid response protocols for detection and delimitation surveys, studying beetle spread through space and time using the location of infested trees so that containment buffers and policies could be implemented to ensure containment or to prevent escape to natural forests, and clarifying the beetle’s life cycle in Ontario.
The research team designed four protocols to address most of these knowledge gaps: one for tree removal, two for tree assessments, and one for allowing A. glabripennis to emerge from samples of infested trees. The protocols were tested by experienced forest health officers from Natural Resources Canada and the Ontario Ministry of Natural Resources. The officers also contributed to the original design of templates to be used to record field and laboratory data on each tree removed.
To achieve some of the study objectives, the research team needed access to a space to process wood samples in a safe and contained environment. The City of Toronto provided access to parts of the building at Emery Yard. A part of this building was retrofitted to become a makeshift laboratory, where samples from tree stems of interest were subsequently processed. Within that space, the CFIA also built a physical containment facility (e.g., Biosafety level 1 or PC-1; 4.9 × 10.4 m) where infested logs could be kept securely in individual tubes to recover emerging adults, to confirm beetle injury patterns, and to determine how long A. glabripennis adults took to emerge from injured logs.
Operation plan and achievements
The operation plan was designed to eradicate the beetle and to generate information that could be used to adjust the plan should the need arise. The CFIA modified some steps of the recommended eradication response (detailed in section, Recommended actions, above). For example, for step 3, the CFIA chose to treat the Beechwood and Thistletown satellite infestations, which had only affected trees with signs of oviposition, differently than Ansley Grove, which had several trees with signs of adult emergence. Specifically, the CFIA decided to remove only affected trees in the satellite areas that had no signs of adult emergence and all high-risk trees in the satellite that had signs of emergence. In another modification to step 3, the CFIA adjusted the size of the removal radius for suitable tree genera at high risk of injury: only trees of those genera within 400 m of an infested or attacked tree would be removed, rather than those within the recommended 800 m.
Key elements of the CFIA operation plan included the identification of supporting documents, the description of technical processes, the development of fieldsheet templates, a constant review of processes, and the documentation of activities and decisions during the response. Information about each of these elements is detailed below.
Identification of supporting documents
Before starting tree removal, the CFIA had to adhere to a requirement under the Plant Protection Act and Regulations (Canada Department of Justice 1990), whereby an inspector must deliver a “notice to dispose” (Supplementary material, Appendix 2) to the owner of each property with trees targeted for removal. This notice indicated the property details, the owner’s name and address, the location(s) of the tree(s) in question, how each tree would be treated, and the period during which the treatment would occur. Only owners affected by treatment would receive a notice to dispose. The cities of Toronto and Vaughan made their municipal taxation rolls available to the CFIA to assist with identification of landowners and also provided high-resolution satellite imagery of the regulated area (Fournier and Turgeon Reference Fournier and Turgeon2017), which proved useful for determining the exact location of trees and for visualising the landscape associated with each affected area.
Description of technical processes
The science subcommittee relied on information and data gathered during the operation phase to recommend changes to the eradication plan. For example, they used the information and data to modify categories of host suitability and to adjust the response actions for each tree genus. They also used the data to distinguish a core infestation from a residual infestation. Finally, based on the data, they developed and implemented grid-based area-wide survey protocols and training and quality assurance programmes.
The technical portion of the operation phase began with the removal first of affected stems and then of high-risk trees. At the onset of this phase, one team performed the tree removals. This approach was chosen because of the need to assess the process’s adequacy and to ensure the appropriate information could be collected in the prescribed manner. The approach showed that the protocols required only minor adjustments, and once these amendments were completed, the number of removal teams increased gradually. New teams were trained for a week by the initial team to ensure familiarity and consistency in data acquisition and recording. The number of teams assigned to tree removal increased from one in November 2003 to five in December 2003, and then to a peak of 35 teams by early March 2004. Each team consisted of a data collector, a specialist – either a forest health officer, a trained and experienced arborist, or an inspector with a strong forestry background (Turgeon et al. Reference Turgeon, Pedlar, de Groot, Smith, Jones, Orr and Gasman2010) – who was familiar with signs and symptoms of A. glabripennis injury, a chainsaw operator, and a variable number of assistants to handle host material or special equipment involved in tree removal.
Each morning, data collectors and specialists reported to Emery Yard, where the operations coordinator assigned daily work schedules, with the locations of trees to be removed or, if the monitoring phase was underway, examined. The work assignments were also displayed for each crew on a large board. At the start of the day, each data collector and specialist met with their cutting crew at the yard and left together to perform their assignment or duties. At the end of the day, the data collectors submitted the paperwork for the completed work, and the crew reported any issues they may have experienced during the day (e.g., difficulty accessing properties, presence of dogs – the CFIA hired a dog trainer to instruct field staff on what to do if dogs were present – difficulty in locating or accessing trees on industrial properties because of the presence of junk or because they were fenced off by equipment, traffic-related issues such as parking on busy streets or being stuck in traffic, or a need for escorts from the Ontario Ministry of Transportation when accessing highways or from Canadian Pacific Railways or Canadian National Railway when working along railways).
Stem removal
To determine the information that could be acquired in the field or laboratory, members of the research team first removed a short (< 5 m) injured tree from the core infestation area and brought it into a garage bay of the building at Emery Yard to be examined. Based on this examination, the researchers designed a removal process to meet the regulatory, operational, and research objectives (identified above, in section, Science input) that was the same, irrespective of the response phase (delimitation, operation, or monitoring) in which it occurred (Fig. 7). Upon arrival at a tree, the data collector and specialist undertook the following steps (similar to those listed in Turgeon et al. Reference Turgeon, Jones, Smith, Orr, Scar and Gasman2016; Fig. 8).
-
1) They recorded the number of the tree on an aluminium tree tag attached to the tree by the inspectors who had performed the delimitation survey.
-
2) They identified and recorded the tree’s genus and, when possible, species (determined using branching, bark, leaf, or bud characteristics).
-
3) They counted and recorded number of stems at 130 cm above ground.
-
4) They measured and recorded the tree’s stem diameter at 130 cm above ground (D130) using a Lufkin Executive Thinline metal tape (W606PM; Crescent Tools, Apex Tool Group, Sparks, Maryland, United States of America). When encountering an affected multistemmed tree, the information for each stem was recorded separately, but for unaffected multistemmed trees, only the diameter of the largest stem was recorded.
-
5) They measured and recorded the height of only affected trees using a Forestor Vertex digital hypsometer (Grube Hützel, Bispingen, Germany) if the tree was standing or a measuring tape after felling.
-
6) They recorded spatial location using easting–northing pairs (UTM zone 17, Garmin GPSMAP 76 set to North American Datum of 1983 [NAD 83]; Garmin Ltd., Olathe, Kansas, United States of America).
-
7) They recorded the street address of the property on which each tree was located.
-
8) They recorded the type of property or land use (commercial, industrial, institutional, open (nonforested), open (sparse), open (forested), residential, or transport/utility).
-
9) They recorded the tree’s location on the property relative to the street(s).
-
10) They determined and recorded the live/dead status of tree, based on the presence or absence of living buds on twigs during winter and of leaves during summer.
-
11) They determined and recorded the tree’s field injury status (i.e., presence of oviposition pits, emergence holes, or other known signs of A. glabripennis injury) by ground-based visual inspections, using binoculars if necessary, of a standing tree’s bole and major branches.
-
12) They instructed the chainsaw operator on how to fell the tree – in one piece or branch by branch, depending on the tree’s size and shape, its location in relation to buildings, power lines, and other trees, and the abundance and distribution of signs of injury in the crown.
-
13) They recorded the time at which cutting started and the time once the tree was felled.
-
14) They re-examined unaffected trees to confirm their injury status.
-
15) They measured and recorded the height of the lowest and highest signs of A. glabripennis injury on the tree, which was obtained by measuring the shortest length, in centimetres, between the sign and the cut line and then adding stump height.
-
16) They collected a 15-cm-long twig sample with buds from each tree and placed it in a bag bearing the corresponding tree-tag number. These samples were later compared to voucher specimens to verify the original field identification.
-
17) They wrote the tree’s tag and sample numbers on the basal/proximal end of each tree trunk or on the branch section with signs of injury.
-
18) They drew a diagram of the tree, illustrating how all sections with signs of injuries were interconnected.
-
19) They recorded the time when this process was completed, thus providing valuable information for planning purposes on the potential daily rate of removal of trees of different sizes.

Fig. 8. Tree removal process. Upon arrival at a tree targeted for removal, A, the data collector and specialist recorded the necessary information (see section, Treatment tools and techniques: host removal, for details), B, took the required pictures, C, instructed crews on how to fell the tree based on the number of Anoplophora glabripennis injuries (one or multiple specific pieces), D, re-examined the fallen tree to confirm its health and injury status as a tree of interest or not, and recorded additional information, such as the highest and lowest sign of injury (marked with flagging tape), E, collected a stem cookie at 130 cm above ground level and a twig sample for a comparison to voucher specimens of known genera or species, F, cut sections with signs and symptoms of injury into manageable pieces that were labelled and transported to Emery Yard for further assessment, and G, shredded sections without signs or H, sent them to Emery Yard for processing through a tub grinder.
In addition, the data collectors and specialists photographed aspects of each tree removed. The photographs taken were of the tree’s aluminium tree tag, of the tree with a metre stick standing against the trunk, and of the tree’s background so that the tree’s exact location in relation to buildings or other trees could be confirmed later.
All tree sections of interest were transported to Emery Yard for assessment. Small sections without signs or symptoms of injury were chipped curbside. These chips, along with the main stems and branches of unaffected trees too large for curbside chipping, were transported to Emery Yard and stockpiled until they could be shredded with a tub grinder.
Stem assessment: infestation status and injury description
The protocol for the first stem assessment was designed to verify the original field infestation status (i.e., infested, attacked, or suspect) of each stem of interest, to clarify host selection by and suitability to A. glabripennis, and to assess injury level, or abundance of signs, with the goal of improving detectability and survey efficacy (Fig. 7). Typically, this assessment was performed within weeks of removal so that the research team could learn from and act upon preliminary results, as necessary. The stem infestation and injury status assessment consisted of describing each affected log, assessing the abundance of some types of injuries, and characterising those injuries.
Upon arrival at Emery Yard, sections of interest were prepared for the first part of the assessment process (Fig. 9). A chainsaw operator cut each section into logs that were approximately 50 cm in length, avoiding cutting through exit holes whenever possible. An assistant wrote additional relevant information on the basal end of each log to identify main stems, sample sections, log sequence, sample heights, and a base-26 (hexavigesimal) branch code to indicate the sample’s relative position in the branch hierarchy (Fig. 10). This information would enable researchers to reconstruct the tree later, if necessary (Fig. 9).

Fig. 9. Tree assessment process – injury status and injury description. Stems and branches of trees of interest were: A, cut into logs approximately 50 cm in length; B, labelled; C, stored until they could be processed; and D, E, reassembled according to the labels. Assessment consisted of: F, taking several measurements of the logs; G, recording the number and size of oviposition pits and emergence holes by Anoplophora glabripennis and removing the bark surrounding five signs of oviposition; and H, collecting live or dead specimens for molecular studies and determining whether the stem was infested, attacked, or suspect (see section, Data interpretation, for details).
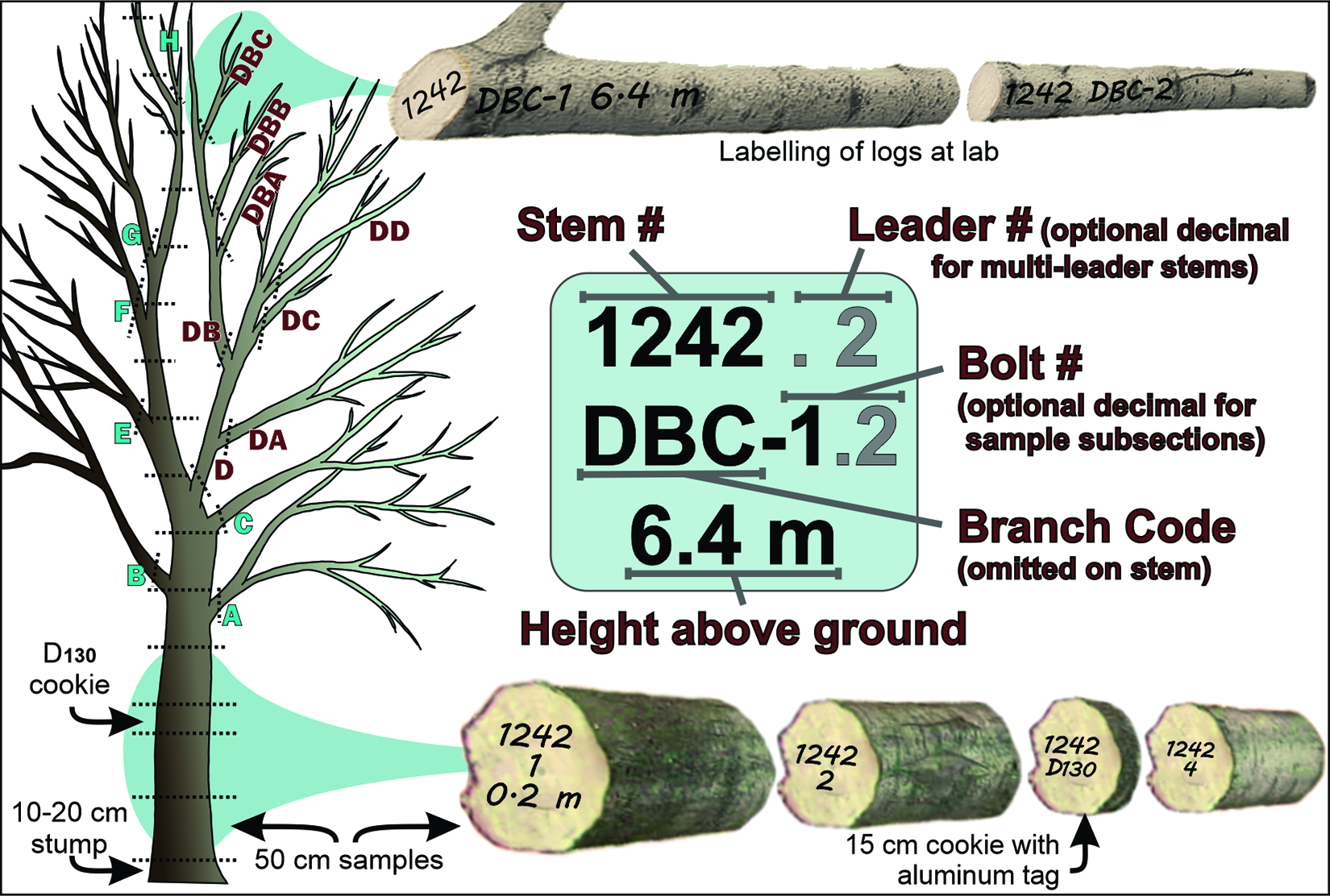
Fig. 10. The labelling scheme used before starting the assessment process for stems affected by Anoplophora glabripennis. This scheme consisted of a mixture of decimal codes to identify main stems, sample log sections (logs), and sample heights, as well as a hexavigesimal branch code to indicate the relative position of a sample in the branch hierarchy.
Once labelled, logs of interest from stems removed between November and May were kept outdoors under natural conditions at Emery Yard until assessed. Logs kept under these conditions represented no risk of A. glabripennis adult emergence or spread because the temperatures typically experienced in Ontario between November and April are below the beetle’s activity threshold and are often well below freezing (Keena Reference Keena2006; Keena and Moore Reference Keena and Moore2010). Logs from trees removed between May and October were placed in metal trashcans (88 or 140 L), sealed with duct tape, and placed in the containment facility until assessed – usually within days to prevent mildew.
The description of each log (Fig. 9) included the following measurements: length, diameter at the basal end, and mean bark thickness (by measuring the minimal and maximal bark thickness at its basal end). The extent of injury was assessed by counting the number of A. glabripennis oviposition pits and emergence holes. Further characterisation of injuries found on each log was achieved by chiselling out up to a maximum of five pieces of bark, about 3 × 3 cm in size, each with an oviposition pit at its centre. The thickness of each of these pieces of bark was measured with calipers, and whether a specimen of A. glabripennis was or had been present under the bark at that location was recorded. If a specimen was present, its development stage (i.e., egg, or small- or intermediate-sized larva, etc.), condition (live – e.g., turgid egg – or dead – e.g., collapsed egg), and location (i.e., inner bark, sapwood, or heartwood) were recorded. All live specimens collected during this process were placed in absolute ethyl alcohol and kept for an assessment of genetic diversity and potential determination of the population’s origin (Carter et al. Reference Carter, Smith and Harrison2009a, Reference Carter, Smith, Turgeon and Harrison2009b; Turgeon et al. Reference Turgeon, Orr, Grant, Wu and Gasman2015; Javal et al. Reference Javal, Roques, Haran, Hérard, Keena and Roux2017). To complete the injury characterisation, the lengths of up to three oviposition pits and the diameters of up to three emergence holes per tree were measured.
Once this assessment was completed, many logs with more than five oviposition pits were placed in individual emergence containers to provide information on life cycle duration (Fig. 11). The containers were stacked horizontally and kept at 22–25 °C in the containment facility starting in May 2004. In December 2004, the logs were heat treated, as previously described (see section, Processing and disposal of host material) and transported to the Great Lakes Forestry Centre, Sault Ste. Marie, Ontario, where the next assessment was carried out.
Fig. 11. Rearing process. Once assessment and description of injury status and intensity were completed, heavily infested 50-cm-long logs were A, propped up with screws, placed in individual containers (concrete forming tubes), and B, stored horizontally at room temperature (22–25 °C) in the containment facility at Emery Yard, Toronto, Ontario, where they were monitored daily to record C, Anoplophora glabripennis adult emergence.
Stem assessment: colonisation behaviour and pattern
The second assessment protocol was designed to clarify larval behaviour in the stem, within-tree colonisation patterns, invasion chronology, and the beetle’s spread across the landscape (Fig. 7). Sixty infested trees were selected to obtain data on within-tree colonisation patterns (Fig. 12).
Fig. 12. Tree assessment process. Colonisation behaviour and pattern: A, logs with a diameter greater than 15 cm were split lengthwise; B, each section was identified and C, cut into cookies about 2.5 cm thick; D, both surfaces of each section or log cut were marked with matching lower-case Roman numerals, so they could be E, matched later and used to F, characterise oviposition and G, emergence events.
Clarification of larval and pupal behaviour and assessment of host suitability were based on a step-by-step examination of logs from a select number of affected trees of the genus Acer. The first step was to measure a log’s diameter at the proximal end. The second step was to locate all emergence holes on that log, number each one sequentially, and measure their diameter. In the next step, all visible oviposition pits were located and numbered. The fourth step consisted of cutting small-diameter (< 15 cm) logs through the centre of each oviposition pit or emergence hole into slices from 2.5 to 5 cm in thick (hereafter called “cookies”) using a band saw and of labelling both surfaces of each sectional cut with matching lower case Roman numerals, so that the log or each larval tunnel could be reconstructed later (Fig. 12). However, because this step was not possible on logs that were heavily infested with multiple pits or holes, one pit or hole was chosen at random among those too close to be separated. Logs with a diameter exceeding 15 cm were split into manageable pieces using an electric log splitter, their corresponding surfaces were labelled with matching uppercase letters (A, B, C, etc.), and then each piece was cut into cookies and matched with lowercase Roman numerals, as necessary.
Each oviposition pit or event on the log was assigned to one of six outcomes based on the signs of injury observed: oviposition pit and stain only; larval feeding gallery in bark and/or wood, without evidence of a tunnel; larval feeding gallery and a tunnel entrance into the wood, without evidence of a pupal chamber; larval feeding gallery, tunnel entrance, and pupal chamber, without evidence of emergence; larval feeding gallery, tunnel entrance, pupal chamber, and emergence hole; and unknown outcome because the tunnel continued into another log that was not available for assessment. The year each adult exit hole was made was later determined by referring to annual growth rings and following dendrochronology procedures similar to those reported by Sawyer (Reference Sawyer2007a), Favaro et al. (Reference Favaro, Battisti and Faccoli2013), Dodds et al. (Reference Dodds, Hull-Sanders, Siegert and Bohne2014), Turgeon et al. (Reference Turgeon, Orr, Grant, Wu and Gasman2015), and others (e.g., Todd Reference Todd2018).
Development of fieldsheet templates
Before the beginning of tree removal, the CFIA investigated the idea of collecting data digitally, but given the speed at which data collection needs were evolving and the cost and capabilities of technology available in 2003, the agency decided to use paper despite that medium’s associated problems. As a result, at the onset of the operation phase, the field crews used a paper data acquisition form that was designed to capture on one sheet all the information collected during stem removal for each tree removed. This created considerable paperwork for both field and office staff when a property had many trees, and it created the potential for misplaced or duplicated forms. At the end of March 2004, all known infested trees had been removed, and the focus shifted to follow-up activities, such as stump removal or stump treatment with herbicide, grass seeding, and fence removal, repair, or replacement at each property that had been treated. Each property had to be revisited to determine the work that was needed. To eliminate the need for multiple visits to a property – and to create a seamless link between research and operation – the data acquisition form was modified to track both tree removal and related work activities by property. On the revised form, each page could include information on up to 10 trees, as well as entries to record the type of work that would be needed to restore the property. The final template of the removal fieldsheet (Supplementary material, Appendix 3) was printed on a carbonless copy paper with a white original and yellow and pink copies. The white original sheet was submitted to the research team so that assessment of the infestation status could begin promptly. The operation team retained the yellow and pink sheets so that the various tasks could be assigned to the appropriate crews for action, based on the removal crews’ notes regarding the need for and type of additional work required on the relevant properties. Upon completion of all tasks on a property, the pink sheets were submitted to the CFIA for final data entry. The CFIA recorded the information it needed to fulfil its legal requirements and obligations, including the possibility of compensation. All other assessment data fieldsheets were intended for research purposes only and therefore are not discussed here.
Constant review of processes
The operation phase of the Toronto/Vaughan emergency response plan was modified a few times during this programme due to new knowledge that was acquired during the treatment and assessment of affected stems. As of 6 June 2008, 682 stems (Table 2), either containing live or dead specimens of A. glabripennis at various stages of development or bearing only signs or symptoms of injury consistent with this pest, had been found in the regulated area in the Greater Toronto Area. The elements of the operation plan that were reviewed were the number of suitable tree genera considered at high risk of injury and the radius of buffers around treated affected trees (see section, Changes to processes and protocols, below).
Table 2. Number of tree stems with signs or symptoms of injury caused by Anoplophora glabripennis and removed in each phase of the eradication programme of the Toronto/Vaughan and Mississauga/Toronto outbreaks, Ontario, Canada between 2003 and 2020.
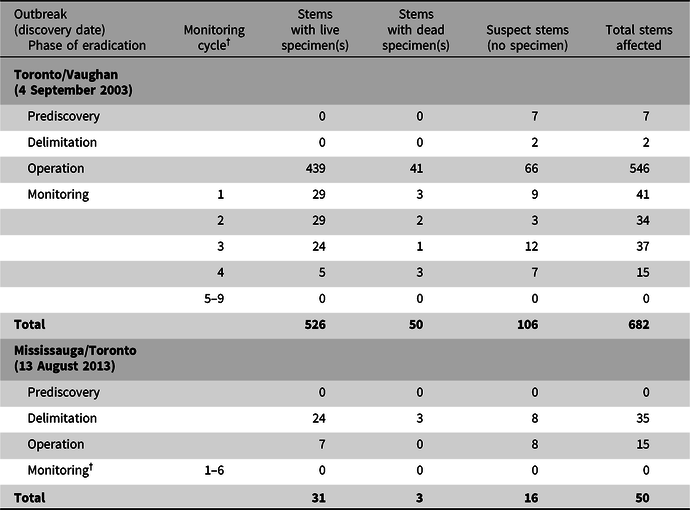
† The beginning of each monitoring or survey cycle coincided roughly with the predicted onset of adult emergence in the Greater Toronto Area, which occurred around 1 July. Each cycle ended on 30 June of the following year. For the Toronto/Vaughan and Mississauga/Toronto outbreaks, the first survey cycles began on 1 July 2004 and 1 July 2014, respectively.
Documentation of treatment activities and decisions
As mentioned earlier (see section, Operation plan and achievements), not all of the science subcommittee’s recommendations were incorporated exactly as advised into the operational plan that was implemented in November 2003. For example, one recommendation was that all trees at high risk of injury found within an 800 m radius of an infested tree should be removed. The CFIA instead chose to set the radius at 400 m from an infested or attacked tree. Another recommendation was to treat the core infestation and all three satellites similarly, whether signs of adult emergence had been found or not. The CFIA chose to remove only affected trees in two satellite areas that showed no evidence of A. glabripennis emergence – Beechwood and Thistletown. To increase the level of confidence in that strategy and to account for the fact that 100% detection was unlikely, intensive detection surveys in these satellite areas were made the highest priority in the final stages of the initial treatment (1 April–30 June 2004). The surveys uncovered more infested trees with oviposition pits in Beechwood. Following the discoveries, the CFIA treated the Beechwood satellite infestation similarly to the core infestation. Because no additional injured tree was found in Thistletown, no further tree removal took place there.
Overall, the CFIA removed about 12 000 trees (or 37 000 stems) from affected areas in the Toronto/Vaughan area between 20 November 2003 and 31 March 2004. Of the trees removed, at least 500 were affected (Table 3). By 7 March 2008, the day of the last treatment in that regulated area, about 29 000 trees or nearly 60 000 stems had been removed. However, these values are slight underestimates of the number of trees removed because discovery of injured trees in woodlots led to slight adaptations in protocols for these areas. In the woodlots, the field teams examined all trees for injury but recorded information only for those with stem diameters (D130) larger than 10 cm. Also, a few hundred trees of suitable genera within the regulated area were removed and destroyed by builders as part of an urban development before being tagged, with no information recorded as a result.
Table 3. Number (cumulative number) of unaffected tagged trees, unaffected stems, and affected stems removed during each calendar year while treating areas affected by Anoplophora glabripennis starting in 2003, when an outbreak was found in Ontario, Canada.
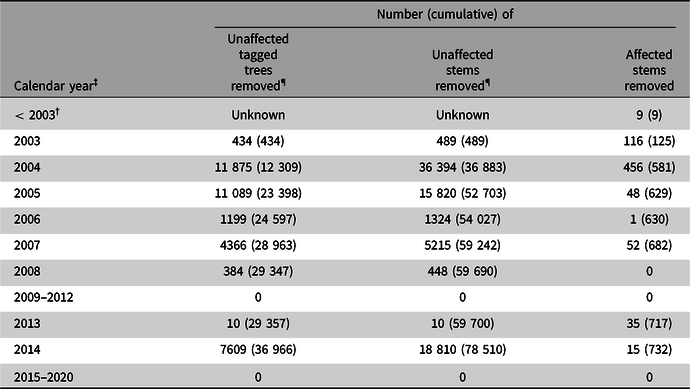
‡ January to December.
† These nine stems were stumps of maple trees with signs of injury typical of A. glabripennis that had been removed by landscapers or other stakeholders either before discovery of the outbreak or at the onset of the operation phase on 20 November 2003.
¶ The number of additional unaffected tags and stems removed with no date information was 359 and 412 in Toronto/Vaughan and 1167 and 2013 in Mississauga/Toronto.
As noted, some affected stems were apparently removed before A. glabripennis was officially detected, as demonstrated by the discovery in mid-September 2003 of seven stumps with signs of A. glabripennis attack. All of these stems were located at the discovery site (Tables 2 and 3). In addition, two affected stems fell during a storm in September 2003, shortly after the delimitation phase had started but before the operational phase had begun; both trees were shredded before any data could be collected (Table 2). When assessed, most (84%) of the affected stems contained specimens (dead or alive) of A. glabripennis (Table 2). The remaining stems contained only multiple signs and symptoms characteristic of A. glabripennis injury that were readily visible at the time the surveys were carried out.
Treatment options
Tree removal was the only type of treatment used in the Ontario A. glabripennis eradication programme. The research and operation teams jointly developed four removal options to account for all potential scenarios that were likely during the operation phase (Fig. 13). The first option guided removal when the entire property was within 400 m of an infested tree. The second one facilitated decision-making and increased transparency and consistency when deciding how to treat properties where trees at one end of a property were within the 400 m radius and those at the other end were not.
Fig. 13. Description of the four tree removal options developed to standardise treatment of properties with similar land use affected by Anoplophora glabripennis in Ontario, Canada between 2003 and 2013. Ravine edge, the area between the boulevard and an imaginary line parallel to the street and crossing the middle of the house or building; ravine top, the area between that line and the ravine (or backyard of property). See section, Treatment options, for a list of criteria used to assign properties or cells to an arbitrary level of risk of injury.
The remaining two options dealt with the treatment of properties bordering on or encompassing woodlots with or without ravines. At the programme’s start, it had been decided that such properties would be dealt with on a case-by-case basis. This approach was necessary because no precedent for how to treat such landscapes existed, the presence of A. glabripennis had the potential to importantly alter woodlot composition (species abundance and richness) and function (Dodds and Orwig (Reference Dodds and Orwig2011) later provided evidence to support those assumptions), and ravines were viewed as sensitive or precarious ecological landscapes. Extensive removal of trees in ravines, especially along their upper edges, increased the risk of erosion, which can lead to both property and environmental damage. To deal with these risks, the research and operation teams agreed that the information derived from each case would contribute to the development of tree removal options or procedures and rules specific to these two landscape types.
Development of a process to facilitate selection of a tree removal option accounted for the land use of the affected cell or property and the risk of infestation. First, each property was assigned to one of six recognised land use categories (commercial, industrial, institutional, open – including forested areas – residential, and transportation or utility; Clark et al. Reference Clark, Wallace and Earle2006) and to one of two risk levels. High-risk properties were those located within 800 m of the core infestation or of a satellite infestation where affected stems had exit holes and fresh oviposition pits, suggesting the infestation was two or more years old. Conversely, low-risk properties were those farther than 800 m from the core infestation or within 800 m of a satellite infestation with affected stems that had no exit holes. Second, a flowchart was developed that directed activities to one of four treatment scenarios deemed appropriate for the property (Fig. 13).
Treatment of forested areas: what we learned
Treatment of forested lands with ravines represented different challenges than did treatment of forested areas without ravines; therefore, different scenarios were developed for each type of situation.
Forested areas without ravines. The first instance of a woodlot being located within 400 m of trees with exit holes occurred in mid-September 2003 in Ansley Grove, at the southeast corner of Blue Willow Drive and Ansley Grove Road (hereafter referred to as “the Blue Willow woodlot”; 43.79030 N, 79.55997 W; 2.0 ha), Vaughan (Fig. 14). Based on treatment option 1, all suitable high-risk trees would have had to be removed. Rather than implement option 1 for treatment of a woodlot, tree climbers intensively surveyed the woodlot and found no evidence of injured trees. Next, field crews felled and re-examined all trees belonging to the 10 suitable high-risk genera (and with a D130 ≥ 10 cm) within the outer 10 m of the woodlot perimeter (Fig. 14). No sign of beetle injury was found; therefore, no further treatment took place. Conversely, discovery of an injured tree in that 10 m band would have triggered removal of another 10-m strip of trees along the woodlot’s edge. The goal of this approach was to find out if A. glabripennis first targeted the edges of densely wooded areas.
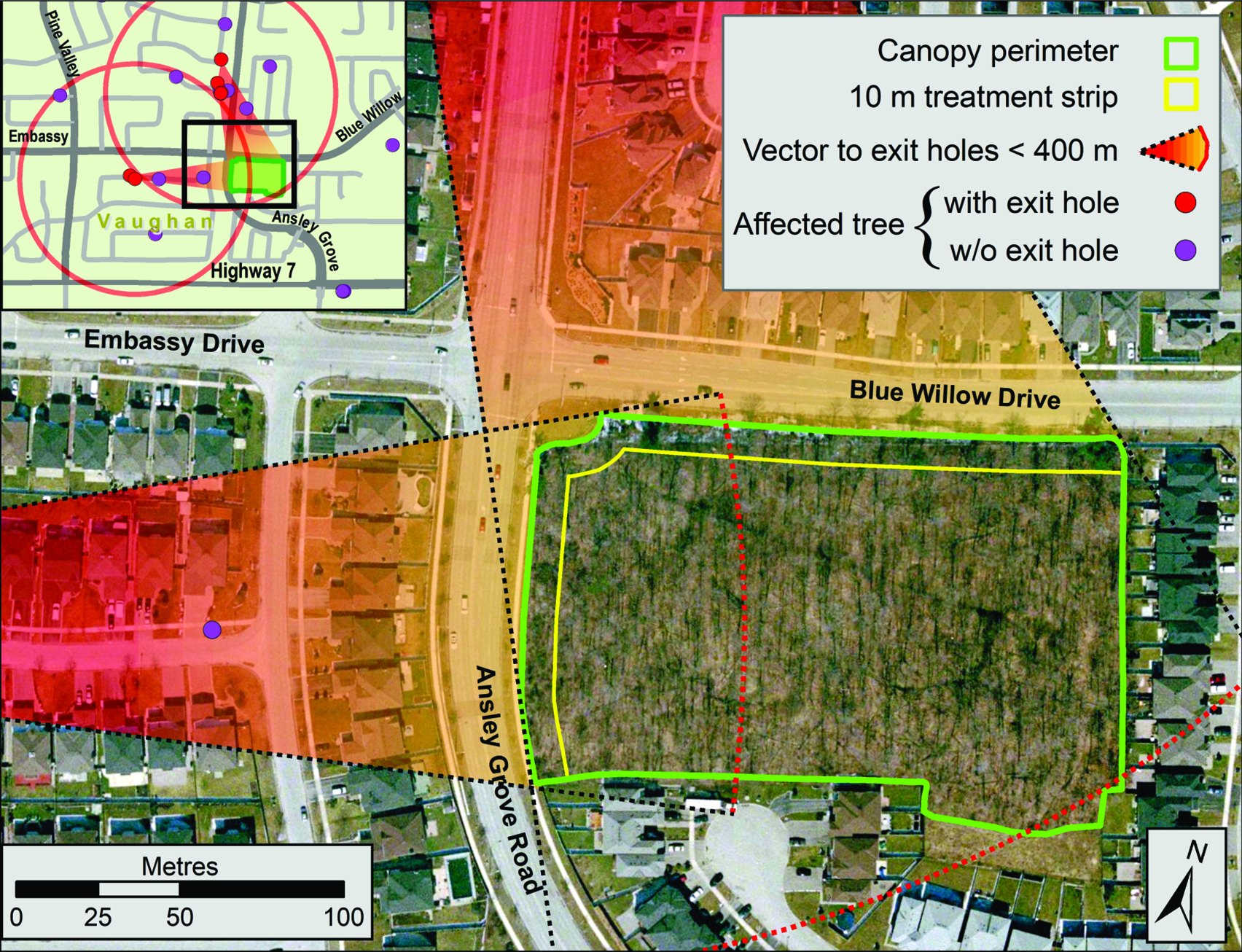
Fig. 14. Aerial view of the Blue Willow woodlot, illustrating the 10-m strip from the canopy perimeter where trees were removed in an effort to develop a treatment option for woodlots without ravines (i.e., whether it was necessary to treat the entire woodlot because it was within 400 m of a tree with an emergence hole). The inset shows the location of affected trees with and without (w/o) emergence holes within 400 m of the woodlot.
The approach was repeated on a second woodlot that also had a few trees with emergence holes outside the woodlot but within 400 m of its outer edges. This woodlot was in the northern end of the core infestation, on Aviva Park Drive (hereafter referred to as “the Aviva Park woodlot”; 43.77474 N, 79.55834 W; 5.7 ha; Fig. 15a), and was bordered by a 210-m-wide electrical transmission corridor and a railway to the south, an industrial park to the east, an 820 × 400-m field to the west, and a 200-m-wide transportation corridor to the north. Several injured trees were found within the woodlot’s outer 10-m strip (Fig. 15a). A second 10-m strip was removed, and again injured trees were found. A third 10-m strip was then removed, and more injured trees were found. At that point, the CFIA decided to treat the entire woodlot. Subsequently, when studying the location of injured trees within the Aviva Park woodlot in relation to removed trees that had diameters (D130) no smaller than 10 cm, most injured trees (28 of 36) were found within 10 m of the woodlot’s outer canopy edge (Fig. 15b), and the remainder were within 19.7 m of the canopy edge. Affected trees were located, on average, 7.2 ± 0.8 m from the canopy edge.
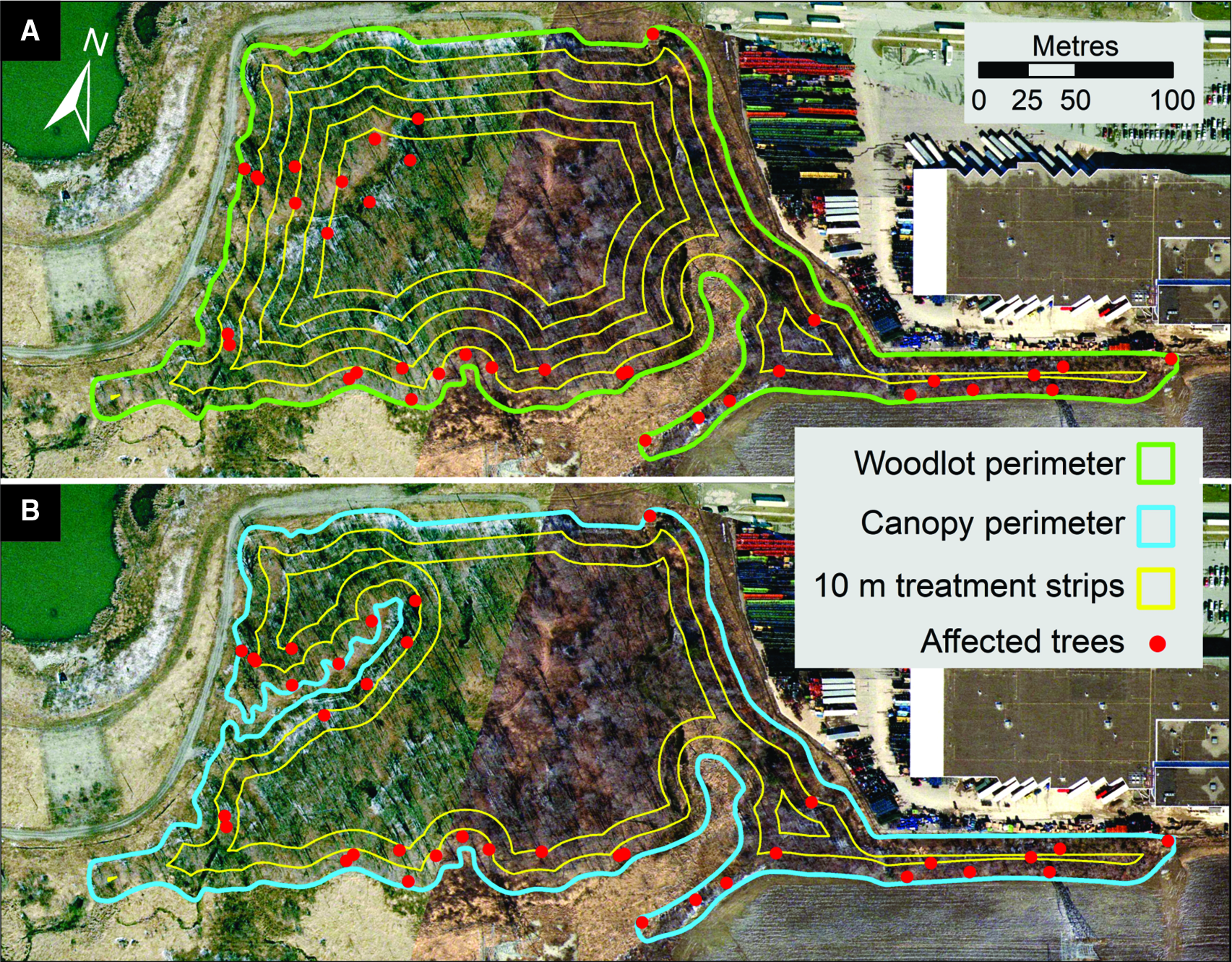
Fig. 15. Aviva Park woodlot. A, Aerial view, illustrating the woodlot perimeter and the five 10-m strips from the perimeter where trees were removed sequentially in an effort to develop a treatment option for woodlots without ravines (i.e., whether it was necessary to treat the entire woodlot). B, Aerial view of the location of affected trees within proposed 10-m bands based on the outer edge of the stand canopy instead of the woodlot edge.
This information was used to develop a tree removal option for woodlots without ravines. The CFIA used treatment option 3 to treat another infested woodlot located at Emery Yard (Fig. 13). In that woodlot, all suitable high-risk trees with a minimum 10-cm diameter (D130) and located within 50 m of each infested and attacked tree were removed. No additional affected trees were found at that site during that removal or during subsequent monitoring surveys.
Forested areas with ravines. A few instances occurred where a tree with at least one emergence hole was located within 400 m of a forested ravine. Because of ravines’ precarious terrain and because any type of treatment implemented in a ravine could have substantial operational, property, and environmental consequences, the goal was to avoid treating forested areas within ravines unless irrefutable evidence showed that trees in the ravine were injured by A. glabripennis. Therefore, to develop option 4, the research and operation teams examined the data on the locations of injured trees on various property types. Most such trees were found in industrial, commercial, and residential landscapes (Table 4) and were located between the street and the house or building on the property next to a driveway. Few of the injured trees were found in side or backyards. That information was used to design a protocol consistent with this information and specific to this landscape type (Fig. 13). For this protocol, “ravine edge” was defined as the edge of the nearest street. More specifically, “ravine edge” was defined as the area between the street curb and an imaginary line parallel to the street and crossing the middle of the house or building. This definition would apply to all ravines surrounded by developed land. By defining the ravine edge as the street edge, the treatment option for wooded ravines could now be applied without compromising the ravine’s structural integrity. It was recognised that, should properties with different characteristics ever be encountered, a different definition may be required. “Ravine top”, on the other hand, was defined as the area between the imaginary line crossing the middle of the house or building and the physical edge of the ravine. In operational terms, this meant that all suitable high-risk trees located on the ravine edge (front yard) would be removed and examined for signs of injury but high-risk trees in the ravine top (backyard) area and those in the ravines would remain in place and would be intensively monitored. This approach eliminated most of the risks associated with removing trees located within a ravine or trees in the backyards of properties at a ravine’s edge unless they were affected. This option for the treatment of wooded areas with ravines was applied subsequently to all properties adjoining ravines. No tree of interest was ever found on a ravine top or ravine edge.
Table 4. Comparison of percentages of stems affected by Anoplophora glabripennis among various types of land use within the regulated area of the Toronto/Vaughan outbreak, Ontario, Canada between 2003 and 2013.
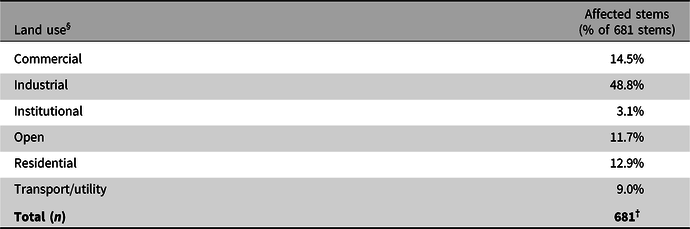
§ Listed alphabetically according to Clark et al. (Reference Clark, Wallace and Earle2006).
† Land use could be confirmed for 681 of the 682 affected stems.
Timing of treatment
Because the discovery of the infestation had occurred in the summer of 2003, the first wave of treatment, which included the removal of affected and suitable high-risk trees, occurred during the winter of 2003–2004, before the expected onset of adult beetle emergence in summer 2004.
The discovery of more affected trees in September 2004 raised the issue of whether treatment of affected and unaffected trees should always occur in winter, irrespective of the time of year that trees of interest were found after the first treatment. The science subcommittee recommended that all trees of interest in residual populations, irrespective of their infestation status, be removed soon after they were found and that treatment of unaffected high-risk trees be scheduled during winter. Removing trees of interest immediately would provide opportunities to confirm the tree’s infestation status, to determine the extent of the infestation, to prevent additional beetle emergence, feeding, mating, oviposition, and dispersal (especially if the infestation was discovered in summer), to notify affected property owners, and to plan treatment operations.
Indeed, some live A. glabripennis adults were recovered on the stems of trees felled in August 2005. This suggested that felling trees in summer, after adult beetle emergence had begun, would not necessarily result in dispersal of all emerging beetles. It also suggested that an immediate inspection of the bole and branches of trees felled during summer could remove both the immature population still developing within the tree and any A. glabripennis adults found on the tree. Removing injured trees at the time of discovery would also prevent reinvasion between seasons (Fournier and Turgeon Reference Fournier and Turgeon2017).
Postponing treatment of surrounding high-risk trees until winter would provide an opportunity to re-examine them and to determine if their infestation status – “unaffected” at the time of the infestation’s discovery – had changed. If it had, boundaries of the affected area were adjusted to satisfy the 400 m rule, and tree removal expanded accordingly.
Changes to processes and protocols
Three modifications to the removal protocols occurred during the operation phase of the eradication programme. These included an increase in the number of tree genera categorised as suitable, a decrease in the number of genera considered at high risk of injury, and changes to the size of the buffer around affected trees.
Number of suitable genera. Starting in 2003, the CFIA had considered 10 genera of broadleaf trees to be suitable for the complete development of A. glabripennis in Canada (see section, Targeted hosts, above). In July 2011, the CFIA added two genera, each of which is represented by a single species and was previously classified as of unknown suitability, to its list of suitable and regulated genera (CFIA 2016). The addition of Cercidiphyllum japonicum Siebold and Zuccarini (Cercidiphyllaceae) and Koelreuteria paniculata Laxmann (Sapindaceae) was based on evidence that A. glabripennis had completed development on C. japonicum in the United States of America (National Plant Board 2009) and on K. paniculata in China under field conditions (National Plant Board 2011).
Number of high-risk genera. At the onset of the response phase in November 2003, the CFIA assumed all 10 suitable genera were at high risk of injury by the beetle. In January 2007, after a review of removal data by the science subcommittee, the CFIA shortened that list from 10 to four. The decision was based on evidence that no tree of the genus Albizia was present in the regulated area, that no trees from the genera Aesculus, Celtis, Platanus, and Sorbus had been affected (Turgeon et al. Reference Turgeon, Smith, Pedlar, Fournier, Orr and Gasman2022), that only three of the more than 10 000 available stems of Ulmus had been infested, and that only one stem each of two genera of questionable suitability (Fraxinus and Tilia) and no stems from genera deemed unsuitable within 400 m of trees with an emergence hole had been injured (Turgeon et al. Reference Turgeon, Jones, Smith, Orr, Scar and Gasman2016). This evidence removed support for the argument that each of these suitable genera was at high risk of injury.
The science subcommittee’s resulting recommendation was to consider these six so-called “suitable” genera as representing a moderate risk of injury instead and to treat them differently. As such, trees of these genera would no longer be removed when an affected area was being treated unless they were affected; however, they would be intensively surveyed in buffer 1 zones. The reduced number of high-risk genera led to an overall decrease in the number of trees to remove during treatment. This decrease shortened the operation phase and reduced equipment and staff requirements, thereby also lowering operation costs.
One drawback of reducing the number of high-risk genera was that the affected area could no longer be considered “sanitised” (see section, Evaluation of operation efficacy, below, for definition) because it would contain stems from six suitable moderate-risk genera. To ensure these stems would not provide A. glabripennis with an avenue to escape detection, the monitoring phase was modified to include intensive surveys of all moderate-risk genera in affected areas. The two genera added in 2011 to the list of suitable genera were also categorised as moderate risk. The CFIA considered all other tree genera of unknown or questionable suitability as low risk.
Size of affected area. From 2003 to 2006, the radius of affected areas for infested or attacked stems was set at 400 m from an infested or attacked tree, and just the tree itself for suspect trees. Starting in January 2007, the CFIA used the information collected in the previous years to justify a reduction of the radius of affected areas in the Toronto/Vaughan regulated area from 400 m to 200 m when the number and distance between affected trees suggested it was a small, residual infestation. This reduction would not apply if the infestation status suggested it was a new core infestation.
Health and environmental implications
Typically, several key actions that address health and environmental concerns must be undertaken when planning to eradicate a pest. These include identifying the health implications of the proposed response, evaluating the public acceptability of the proposed materials and methods, and performing other assessments, which could include initiating the necessary health risk assessments, establishing preferred materials and delivery systems, and initiating environmental impact assessments for pesticide and application methods.
Identifying the health implications of the proposed response
As indicated earlier (see section, Recommended actions), the proposed response was the removal of all infested trees and any surrounding trees belonging to high-risk genera. This plan limited the known health risks to a reduction in ecological services (e.g., summer cooling) provided to residents by trees removed from affected areas and to the physical safety of staff involved in tree removal and monitoring surveys throughout the calendar year. To address these health implications, health and safety committees were established for field crews. The extent of reduction in summer cooling for residents was not assessed.
Two lost-time injuries occurred during the eradication plan’s operation phase. The first involved an arborist using a chainsaw while working from the bucket of an aerial tower. The chainsaw slipped out of one hand and injured the arborist’s other arm. The Ontario Workplace Safety and Insurance Board investigated the accident and found no wrongdoing (J. Doyle, unpublished documentation). This accident had no impact on the other activities of the operation.
The second injury involved an arborist–climber who fell to the ground on 26 April 2004, while inspecting a large tree for signs of A. glabripennis. The injured climber was taken to the hospital and released the same day. Because of its nature, this accident was reported to the Ontario Ministry of Labour, which sent a representative the next day to investigate. The investigator issued two orders to the City of Toronto: the first order dealt with the climbing system (split tail) used by the climber; the second was a stop-work order until withdrawn. While being investigated by the Ministry of Labour, the City of Toronto fall protection procedures were reviewed by three outside parties. The review concluded that the City of Toronto’s standard practices and equipment used were equivalent or exceeded the standards for fall protection used within the industry. The City of Toronto appealed the Ministry of Labour orders. The stop-work order was withdrawn on 5 July 2004. This accident impacted survey operations carried out before the monitoring phase’s start and was not limited to the eradication programme; the stop-work order affected the more than 120 arborists employed by the City of Toronto and all those working under contract for the city.
Evaluating the public acceptability of the proposed material and methods
Most encounters with the public occurred before the eradication plan was finalised. The CFIA used public meetings to inform residents living in affected areas of the proposed eradication plan and to obtain feedback on it. Most property owners accepted the concept of treating affected areas rather than just injured trees, finding it a reasonable approach to reach the goal of eradication; a few, however, were vehemently opposed to the removal of trees at risk and considered it totally unacceptable and destined to fail.
Indeed, most citizens agreed that the approach of removing all affected trees rather than treating them with insecticides made sense, given our knowledge of A. glabripennis at the time. For several citizens and elected officials, however, the most contentious part of the plan was the proposed removal of all trees belonging to high-risk genera within 400 m of an infested or attacked tree. Their view was that the removal of these seemingly “healthy” trees was pointless, especially if there was no compensation. Instead, these opponents wanted the CFIA to inject high-risk trees with insecticides as a means to control the beetle. These individuals continually challenged the validity of the proposed removal of trees surrounding affected trees to contain and control this outbreak. They held the opinion that an insecticide should be applied to treat apparently unaffected trees, as had been done in the earlier outbreaks in the states of New York and Illinois, instead of removing trees, as had been done in New Jersey. This lack of acceptance was also linked in part to a general lack of trust in the CFIA because of similar yet ineffective approaches taken with other invasive species (e.g., emerald ash borer; Mackenzie and Larson Reference Mackenzie and Larson2010). At the same time, many citizens opposed the use of any insecticide to control A. glabripennis in affected trees, especially near ravines.
Some property owners expressed concerns that the removal of trees in the selected genera could lower property resale values. This risk was not monitored. However, not every citizen liked all the trees on their properties. For example, during the surveys for A. glabripennis, inspectors found a tree on one property into which a hole about the size of a real emergence hole had been drilled in the trunk, presumably in an attempt to trigger tree removal activities. The inspectors recognised that this was not an A. glabripennis emergence hole (see Ric et al. Reference Ric, de Groot, Gasman, Orr, Doyle and Smith2007, photo 228).
Another factor cited for some individuals’ cool reception to certain aspects of the response plan was the absence of a tree replacement plan or compensation. A tree replacement programme was announced on 12 May 2004, two months after tree removal in the core and one satellite infestation area had been completed. It was hoped this new component of the emergency response plan would also increase public engagement, making citizens more likely or willing to report additional beetle finds.
Performing other assessments
For the reasons listed above (see section, Identifying the health implications of the proposed response), no health risk assessment was carried out. Furthermore, because the CFIA’s proposed eradication response against A. glabripennis did not include pesticides, there was no need for other health or environmental assessments (e.g., establishing a list of preferred materials and delivery systems and initiating environmental impact assessments for pesticide and application methods). Research on the environmental impact of pesticide application was already underway, however, and later determined that injecting tree stems with imidacloprid to control A. glabripennis–injured trees results in senescent leaves with residue levels sufficient to reduce natural decomposition processes in aquatic and terrestrial environments through adverse effects on nontarget decomposers (Kreutzweiser et al. Reference Kreutzweiser, Good, Chartrand, Scarr and Thompson2008b). In addition to these trials, the stems of about 100 trees were injected with Confidor, a formulation of imidacloprid, in an experimental trial designed to compare the uptake and fate of this insecticide in each of the various tree genera considered suitable for the development of A. glabripennis. The results from this study have yet to be published.
Documentation
The CFIA prepared its first overall management plan on 31 March 2004 (CFIA, unpublished) and then subsequently updated it (CFIA, unpublished). These plans summarised the various activities and methods to be used and the public awareness objectives and were endorsed by the agency’s Director of Plant Protection.
Few documents detail the chronology of events related to the discovery and eradication of A. glabripennis in Ontario and the type of issues arising as the eradication programme unfolded. This section’s focus therefore is primarily on describing the operational and scientific events and the issues that arose during this operation.
The CFIA received a copy of all 10 reports prepared by the science subcommittee for the area response team leader. In these documents, the subcommittee typically answered specific questions raised by the area response team leader by providing the facts as they were known at the time and a list of recommendations on potential courses of action based on that evidence.
As mentioned above (see section, Changes to processes and protocols), the CFIA adjusted its policies on treatment and monitoring of trees surrounding those affected by the beetle. More specifically, the CFIA reduced the number of host genera to target for removal and reduced the size of the affected area for residual infestations. These adjustments, which were implemented in January 2007, led to changes in the amount and type of data available for research over time. For this reason, tree data were grouped on the basis of discovery year – trees discovered between September 2003 and December 2006, and trees discovered between January 2007 and December 2007 (Table 5; Fig. 16).
Table 5. Size of affected areas and number of main stems available to Anoplophora glabripennis by category of tree suitability within each period of time affected trees were discovered in Ontario, Canada.
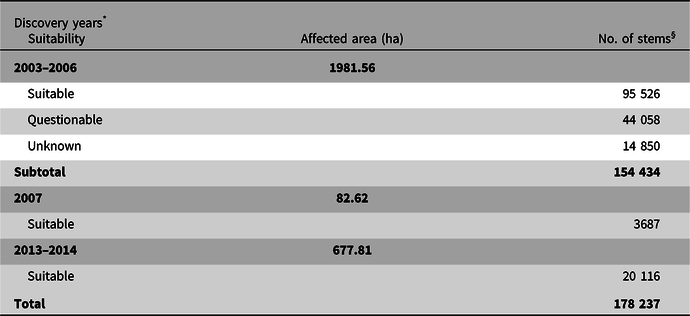
* Radii (r) of affected areas varied among discovery years. It was 400 m for those discovered between 2003 and 2006, 200 m for those discovered in 2007, and 800 m for those discovered in 2013 and 2014. See text for details.
§ Of the 178 237 stems available within r, 7456 stems were dead (5082), without a valid D130 (1693), or without a genus (1188); 507 stems suffered one or more of these three conditions. D130, diameter of tree at 130 cm above ground.
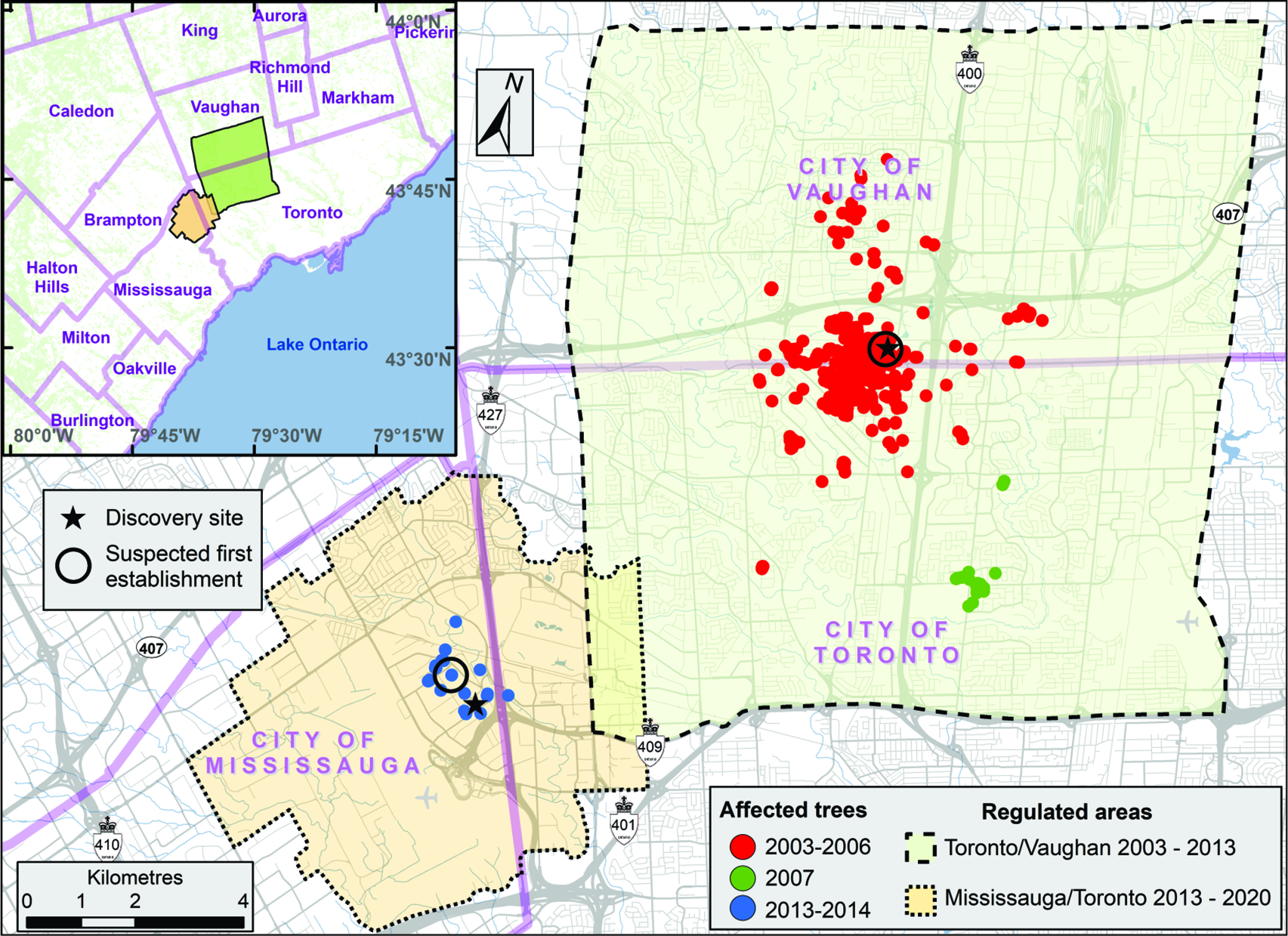
Fig. 16. Locations of Anoplophora glabripennis–affected trees discovered between August 2013 and April 2014 in the Mississauga/Toronto regulated area (blue dots in orange-shaded area) in relation to those found between September 2003 and December 2006 (red dots) and between January 2007 and December 2007 (green dots) in the Toronto/Vaughan (green-shaded area) regulated area. The inset shows the locations of the Toronto/Vaughan and Mississauga/Toronto regulated areas in the Greater Toronto Area.
Financial management
In 1999, the CFIA had met with, received input from, and conducted emergency simulations with potential cooperating organisations across Canada while preparing its draft emergency response plan (see section, Detection preparedness and facilitation). These agencies were invited to meet again with the CFIA to be briefed on the discovery of A. glabripennis before the emergency response plan was released to the public. At the meeting, the organisations – which included provincial and federal agencies – offered, on an ad hoc basis and without requesting compensation, staff who could be shared and trained quickly to help the CFIA’s Plant Protection staff and staff from other CFIA programmes to complete the delimitation surveys.
Resourcing for an eradication programme is not a normal line item in a regional budget for any agency, including the CFIA. Several steps must be taken before special resourcing is provided. Once the CFIA declared an emergency, the agency’s regional office was allocated additional financial resources to meet the increased staffing needs for the programme’s operation and monitoring phases. Access to these resources would be challenging using government hiring practices. Therefore, the CFIA received assistance from the affected municipalities and other organisations to handle these challenges. In addition, the municipalities facilitated access to specialised equipment, buildings, and sites (e.g., Emery Yard) to implement the emergency response plan.
Monitoring phase
The primary objective of a monitoring phase in an incursion response plan is to determine the impact or assess the success of the response strategy on the target organisms (Hosking Reference Hosking2001). Activities carried out during this phase should also make it possible to evaluate progress towards eradication. In terms of the A. glabripennis infestations in Ontario, key elements of this phase included evaluation of the operation’s efficacy, pest population monitoring, financial management, health and environmental monitoring, and community support monitoring. As indicated (see section, Operation phase), detection of residual A. glabripennis populations during the monitoring phase led to a temporary and partial return to the operation phase, and once additional treatment was completed, the programme reverted completely to monitoring activities. This sequence continued until the regulated area was declared pest-free.
Evaluation of operation efficacy
One aspect of the operation phase that needed to be evaluated critically was whether the treated areas had indeed been sanitised of all high-risk host material – or fully treated – as expected. Between 2003 and 2006, a property was considered “sanitised” when all trees within any of the 10 suitable high-risk genera had been removed and all affected stumps had been either removed or sprayed with an herbicide to prevent sprouting. Beginning in January 2007, a fully treated property was one where all four suitable high-risk genera had been removed and stumps had been treated but moderate-risk genera remained.
To evaluate the integrity of the treatment activities, the CFIA performed two types of sweeps – stump sweeps and sanitisation sweeps. Stump sweeps were conducted in affected areas between the end of treatment and the start of the first monitoring cycle – specifically, between 31 March and 1 July – to confirm that all stumps of treated suitable high-risk trees had also been treated adequately. These sweeps became a routine process after a dozen stumps with signs of A. glabripennis injury were found the first time this survey was undertaken in April 2004. The lingering signs of injury had escaped earlier notice because snow accumulation had prevented a complete examination of the base of trees at the time of tree removal.
The objective of the sanitisation sweeps was to confirm that properties classified as sanitised after treatment remained free of suitable high-risk hosts at the end of the monitoring phase. This sweep consisted of a systematic inspection of all treated properties considered sanitised and was carried out during the penultimate cycle of the monitoring phase. No sign of A. glabripennis injury was found on the few suitable high-risk trees found in these sanitised areas. This evaluation also revealed that, contrary to instructions to property owners, some trees of suitable high-risk genera had been replanted on private properties within the treatment area soon after the end of treatment.
Pest population monitoring
The availability of technology for efficient detection of an alien pest at low densities (Bulman et al. Reference Bulman, Kimberley and Gadgil1999; Myers et al. Reference Myers, Simberloff, Kuris and Carey2000; Mehta et al. Reference Mehta, Haight, Homansa, Polaskya and Venette2007; Tobin et al. Reference Tobin, Kean, Suckling, McCullough, Herms and Stringer2014) and the ease of detection (Food and Agriculture Organisation of the United Nations 2016) are two of several factors that influence the duration and success of eradication programmes (Fournier and Turgeon Reference Fournier and Turgeon2017). At the time of the 2003 discovery of A. glabripennis in Ontario, progress was being made towards the development of a trapping system for this insect (Nehme et al. Reference Nehme, Keena, Zhang, Baker, Xu and Hoover2010, Reference Nehme, Trotter, Keena, McFarland, Coop and Hull-Sanders2014; Wickham et al. Reference Wickham, Xu and Teale2012; Meng et al. Reference Meng, Trotter, Keena, Baker, Yan, Schwartzberg and Hoover2014), but no effective long-range attractant was yet available that would have increased detection efficacy. This left only visual inspections of tree boles and branches as a detection tool. Because of this, the main activity of the monitoring phase was to visually inspect trees throughout the regulated area. As indicated earlier (see section, Survey design and specifications), the experience in other jurisdictions indicated that the accuracy of visual inspections was low, especially when the densities of the beetles and their related signs of injury were low, and that it varied with the techniques used and with the time of year when inspections were performed (J. McCarthy, City of Chicago, personal communication). The key elements of this monitoring phase therefore involved developing a monitoring strategy that took into consideration the existing limitations, developing a plan and determining its duration, summarising what happened during monitoring, and validating the survey techniques and results.
Developing a monitoring strategy
The lack of sensitive detection tools meant that the CFIA’s monitoring strategy had to incorporate redundancies to maximise detection of the pest and its signs (Myers et al. Reference Myers, Savoie and van Randen1998; Hosking Reference Hosking2001; Panetta and Lawes Reference Panetta and Lawes2007; Brockerhoff et al. Reference Brockerhoff, Liebhold, Richardson and Suckling2010b; Food and Agriculture Organisation of the United Nations 2016). As such, the strategy would have to include repeated visual examinations of untreated trees within the entire regulated area by different inspectors over multiple years – equivalent to at least two complete life cycles of A. glabripennis – to increase the likelihood of finding further signs or symptoms in A. glabripennis–injured trees. Also, the strategy would have to rely on a variety of approaches to visually inspect the bark on each tree’s bole and branches because shapes, sizes, and appearances of signs and symptoms changed over time and not all signs were readily apparent from the ground or easily discernible – especially when signs were old and densities were low (Ric et al. Reference Ric, de Groot, Gasman, Orr, Doyle and Smith2007; Haack et al. Reference Haack, Hérard, Sun and Turgeon2010). Finally, the strategy had to take into consideration the financial and human resources available. This proved difficult to implement and time consuming to complete because the size of the regulated area, which expanded beyond buffer 2, was too large to allow intensive surveillance of every tree on an annual basis, given the available resources. To give the CFIA some level of confidence that the regulated area eventually could be declared pest-free based only on visual inspections, the examinations had to be rigorous and systematic. A monitoring plan was needed.
Developing a monitoring plan and determining its duration
When the Toronto/Vaughan outbreak was discovered, the United States Department of Agriculture had already developed and implemented three types, or levels, of survey that were based on distance from an infested tree (Haack et al. Reference Haack, Hérard, Sun and Turgeon2010; United States Department of Agriculture 2014). The survey plan and methodology used by the CFIA during this phase were based roughly on some components of these three survey levels.
All of the surveys conducted during the Ontario outbreak, regardless of the programme phase during which they were performed, had common elements. They included considerations of what hosts should be targeted, where, when, and how frequently the surveys should take place, and how the surveys should be conducted. These essential elements would affect survey quality, treatment options, treatment duration, recovery plans and activities, and programme costs.
The development of the CFIA’s monitoring plan comprised six steps. The first step was to subdivide the regulated area into 2059 manageable and easily visualised spatial units, or cells. These cells were not the same as the grid-based cells used for surveys carried out during the delimitation survey; instead, they were designed to follow anthropogenic and landscape features to assist inspectors in maintaining their whereabouts during the surveys, relative to their assigned work areas and to separate different land uses, which often had different operational needs.
The second step saw each cell assigned to an infested area or to one of the buffers defined for the mitigation phase. In the third step, each cell was assigned one of the four survey intensities recommended for buffers. In addition, minimum survey requirements were established. As before, survey intensity would decrease as distance from an injured tree increased (Fournier and Turgeon Reference Fournier and Turgeon2017). Survey requirements would have to be consistent with the risk associated with each affected area and buffer, based on knowledge of the beetle’s host suitability, selection, and location relative to affected trees (i.e., visual survey intensity should decrease as distance increased), and be undertaken with available human and financial resources.
In step four, the survey cycles were defined. It was impossible to survey the entire regulated area within a year given the number of trained staff available. A survey cycle was defined as the period starting around the approximate date of first adult emergence in a calendar year and ending one year later. Smith et al. (Reference Smith, Tobin, Bancroft, Li and Gao2004) predicted that the onset of adult emergence occurred each year when about 400 degree-days above a threshold of 10 °C had accumulated since 1 January. In Ontario, this accumulation occurs around 1 July (Fig. 17); therefore, each survey cycle would begin on 1 July and end the following year on 30 June.
Fig. 17. Degree-day accumulation above a 10 °C threshold in each year between 1995 and 2004 recorded at the Toronto Pearson International Airport weather station. For that 10-year period (grey lines), the minimum, maximum (solid black lines), and mean annual (dashed black line) accumulations were 1256 degree-days (1997), 1641 degree-days (2002), and 1454 degree-days, respectively.
Step five addressed the need to establish survey priorities for each cycle and survey requirements for each cell before it could be considered pest-free. Survey priorities for each cycle were based on the outcome of survey activities carried out during the previous cycle. In brief, if additional affected areas were discovered during the earlier period, buffer zone boundaries would be adjusted and all cells within 800 m of an infested tree would see their progress towards survey requirements reset to zero. If no additional affected area was discovered, survey priorities for each cell would be set by a survey model. Survey requirements for a cell would be based on two pieces of evidence. The first was the awareness that some A. glabripennis specimens spent at least two winters in the wood of infested trees before emergence (Fig. 18). The second piece of evidence was data showing that both the number of affected trees and the number of signs of injury per affected tree in the satellite infestations were much lower than in the core area (Table 6). For a cell to be considered A. glabripennis-free, it was agreed that all suitable hosts would have had to have been surveyed, with no A. glabripennis found, at least three times in three survey cycles carried out over a span of at least five years since the cell had first been assigned to an area or buffer (Fournier and Turgeon Reference Fournier and Turgeon2017).
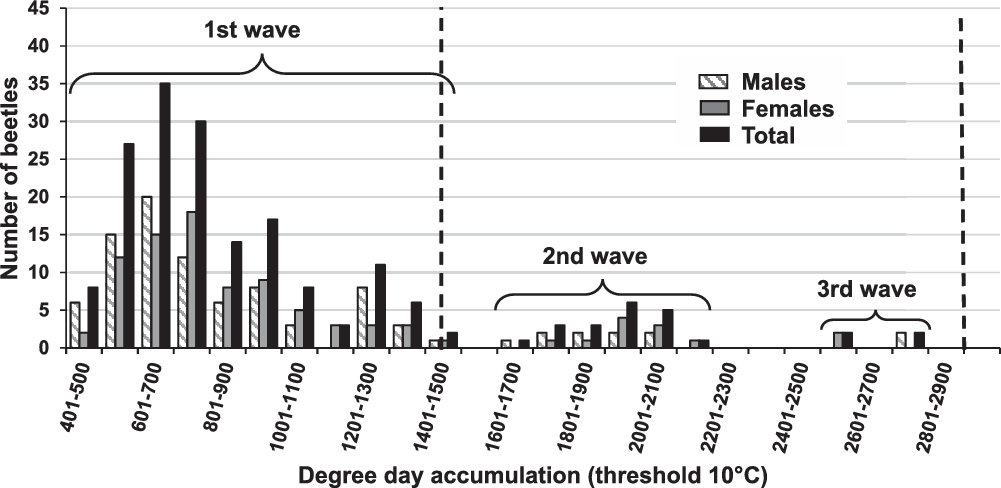
Fig. 18. Number of female (n = 91) and male (n = 93) Anoplophora glabripennis emerging from infested logs collected in Toronto/Vaughan and kept in a containment facility as a function of degree-day accumulation above 10 °C – the development or activity threshold for many life stages of A. glabripennis (Keena Reference Keena2006; Keena and Moore Reference Keena and Moore2010). The vertical dashed lines represent the average annual degree-day accumulation recorded at the Toronto International Airport weather station between 1995 and 2004. Average female emergence in rearing facility lagged behind that of males by 42 degree-days for all three waves combined.
Table 6. Number of stems injured by Anoplophora glabripennis that were infested, attacked, or suspect in each infestation discovered within the regulated area of the Toronto/Vaughan, Ontario outbreak between September 2003 and May 2013, and the timing of these discoveries†.

† The beginning of each monitoring or survey cycle coincided roughly with the predicted onset of adult emergence. In the Greater Toronto Area, this occurred around 1 July. Each cycle ended on 30 June of the following year. For the Toronto/Vaughan outbreak, the first survey cycles began on 1 July 2004.
Step six dealt with the duration of the monitoring phase: the phase would end when all cells of the regulated area had been declared pest-free.
To spatially and temporally track the outcome and progress of monitoring surveys in the regulated area, a spatial decision support system was developed and parameterised with data collected by the CFIA during the operation phase and with information based on existing policy and scientific and expert knowledge (Fournier and Turgeon Reference Fournier and Turgeon2017). The system was used to support the effective and efficient allocation of survey efforts, forecasting, and reporting of surveillance activities and to ensure that established requirements were met within the shortest period possible.
The plan for the monitoring phase also included surveillance activities outside of the regulated area to support the CFIA’s claims of those areas’ pest-free status. The science subcommittee recommended that area-wide grid-based surveys across Canada, interrupted in 2001 because of the arrival of plum pox virus, be resumed as soon as possible, especially in high-risk areas such as those where A. glabripennis interceptions had occurred earlier (Table 1). An area-wide grid-based survey protocol that maximised the likelihood of detecting an infestation of about the same size as that discovered in Ontario was developed and implemented as a pilot project in Ontario in 2008 and across Canada in 2009 (Bullas-Appleton et al. Reference Bullas-Appleton, Fournier and Turgeon2021).
Documentation of monitoring activities and decisions
The monitoring phase began on 1 July 2004 and followed the monitoring and operation plans and rules for nine survey cycles. The surveys carried out during this phase were performed by teams of inspectors looking for the beetle or its signs and symptoms of injury, in the manner previously noted (see section, Survey design and specifications). As happened during the delimitation survey, forest health officers performed visual inspections from the ground of the bark surfaces of exposed roots (if any), bole, and main branches larger than 2.5 cm in diameter for evidence of A. glabripennis injury (Ric et al. Reference Ric, de Groot, Gasman, Orr, Doyle and Smith2007), and arborists certified to climb trees focused on stems and branches that could not be fully inspected from the ground.
A field study was also conducted in the first monitoring survey cycle to assess the relative attractancy, feasibility, and practicality of using two types of sentinel trees – potted saplings and landscape trees – to detect A. glabripennis. Previous studies (M.T. Smith, unpublished data) had identified a species of tree that was highly attractive to free-flying adult A. glabripennis, Acer pictum subsp. mono (Maximovich) Hiroyoshi Ohashi. Three types of sentinel trees were deployed in affected areas. The first type consisted of two side-by-side pairs of potted 1- to 1.5-m-tall Acer pictum and Acer platanoides Linnaeus treated with the insecticide permethrin. Between each potted tree from the same pair was a permethrin-treated 12-unit Lindgren funnel trap to increase the visual attractiveness of the trees. The second type of sentinel-tree unit consisted of side-by-side 2-m-tall potted Acer platanoides, the tree that was most frequently injured in the Toronto/Vaughan infestation. The third type of sentinel-tree unit consisted of a landscape tree (D130 >10 cm) of either the genus Tilia or the genus Elaeagnus and a pair of potted Acer mono and Acer platanoides in each hemisphere. No A. glabripennis adult was caught in the traps, and none of the potted trees showed signs of injury.
Most of the 682 affected trees found in the Toronto/Vaughan regulated area were removed before the start of the monitoring phase. Ninety-two affected stems were discovered during the first three survey cycles, impacting survey priorities for the first four cycles (Table 6). Each discovery triggered an immediate pretreatment survey of all four high-risk and six moderate-risk genera within a 400-m radius until no new affected trees were found. Only trees of interest discovered during this pretreatment were removed within days of discovery, regardless of the time of year, to ascertain their infestation status. Unaffected trees within that radius were left standing until treatment of the affected area could begin. This practice provided residual adults that might have emerged and flown away before or during pretreatment removal with prospective oviposition sites in nearby trees. The number of affected trees and the extent of injuries were used to determine whether the new infestation was that of a residual population or a new core infestation. All new infestations were determined to be residual infestations. The next steps were to identify the most appropriate period for treatment of the new infestation and to issue and deliver notices to dispose to the affected property owners. During treatment, all felled trees were re-examined before chipping in an effort to locate additional injured trees. The discovery of a few additional infested trees during treatment of new affected areas also resulted in a further expansion of the affected areas.
All residual infestations consisted, similarly to the satellite infestations discovered in 2003 (Table 6), of a few infested trees with viable A. glabripennis life stages, but they mostly contained only fresh oviposition pits or recent (i.e., current-year) emergence holes with or without oviposition pits. Conversely, the core infestation had contained many infested trees (> 300) with many viable life stages (turgid egg, larva, pupa, and adult stages) and most signs of injury (e.g., exit holes, frass or shavings, exposed feeding galleries, oviposition pits, etc.), which suggests the beetles had been present for many years.
As mentioned above (see section, Changes to processes and protocols), three key aspects of the removal protocol were modified during the operation phase. The consequence of these changes led to the following concomitant changes during implementation of the monitoring plan. The addition of two genera, Cercidiphyllum and Koelreuteria, to the CFIA’s list of 10 suitable hosts resulted in the implementation of a targeted survey that found that all trees of these genera were free of injury. The reduction of the number of high-risk genera from 10 to four meant that treated areas were no longer deemed sanitised because six to eight suitable genera remained untreated, thereby resulting in a slight increase in the number of suitable moderate-risk genera to inspect in affected areas discovered after January 2007 to ensure no shift in the beetle’s host selection patterns had occurred. Finally, reducing the length of radii of affected areas from 400 m to 200 m increased the number of trees being surveyed in affected areas and in buffer 1 zones. Specifically, starting in January 2007, all trees from suitable moderate-risk genera located between 0 and 800 m from an affected tree were surveyed, as were all those of the four suitable high-risk genera between 200 and 800 m from affected trees. The survey protocol for buffers 2 and 3 was unaffected. In buffers 2 and 3 (800–2400 m and > 2400 m, respectively) only one high-risk genus – Acer – was surveyed but at different intensities (Fournier and Turgeon Reference Fournier and Turgeon2017).
The last date on which an affected tree was found in the Toronto/Vaughan regulated area was 7 December 2007. Treatment based on that last discovery ended on 31 March 2008. The first survey cycle of the monitoring phase without a new discovery began on 1 July 2008 and ended on 30 June 2009. On 5 April 2013, the CFIA declared that A. glabripennis had been eradicated from the Toronto/Vaughan regulated area and repealed the ministerial order that had been in place (CFIA 2013). At that time, each of the 2059 monitoring cells had either been inspected at least three times over at least five years or had been completely sanitised. Some cells had been surveyed as many as nine times because of their proximity to successive detections of affected trees (Fournier and Turgeon Reference Fournier and Turgeon2017).
Validating the survey techniques and results
A landfill survey was performed in March 2004 by forest health specialists from the Ontario Ministry of Natural Resources and Natural Resources Canada. The specialists were sent to landfills across the Greater Toronto Area that had received household, yard, and commercial waste from homes and businesses in the regulated area during the previous few years. All suitable host genera in open areas and up to 20 m into wooded areas in all directions surrounding each landfill were inspected. No sign or symptom of A. glabripennis injury was found. This outcome was consistent with results from a pathway analysis by Auclair et al. (Reference Auclair, Fowler, Hennessey, Hogue, Keena and Lance2005) that indicated that the risk of establishment and spread in or around landfills located outside areas regulated for A. glabripennis was low. Regardless of the outcome of this Ontario survey, the science subcommittee recommended that yard waste be kept and processed (see section, Processing and disposal of host material) within the regulated area for the duration of the eradication programme.
In 2005, the CFIA found trees with signs of A. glabripennis emergence growing within Emery Yard, a disposal site for yard waste established before the discovery of the Toronto/Vaughan infestation. Dendrochronology of these affected stems suggested that the beetle had arrived at that site before 2003, negating the concern that infested wood being stored and processed at the facility for research purposes was the origin of the infestation at that location.
The CFIA relied on two tools to increase confidence in the monitoring survey results and to improve detection of signs of injury by inspectors during the monitoring phase. The first tool was a structured training programme that assessed the efficiency of inspectors in detecting low densities of simulated signs of oviposition and emergence. Training began in 2008 and was based on an experimental design used to assess the effect of density and location of simulated signs of injury on their detectability from the ground and the time required to find these signs (Turgeon et al. Reference Turgeon, Pedlar, de Groot, Smith, Jones, Orr and Gasman2010). Before 2008, training consisted of requiring inspectors to frequently examine displays containing stems with the entire range of known signs and symptoms of injury to refresh their search image.
The second tool was a quality assurance programme – another by-product of the study by Turgeon et al. (Reference Turgeon, Pedlar, de Groot, Smith, Jones, Orr and Gasman2010). Each year, starting in 2008, one or two maple trees were randomly selected in a variable number of survey cells, and in each selected tree, three simulated signs of oviposition and one of adult emergence (Fig. 19) were made by a tree climber who was not involved in the survey operations but who was familiar with signs of A. glabripennis injury. The tag numbers of the selected trees were recorded, and pictures were taken of the exact location of simulated A. glabripennis signs so they would not be confused with real signs should these trees become infested at a later date. The simulated signs were allowed to age under natural conditions for several weeks. The cells were then surveyed as part of the regular monitoring survey schedule, and inspectors reported all trees of interest to supervisors, who confirmed with the tree climber that the tree was part of the quality assurance programme. Between 70% and 100% of the trees imbedded with simulated signs were detected each year.

Fig. 19. Part of the Canadian Food Inspection Agency survey quality assurance programme. A, A forest health specialist marks B, simulated signs of Anoplophora glabripennis oviposition (pits) and C, emergence (holes), without the knowledge of inspectors conducting operational visual surveys from the ground or by climbing trees.
From 2003 to 2006, inspectors also recorded the locations of trees of unknown and questionable suitability. To mitigate against the possibility that these genera could be targeted by A. glabripennis in Toronto/Vaughan more frequently than they had been in other outbreaks or in the beetle’s native range – and thus escape detection – the science subcommittee suggested that a host-confirmation survey of trees from these two categories be undertaken. A host-confirmation survey of more than 3000 trees, all within 400 m of a tree with an A. glabripennis emergence hole, was initiated on 1 April 2004 and lasted three years (Turgeon et al. Reference Turgeon, Jones, Smith, Orr, Scar and Gasman2016). All that was found were signs of oviposition on a single ash (Fraxinus sp.) tree during the first year of the survey. That attacked tree, which was within 200 m of what is considered the epicentre of the infestation, was removed.
Several cells located just outside the regulated area were also surveyed because the boundary of buffer 2 (800–2400 m) for affected trees found in 2007 extended a few metres beyond the southern limit of the regulated area. Rather than expanding the regulated area, new cells were created and surveyed to confirm the absence of affected trees.
Finally, all wooded areas within buffer 1 and 2, including ravines, were intensively surveyed. A survey protocol for wooded areas in buffer 3 (> 2400 m from an affected tree) was developed, wherein surveys focused on trees within 10 m of parking areas and along pathways leading into wooded areas. No affected tree was detected during these surveys.
Health and environment monitoring
Unlike other eradication programmes, such as those in the United States of America, that used insecticides as a prophylactic treatment during the operation phase (United States Department of Agriculture 2014) to protect suitable high-risk stems within 400 m of an infested tree, the CFIA’s programme did not use insecticides in this way. In addition, no trees in ravines had had to be removed, which might have required different treatment options or necessitated work to stabilise soil or terrain or to reconstruct the ravine edges or tops. For these reasons, health and environmental monitoring was not required during the programme’s monitoring phase.
Community support
Because the monitoring phase did not include the use of insecticides, the CFIA was not aware of any concern within the community about this phase of the response plan. This absence of concern was interpreted as a sign of support for the monitoring phase.
The cities of Toronto and Vaughan took advantage of the compensation offered by the CFIA to supplement their own respective budgets to reforest some of the areas most affected by tree removal with trees from genera of questionable or unknown suitability. Municipalities and property owners were eligible for compensation only if they replanted with trees belonging to genera of questionable or unknown suitability for A. glabripennis. About 700 requests for compensation were submitted by property owners (CFIA, unpublished data).
Review phase
During the review phase, all decisions and actions taken during the eradication campaign against A. glabripennis in Toronto/Vaughan, along with their rationale and outcomes, would have been re-examined, assessed, and rated. It was critically important that a programme such as this, from its development to the declaration of the affected area as pest-free, be reviewed by individuals familiar with the entire programme and by outsiders to identify factors that influenced the programme’s outcome and to determine whether they contributed positively or negatively to it.
This step is critical because of the limited number of successful eradications worldwide. Documenting the entire process of each such programme from start to finish is essential to ensure proper knowledge transfer for a critical review of the current programme and the betterment of future programmes (Myers et al. Reference Myers, Savoie and van Randen1998, Brockerhoff et al. Reference Brockerhoff, Liebhold, Richardson and Suckling2010b, Tobin et al. Reference Tobin, Kean, Suckling, McCullough, Herms and Stringer2014; Porth et al. Reference Porth, Dandy and Marzano2015; Kean et al. Reference Kean, Suckling, Sullivan, Tobin, Stringer and Smith2021). The review phase for the Toronto/Vaughan outbreak did not occur, because just as the CFIA was preparing itself for this review in 2013, another breeding population of A. glabripennis was discovered in Ontario.
Another satellite outbreak
About five months after the CFIA had declared the regulated area of the Toronto/Vaughan outbreak pest-free, another breeding population of A. glabripennis was discovered in Ontario. This second population was discovered in a light industrial area of Mississauga and Toronto (hereafter Mississauga/Toronto) on 13 August 2013. The discovery site and the epicentre of this new outbreak were outside of the Toronto/Vaughan regulated area and nearly 10 km away from its epicentre (Fig. 16). Turgeon et al. (Reference Turgeon, Orr, Grant, Wu and Gasman2015) published a detailed account of this outbreak’s detection event, which was much smaller than that of Toronto/Vaughan. The account covers most of the events, strategies, and plans of the outbreak’s evaluation, response, and operation phases.
The eradication plan used for the Mississauga/Toronto outbreak also called for tree removal of affected and of suitable high-risk trees as the sole mitigation tool. However, two differences between the two programmes existed. First, the boundaries of the second regulated area were limited to only cells within 2400 m from an affected tree. The second difference was the size of affected areas. After deliberation, the CFIA decided that the radius of the Mississauga/Toronto affected area would be 800 m from an infested or attacked tree instead of the 400 m or 200 m used in Toronto/Vaughan (Fig. 2). This change in radius resulted in the creation of a third group of trees, all discovered and removed between August 2013 and April 2014 (Table 5; Fig. 16). The reason for the change was that, in the Mississauga/Toronto outbreak, the distance among affected trees often exceeded 400 m. This increased distance may have been due partly to the low density of suitable trees in the area.
The operation phase of this second outbreak started on 19 February 2014 and ended on 1 May 2014. Most (68%) affected stems contained specimens identified as A. glabripennis (dead or alive; Table 2). The remaining stems (32%) were suspect.
The subsequent monitoring phase began on 1 July 2014 and lasted six survey cycles. This regulated area was divided into 435 cells. Several cells covered the Pearson International Airport, which presented its own unique challenges for inspectors because of the security concerns associated with access to an international airport. The rule for ending the monitoring phase was similar to that used for the Toronto/Vaughan outbreak: each cell had to be surveyed – and found free of A. glabripennis – at least three times over at least five years. No residual population was found during the six survey cycles (Table 2). On 25 June 2020, the CFIA declared the Mississauga/Toronto regulated area pest-free (CFIA 2020).
What was learned
This section summarises lessons learned, observations made, and some results attained over the course of the eradication programme for both the Toronto/Vaughan and Mississauga/Toronto outbreaks, including how research on the beetle that was undertaken while implementing the programme adaptively informed the response. It also provides a critical look at the requirements and key factors that contributed to the successful eradication of A. glabripennis in Canada – what worked and what could be improved on – for the betterment of future programmes to control outbreaks by A. glabripennis and other similar invasive forest pests.
A greater understanding of Anoplophora glabripennis and the local outbreaks
Arrival
The authors of the present report hypothesise that the Toronto/Vaughan outbreak started in the early to mid-1990s, based on three facts. First, a few of the injured trees on the property at the outbreak’s putative epicentre were dead and still standing at the time of treatment. The number of years these trees had been under attack could not be determined with certainty; however, it was clear they had been under attack for many years. Some of these signs of injury could be observed all the way to the ground. Second, at least nine trees on that same property had been removed before September 2003, and their respective stumps all contained characteristic A. glabripennis larval tunnels and exit holes. The presence of A. glabripennis injury signs on the stumps suggested these trees had also been injured for many years before they were removed and had likely died or been severely weakened as a result of those injuries. This information prompted the trace-back survey of landfills (see section, Validating the survey techniques and results, above) to find out how and where these trees had been disposed. Third, the assumed arrival of the beetle in Canada is consistent with the years of the earliest interceptions of A. glabripennis at ports of entry and after entry in warehouses (Table 1). In the Mississauga/Toronto outbreak, a cursory visual examination of slices from the trunk and branches of the most heavily infested stem revealed the presence of signs of oviposition dating back to 2004 (Turgeon et al. Reference Turgeon, Orr, Grant, Wu and Gasman2015).
Origin
Asian longhorned beetle populations found in North America and Europe have a more limited genetic diversity than those from Asia (Carter et al. Reference Carter, Smith and Harrison2010). Analysis of the genetic diversity of beetles found in Toronto/Vaughan suggested that the population had originated from an introduction event that was separate from those of the American infestations and that it had been founded either by a small number of beetles or by a large number that had later suffered genetic bottlenecks (Carter et al. Reference Carter, Smith, Turgeon and Harrison2009b). Turgeon et al. (Reference Turgeon, Orr, Grant, Wu and Gasman2015) hypothesised that the Mississauga/Toronto outbreak was a satellite of the Toronto/Vaughan outbreak.
Establishment
During the response programme, we learned more about how A. glabripennis became established in the area, the beetle’s preferences for available tree species, how it colonised and spread within trees, and the length of its life cycle in the region.
Size of outbreak
The Toronto/Vaughan regulated area was much larger than that of Mississauga/Toronto: 15 217 ha versus 4589 ha, respectively (Fig. 16). This difference stemmed in part from variances in the way these regulated areas were established. The Toronto/Vaughan regulated area was designed to encompass all buffers around all known infested trees and to follow easily recognisable highways and boulevards, whereas the boundaries of the Mississauga/Toronto outbreak were fixed on the nearest suitable landscape feature at a minimum of 2400 m from all affected trees, regardless of the complex shape that would result.
In both regulated areas, the discovery of most affected trees occurred during the delimitation phase. In Toronto/Vaughan, most affected trees were removed during the initial operation phase (Table 2). In Mississauga, most affected trees were removed within days of their discovery, during the delimitation phase. Treatment of the Toronto/Vaughan outbreak was accomplished by the removal of 682 affected and 59 690 unaffected stems (Table 3). Affected stems contained either live or dead A. glabripennis specimens or exhibited only signs and symptoms of injury. Only 171 of the affected stems had exit holes. In Mississauga/Toronto, 50 affected stems and 18 820 unaffected stems were removed (Table 3), bringing the total number of affected and unaffected stems removed in an attempt to eradicate A. glabripennis in Ontario to 732 and 78 510, respectively.
During the first three cycles of the monitoring phase of the Toronto/Vaughan outbreak, 14 residual infestations were discovered in addition to the core infestation and the three satellite infestations (Table 6). Most of the residual infestations and the three satellites comprised small numbers of affected stems (1–28 stems; Table 6): one satellite and 11 residual infestations contained no more than 10 affected trees. Discoveries of new infested or attacked trees led to expansions of the affected area. Most residual infestations were outside previously treated areas, and all were within the boundaries of the regulated area established in January 2004 (Fig. 2).
The number of affected stems in the Toronto/Vaughan outbreak was greater than that of most A. glabripennis outbreaks in Europe (Straw et al. Reference Straw, Fielding, Tilbury, Williams and Inward2015) but much smaller than most outbreaks in the United States of America (Haack et al. Reference Haack, Bauer, Gao, McCarthy, Miller, Petrice and Poland2006, Reference Haack, Hérard, Sun and Turgeon2010; Haack Reference Haack2020). The number of affected stems reported herein from the two Ontario outbreaks likely were slight underestimates. This is in part because property managers near the epicentre in Toronto/Vaughan removed stems before the official discovery of the outbreak, leaving some stumps (< 10 cm in height) that had signs of oviposition and larval tunnelling and other stumps that had no visible signs of injury but likely also came from affected trees. If a stump showed no sign of injury at the time of discovery but came from an affected stem, that stem may not have been included in the data sets. False negatives (i.e., unaffected trees actually affected; Dodds and Orwig Reference Dodds and Orwig2011) may also have been recorded because none of the inspection methods was 100% accurate for detecting affected trees during the first treatment of the regulated area (Turgeon et al. Reference Turgeon, Ric, de Groot, Gasman, Orr and Doyle2007, Reference Turgeon, Pedlar, de Groot, Smith, Jones, Orr and Gasman2010), especially when inspections occurred during poor weather (Fig. 4; Ric et al. Reference Ric, de Groot, Gasman, Orr, Doyle and Smith2007, Fig. 261). In addition, inspectors discovered a small number (n = 11; < 0.05%) of affected stumps during sweeps of more than 2000 sanitised properties undertaken between the end of the treatment phase (1 April 2004) and before the beginning of the monitoring phase (1 July 2004) to ensure all suitable high-risk stems had been removed. The location of these stumps did not match any of the known locations of affected trees removed during the previous winter. Regardless, the number of false negatives is unlikely to change the trends reported herein in any important way.
Tree selection
In Toronto/Vaughan, beetle attacks were found on seven genera of broadleaf trees (Table 7). Of these genera, the beetle completed its development, as demonstrated by emergence holes that could be unequivocally attributed to A. glabripennis or on emergence from the stems in the containment facility, in only four genera: Acer, Betula, Populus, and Salix. Two of the most important signs of injury – oviposition pits and emergence holes – were observed predominantly on Acer spp. (Tables 8 and 9). In Mississauga, 49 of the 50 affected stems were Acer spp. (Turgeon et al. Reference Turgeon, Orr, Grant, Wu and Gasman2015).
Table 7. Comparison of the percentage of stems affected by Anoplophora glabripennis among tree genera and the percentage of stems of each genus with signs of oviposition (pits) or emergence (holes) visible at the time of detection in the field within the regulated area of the Toronto/Vaughan outbreak, Ontario, Canada between 2003 and 2013.
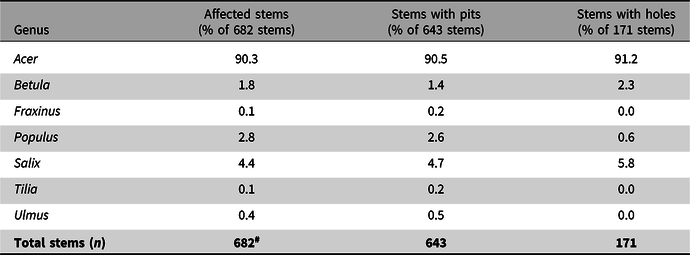
# Nine affected stems had no visible oviposition pit or emergence hole, only other signs and symptoms of injury.
Table 8. Relative abundance of stems † with signs of oviposition (pits) and emergence (holes) characteristic of Anoplophora glabripennis and relative abundance of these signs per tree genus within affected areas of two outbreaks found in Ontario, Canada.
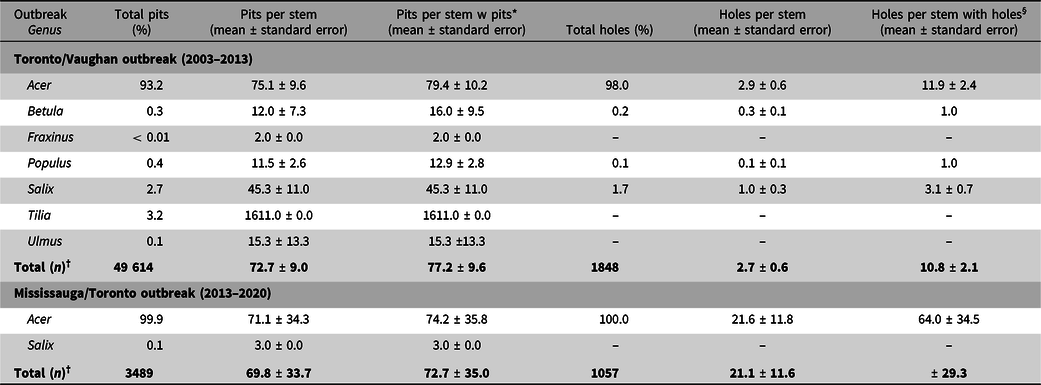
† Total number of affected stems in Toronto/Vaughan and Mississauga/Toronto outbreaks were 682 and 50, respectively.
* Total number of affected stems with oviposition pits in Toronto/Vaughan and Mississauga/Toronto outbreaks were 643 and 48, respectively.
§ Total number of affected stems with emergence holes in Toronto/Vaughan and Mississauga/Toronto outbreaks were 171 and 19, respectively.
Table 9. Abundance of signs of oviposition (pits) and emergence (holes) by Anoplophora glabripennis on injured stems removed before the monitoring phase and during each survey cycle of the monitoring phase of the Toronto/Vaughan, Ontario, Canada outbreak between 2003 and 2013.
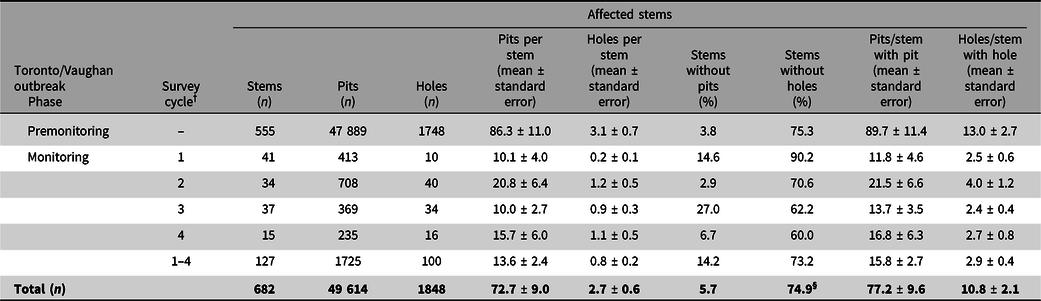
† The beginning of each monitoring or survey cycle coincided roughly with the predicted onset of adult emergence. In the Greater Toronto Area, this occurred around 1 July. Each cycle ended on 30 June of the following year. For the Toronto/Vaughan outbreak, the first survey cycle began on 1 July 2004.
§ Thirty-nine affected stems had no oviposition pits, and 511 had no emergence holes.
Anoplophora glabripennis has a wide host range, but in China, it prefers the genera Populus, Salix, Ulmus, and Acer (Haack et al. Reference Haack, Law, Mastro, Ossenbruggen and Raimo1997; Lingafelter and Hoebeke Reference Lingafelter and Hoebeke2002; Luo et al. Reference Luo, Wen and Xu2003; Pan Reference Pan2005; Yin and Lu Reference Yin and Lu2005; Smith et al. Reference Smith, Turgeon, de Groot and Gasman2009). In Europe, most trees infested by A. glabripennis belonged to the genera Acer and Betula, although attacks have also been reported on Aesculus, Carpinus, Fagus, Platanus, Populus, Prunus, Salix, and Ulmus (Cocquempot et al. Reference Cocquempot, Prost and Carmignac2003; Cocquempot Reference Cocquempot2006; Hérard et al. Reference Hérard, Ciampitti, Maspero, Krehan, Benker and Boegel2006; Reference Hérard, Maspero, Ramualde, Jucker, Colombo, Ciampitti and Cavagna2009; Maspero et al. Reference Maspero, Jucker and Colombo2007). In North America, attacks have been reported on many additional genera (Wang Reference Wang2015).
Within-tree colonisation
Data on the abundance and distribution of signs of injury that were collected during the eradication programme, coupled with a labelling scheme developed to identify and indicate the relative position of sample logs in the branch hierarchy of each tree (Fig. 10), can provide valuable information on the within-tree colonisation process of A. glabripennis and facilitate early detection of affected trees. More than 49 000 oviposition pits on 682 affected stems and stumps removed in Toronto/Vaughan were inventoried (Table 8). The abundance of oviposition pits on the stems ranged from 1 to 3829, with 47.5% of affected stems having 10 or fewer pits (Fig. 20a). Almost 40% of affected stems had between 11 and 100 pits. Only 1% of the stems had more than 1000 pits each. Most of these pits were found on Acer spp.
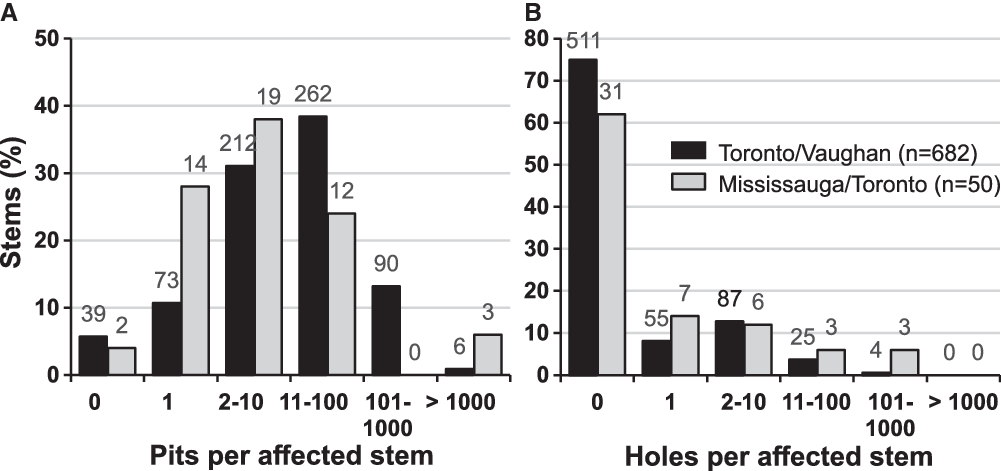
Fig. 20. Percentage of stems affected by Anoplophora glabripennis in each arbitrary class of abundance of A, oviposition pits and B, emergence holes in Toronto/Vaughan (2003–2007) and Mississauga/Toronto (2013–2020). Stems without oviposition pits or exit holes in Toronto/Vaughan (n = 39 or n = 511, respectively) and Mississauga/Toronto (n = 2 or n = 31, respectively) included nine and one stems without a sample submitted for assessment, respectively.
More than 1800 emergence holes were also inventoried on the 171 affected stems that had these holes (Table 8). The majority of affected stems presented no sign of adult A. glabripennis emergence (Fig. 20b). The abundance of emergence holes on each stem with holes ranged from 1 to 272. Slightly more than 30% of stems with emergence holes had only one hole, and about 50% had between two and 10 holes. Only 2% of stems with emergence holes had more than 100 holes. Most of these emergence holes were on Acer spp.
Despite having a dissimilar number of affected trees, the Toronto/Vaughan and Mississauga/Toronto outbreaks show a similar distribution in the proportions of the signs of oviposition and emergence among arbitrary classes of abundance (Fig. 20). In both outbreaks, at least 47% of affected stems had 10 or fewer oviposition pits, and over 60% had no emergence holes. The average numbers of oviposition pits and emergence holes per affected stem removed before the monitoring phase were 86 and three, respectively, compared to only 14 and one on those detected during the monitoring phase (Table 9). Dodds et al. (Reference Dodds, Hull-Sanders, Siegert and Bohne2014) reported similar trends in Massachusetts, where the range of oviposition pits per affected stem ranged from one to 208 in Delaval and from one to 340 in Boylston, with the majority having between one and 10 pits and no exit holes. The high percentage of stems with low numbers of oviposition pits illustrates the challenge of finding infested trees when conducting intensive detection surveys in areas where sign densities are low and when conducting extensive or area-wide surveys outside of an infested zone. It also speaks to how well the CFIA’s field teams performed at finding these signs during the surveys in Toronto/Vaughan and Mississauga/Toronto.
In the United States of America, the level of beetle infestation within a tree is currently determined on the basis of the quantity of signs of emergence (Trotter and Hull-Sanders Reference Trotter and Hull-Sanders2015), with the infestation levels defined as follows: “A” – trees contain female oviposition marks but no emergence hole; “B” – trees have 1–10 emergence holes; “C” – trees contain 11–100 holes; and “D” – trees have more than 100 holes. In the Ontario outbreaks, 74%, 21%, 4%, and 1% of affected stems would have been identified at infestation levels A, B, C, and D, respectively, in Toronto/Vaughan, compared to 62%, 28%, 2%, and 7% in Mississauga/Toronto. The 7% of affected stems with more than 100 emergence holes in Mississauga/Toronto is misleading because most of the instances of these high-density holes occurred on three stems growing from the same root system.
Although the majority of main stems in the Toronto/Vaughan and Mississauga/Toronto outbreaks had fewer than 10 holes (Fig. 20b), some trees were heavily attacked. For example, one tree with a 39.5-cm diameter (D130) in the Toronto/Vaughan outbreak had 3829 oviposition pits and 227 emergence holes and was still alive. More than 100 emergence holes were found on only four of 682 trees (two Acer saccharinum Linnaeus specimens with 105 and 113 exit holes, and two Acer platanoides specimens with 210 and 227 emergence holes). In their study, Trotter and Hull-Sanders (Reference Trotter and Hull-Sanders2015) referred to trees with more than 100 emergence holes as origin trees. However, it is unlikely the four trees in Toronto/Vaughan were the actual origin trees of this outbreak, given the presence of stumps with exit holes at the discovery site. In the Mississauga/Toronto outbreak, the tree with three stems, each of which had more than 100 exit holes, does appear to be the origin tree.
Straw et al. (Reference Straw, Fielding, Tilbury, Williams and Inward2015) noted that the rate for detecting trees with A. glabripennis emergence holes was greater than that of trees without holes, whereas Turgeon et al. (Reference Turgeon, Pedlar, de Groot, Smith, Jones, Orr and Gasman2010) showed that the average rate for detecting trees with simulated signs of oviposition, which varied among inspectors, was greater than that for trees with only simulated signs of emergence. In the Ontario outbreaks, fewer than 40% of affected stumps and stems had emergence holes, and some of the emergence holes were completely covered with callus tissue (Turgeon et al. Reference Turgeon, Pedlar, de Groot, Smith, Jones, Orr and Gasman2010), making detection even more difficult. Indeed, in a few instances, signs of injury were not discovered until the second examination of the same tree (J.J. Turgeon, unpublished data). These results had implications for the design of survey procedures, especially because the majority of affected trees had no emergence holes.
Life cycle duration
When the researchers dissected A. glabripennis oviposition pits in winter and spring 2003/2004, they recorded many turgid eggs that seemed viable and live early instar larvae under the bark. These observations, which are consistent with Keena’s (Reference Keena2006) conclusions that the eggs can overwinter, suggested some A. glabripennis specimens may not enter their host tree’s heartwood until the following spring, when the larvae were in their second season. Eggs or larvae that hatched during a given year but entered the wood the following spring would not have experienced enough heat accumulation in Toronto/Vaughan to emerge as adults that year and therefore likely would have spent at least two winters in the tree, only one of which would have been in the wood. In Toronto, annual degree-day accumulation above 10 °C, the approximate lower threshold for many A. glabripennis life history parameters (Keena Reference Keena2006), rarely exceeds 1600 degree-days (Fig. 17). The records of the daily emergence of A. glabripennis adults in the Emery Yard containment facility show three peaks or periods of emergence, each about 1400 degree-days apart. These emergence peaks were hypothesised to indicate a life cycle lasting 2–3 years (Fig. 18), a range consistent with the number of years required to complete development in Toronto/Vaughan that had been predicted by Trotter and Keena’s (Reference Trotter and Keena2016) phenology model.
Requirements and key factors that contributed to the successful eradication of Anoplophora glabripennis in Canada
At the time of writing, the A. glabripennis response programme detailed in the present report has been successful in eradicating A. glabripennis from the two outbreak areas in the Greater Toronto Area. This positive outcome is a tribute to the planning, organisation, and hard work of the many groups and individuals who came together to battle this invasive insect. The role that each key requirement for successful eradication (Myers and Hosking Reference Myers, Hosking, Hallman and Schwalbe2002; Brockerhoff et al. Reference Brockerhoff, Liebhold, Richardson and Suckling2010b) played in this accomplishment is summarised here, and some key factors that contributed to this success are also listed (Myers et al. Reference Myers, Savoie and van Randen1998).
Transparency and clear lines of authority
Several organisations were affected by the establishment of A. glabripennis in Canada. Given the magnitude of the problem, which transcended the resources and expertise of any single organisation, it was determined early on that it would be best for all stakeholders to join together and work under the leadership of one organisation. The CFIA was the only agency with the necessary legislative authority to fill this role. As the lead organisation, the CFIA steered the surveying and treatment operations, coordinated the contributions of other stakeholders, and ensured that messaging to political stakeholders and the public was coherent and consistent. The roles and contributions of the other stakeholders were based on resources, expertise, and needs. For example, the City of Toronto led tree removal and disposal, the Regional Municipality of York supplemented existing forest inventories – which proved critical for planning and implementing the emergency response – and Natural Resources Canada’s Canadian Forest Service contributed science-based expertise and input.
Cooperation among senior levels of government was also necessary, and a federal–provincial critical pest management committee comprised of senior government officials was established. This committee shared information and perspectives and endorsed resource-sharing arrangements in a cooperative, apolitical, and noncompetitive spirit.
Effective survey tools
From the science subcommittee’s perspective, the only missing requirement in the programme against A. glabripennis in Ontario was an effective tool to facilitate A. glabripennis detection at low insect densities. Indeed, the availability of efficient and inexpensive survey tools when population densities are low can lead to the early detection of an adventive species soon after introduction and of residual populations thereafter. Unfortunately, the limited efficiency of visual inspection of trees, the only practical survey methodology available for A. glabripennis, made it difficult to find all infested trees.
All other requirements for the programme’s successful implementation and completion were present.
Biological susceptibility of target
Key biological factors that likely helped further the eradication programme’s success against A. glabripennis were the apparent limited dispersal by adults (Smith et al. Reference Smith, Bancroft, Li, Gao and Teale2001, Reference Smith, Tobin, Bancroft, Li and Gao2004) and the beetle’s slow rate of development in the outbreak area. Observations made early in the programme suggested that the beetle could take up to three years to complete its life cycle in the Greater Toronto Area (Fig. 18). Another contributing biological factor may have been the adult’s relatively low reproductive rate (Keena Reference Keena2002; Smith et al. Reference Smith, Bancroft and Tropp2002).
These factors, combined with the initial discovery of infested trees at a relatively early point in the outbreak, likely contributed to the overall low number of affected trees and the limited natural dispersion observed at the time of the 2003 discovery. These traits also played a role in determining the number of years required before the CFIA could declare the regulated area pest-free. For example, that duration was increased from two years of negative survey results in 2003 to five years in 2007.
No reinvasion
The 2013 discovery of infested trees in Mississauga/Toronto raised an important question: did this newly discovered population represent a reintroduction in Ontario despite the implementation of new standards for wood packaging materials that were designed to prevent reintroduction (Food and Agriculture Organisation of the United Nations 2018b; Haack et al. Reference Haack, Hérard, Sun and Turgeon2010)? A comparison of the genetic material from the Toronto/Vaughan and the Mississauga/Toronto populations revealed that the two outbreaks were of the same population. Dendrochronology suggested that the Mississauga/Toronto population was likely a satellite of the Toronto/Vaughan population (Turgeon et al. Reference Turgeon, Orr, Grant, Wu and Gasman2015). In general, the low number of A. glabripennis interceptions since the implementation of the new wood packaging standards (Table 1) suggests the risk of reintroduction was reduced but not nil.
Reforestation
Replacement of or compensation for trees removed during the eradication programme made sense from an urban forest ecology perspective but was not in place when the programme began. Some of the resistance to tree removal in residential neighbourhoods in the programme’s early months appeared to be directly linked to the lack of a replacement plan. Availability of such a plan at the onset of this programme would likely have fostered greater public acceptance; however, such plans take time to develop because they require funding and must consider the risks posed to tree species by other current and prospective invasive species (e.g., Agrilus planipennis and Tetropium fuscum). A funding contribution from the Province of Ontario to the municipalities of Toronto and Vaughan in February 2004 and the implementation of the CFIA’s tree compensation plan in May 2004 likely alleviated some public concerns. Despite the modest uptake of compensation by property owners, the public seemed to take solace in knowing financial support was available for landowners to replace trees.
Sufficient funding
Early and sustained funding is another key requirement for a successful eradication programme (Myers et al. Reference Myers, Simberloff, Kuris and Carey2000). As indicated previously (see section, Financial management), funding was not in place during the programme’s delimitation phase simply because funding for an eradication programme is not a normal budget item. However, once the CFIA had officially declared the presence of A. glabripennis in Ontario an emergency, the regional office responsible for programme delivery received the financial resources necessary to implement the response. Such a financial commitment was critical in making this eradication effort sustainable until both regulated areas were declared pest-free. Indeed, with the discovery and subsequent treatment and monitoring of the various satellite infestation populations, the programme ended up lasting for 17 years and costing millions of dollars (Haack et al. Reference Haack, Hérard, Sun and Turgeon2010).
Other factors
Preparedness, availability of a highly effective treatment, and integration of operations and research are other factors that seemed to have contributed to the successful outcome of this programme.
Preparedness
There is little doubt among those involved that the state of readiness by the CFIA and its partners played a key role in the successful implementation of the emergency response against A. glabripennis in Ontario. A 1998 visit by representatives of the CFIA, Natural Resources Canada’s Canadian Forest Service, and the Ontario Ministry of Natural Resources to the Chicago outbreak site provided firsthand insight into the challenges that would ensue should A. glabripennis establish in Canada. This visit led the CFIA to identify stakeholders and prepare a pest risk assessment, a draft emergency response plan, detection survey protocols for high-risk sites, and mock emergency response exercises, as well as an education programme and outreach materials.
Because of that preparation, when the discovery in Toronto/Vaughan was confirmed in September 2003, the agency’s response was swift. The operation phase began in mid-November 2003, and the monitoring phase began in July 2004. The short period between phases suggests that everyone affected by this alien species, including the public, was aware that this species was potentially economically important and that they understood the urgency of acting decisively, effectively, and cooperatively. The rapid response likely played a role in curtailing the further spread of A. glabripennis in Ontario.
This level of readiness would not have been possible without exchanges of critical information with counterparts from the United States of America. Indeed, colleagues from three agencies of the United States Department of Agriculture were active participants on the science subcommittee from beginning to end. That participation allowed the sharing of personal experience and insight, two things rarely documented or otherwise available.
Availability of an effective treatment
Although the efficacy of the survey methodology was less than ideal, the treatment methods appear to have been highly effective. The area response team chose between two options: removing affected trees and unaffected trees surrounding affected trees or removing affected trees and injecting insecticide into unaffected trees surrounding the affected trees. The second option would have required annual surveys of injected trees, in addition to all the trees remaining in the regulated area, for several years to confirm their beetle-free status. Conversely, once affected and unaffected trees had been removed from an area, that area needed no further resurveys, and the likelihood that A. glabripennis would emerge from treated areas became nil. This allowed survey activities to focus exclusively outside of known affected areas. The choice of tree cutting (and chipping) proved to be effective for the characteristics of this particular infestation (e.g., size, age, host trees, and geography).
A social licence to proceed with treatment was critical, even if powerful legislative authority to remove trees was already in place. Overall, there was strong support among the public for the treatment option selected, communications were clear and consistent, open houses and public meetings were well attended and professionally conducted, and the programme messages were scientifically sound. The general acceptance of the programme by the public was due, in part, to the decision to not use insecticides. Some detractors, some of whom could not be placated, wanted insecticides to be used to treat unaffected trees, but these people were few, considering the programme’s size and the number of trees being treated. Eradication of both the 2003 and 2013 outbreaks of A. glabripennis, both of which were discovered by members of the public, was achieved without injecting trees with insecticides.
Integration of operations and research
Integrated operation and research activities also contributed positively to the implementation and outcome of the eradication programme. The preliminary research results reduced the uncertainties around the understanding of the predicted insect behaviour and proposed methodology, allayed public concerns, and were used to modify the management programme and associated communications as new information became available.
The research team developed protocols and acquired data during the operation and monitoring phases to address critical knowledge gaps. For example, the researchers assessed the survey accuracy of inspectors, carried out intensive surveys of trees from genera of unknown suitability surrounding trees with emergence holes for several years to confirm the genera’s status as unsuitable, used dendrochronology to determine an infestation timeline, and examined patterns of infestation to develop treatment plans for forested ravines and woodlots.
Some of this research led to science-based decisions and to adaptive management (Fournier and Turgeon Reference Fournier and Turgeon2017). Changes to the eradication plan resulted in improved effectiveness and a reduced environmental impact (Smith et al. Reference Smith, Turgeon, de Groot and Gasman2009; Fournier and Turgeon Reference Fournier and Turgeon2017). For example, the number of tree genera removed during treatment was reduced and the number of tree genera surveyed was decreased as distance from an affected tree increased, and the radii of affected areas were adjusted, based on the type and size of infestation being treated. Research results also proved useful for designing a survey strategy that improved the likelihood of detecting affected trees in various urban landscapes. Finally, experimental results served as a basis for the development of a strategy for continued surveillance, which has since been used to develop an area-wide, grid-based survey protocol to facilitate detection of A. glabripennis in other urban centres in Canada.
Conclusion
The information presented here provides both a record of Canada’s response to an A. glabripennis infestation and a basis for forthcoming in-depth analyses of the beetle’s behaviour and biology during its invasion of a Canadian landscape. It is the authors’ hope that this account and the results of these analyses contribute to the improved management of this pest and of other invasive species with similar behaviour. As information about successful and unsuccessful efforts to eradicate A. glabripennis in North America and Europe becomes available (e.g., Eyre and Barbrook Reference Eyre and Barbrook2021), assessment of the costs and benefits associated with each strategy will become possible. That information should assist managers in rapidly identifying key factors that contributed to the outcomes and in choosing the most appropriate responses to future outbreaks (Hull-Sanders et al. Reference Hull-Sanders, Pepper, Davis and Trotter2017).
Acknowledgements
The authors dedicate this manuscript to their colleague, the late P. de Groot (Natural Resources Canada, Canadian Forest Service, Sault Ste. Marie), who provided advice, guidance, and recommendations in the early stages of the eradication programme and encouraged the authors to collect as much information as possible on all affected trees. The authors also thank S. Grant-George, T. Stork, and T. Ali (CFIA, Toronto) for providing historical records of the discovery; B.D. Gill, G. Thurston (both CFIA, Ottawa), and E. Bullas-Appleton (CFIA, Guelph) for historical information on interceptions of A. glabripennis in Canada; R.A. Haack (United States Department of Agriculture, Forest Service, Michigan – emeritus) and M. Marcotte and G. Henry (both CFIA, Ottawa) for helpful comments on an earlier draft; all forest health officers and other workers (from Natural Resources Canada, Canadian Forest Service, Sault Ste. Marie and from the Ontario Ministry of Natural Resources and Forestry) involved in this programme for providing valuable technical assistance and guidance throughout the programme; the City of Toronto for technical advice, for providing staff for survey and tree removal, and for providing a facility for the survey and removal crews to report to throughout the programme’s duration; the Toronto and Region Conservation Authority for providing survey and tree removal staff; the City of Mississauga for staff resources for survey and tree removal and technical advice; the City of Vaughan for providing survey staff; and the Regional Municipality of York for providing technical advice.
Supplementary material
To view supplementary material for this article, please visit https://doi.org/10.4039/tce.2021.60.