CHD is the leading cause of death in industrialized countries(Reference Thom, Haase and Rosamond1, Reference Sans, Kesteloot and Kromhout2). Elevated levels of fasting(Reference Patel, Barzi, Jamrozik, Lam, Ueshima, Whitlock and Woodward3, Reference Gotto4) and postprandial(Reference Karpe5, Reference Roche and Gibney6) TAG are associated with increased risk for atherosclerosis; therefore, interventions that decrease or prevent an increase in plasma TAG concentrations may be valuable in reducing the risk of CHD. Aerobic exercise has been shown to lower plasma TAG concentrations in the fasting as well as in the postprandial state(Reference Gill and Hardman7). The hypotriacylglycerolaemic effect of exercise manifests acutely, approximately 12 h after the cessation of a single exercise bout, lasts for 1–2 d and does not seem to result from metabolic adaptations to repeated exercise sessions (i.e. training)(Reference Gill and Hardman7–Reference Thompson, Crouse, Goodpaster, Kelley, Moyna and Pescatello9).
Accumulating evidence suggests that the TAG-lowering effect of exercise depends on the energy expended during the exercise bout(Reference Gill and Hardman7, Reference Petitt and Cureton10) and is only evident with an accompanying negative energy balance(Reference Burton, Malkova, Caslake and Gill11). Single, prolonged sessions of moderate-intensity exercise ( ≥ 90 min at ≥ 60 % of peak oxygen consumption, VO2peak), corresponding to gross energy expenditures ≥ 3 MJ, decrease plasma TAG concentrations the next day by 15–26 % in the fasting state(Reference Gill, Frayn, Wootton, Miller and Hardman12–Reference Tsekouras, Yanni, Bougatsas, Kavouras and Sidossis15) and by 16–34 % in the postprandial state(Reference Gill, Frayn, Wootton, Miller and Hardman12–Reference Malkova, Evans, Frayn, Humphreys, Jones and Hardman14, Reference Herd, Kiens, Boobis and Hardman16–Reference Tsetsonis and Hardman19). However, when the exercise duration is shorter ( ≤ 60 min)(Reference Altena, Michaelson, Ball and Thomas20, Reference Zhang, Ji, Fogt and Fretwell21), or the exercise intensity is lower ( ≤ 30 % of VO2peak)(Reference Tsetsonis and Hardman18, Reference Pfeiffer, Wenk and Colombani22), corresponding to gross energy expenditures < 2 MJ, fasting and postprandial plasma TAG concentrations are not altered. The intensity of exercise per se does not appear to play a key role, since manipulating the intensity and duration of exercise while keeping total energy expenditure of the exercise bout constant does not affect plasma TAG response to exercise(Reference Tsetsonis and Hardman19, Reference Crouse, O'Brien, Rohack, Lowe, Green, Tolson and Reed23). Even exercise of low intensity (30 % of VO2peak) is effective in reducing fasting and postprandial TAG concentrations the next day when performed for 2–3 h (total energy expenditure 3·0–4·2 MJ)(Reference Tsetsonis and Hardman19, Reference Aldred, Perry and Hardman24), but not when performed for shorter time (about 90 min; total energy expenditure 1·6–1·7 MJ)(Reference Tsetsonis and Hardman18, Reference Petitt, Arngrimsson and Cureton25). These data indicate that the energy expenditure threshold for exercise-induced lowering of plasma TAG concentrations lies around 2–3 MJ (in a single bout), which corresponds to 60–90 min of exercise at 60 % of V(26).O2peak Unfortunately, most sedentary individuals will have difficulties exercising at this intensity for so long(Reference Ekkekakis and Petruzzello27, Reference White, Croce, Loureiro and Vroman28), while exercising at lower intensities for prolonged periods of time, e.g. 3 h, is clearly impractical(Reference White, Croce, Loureiro and Vroman28, Reference Keller and Trevino29).
Although exercise of low energy expenditure (approximately 1·5 MJ) has been shown not to affect fasting and postprandial plasma TAG concentrations(Reference Tsetsonis and Hardman18, Reference Altena, Michaelson, Ball and Thomas20, Reference Zhang, Ji, Fogt and Fretwell21, Reference Petitt, Arngrimsson and Cureton25), it is currently not known whether the addition of mild energy intake restriction (approximately 1·5 MJ) on top of such exercise could potentiate the manifestation of hypotriacylglycerolaemia. We therefore sought to evaluate fasting and postprandial TAG responses to acute negative energy balance, induced partly by a single bout of exercise of low energy cost and partly by mild hypoenergetic feeding, in young, healthy, sedentary women.
Methods
Subjects
Eight healthy, lean, young premenopausal women participated in the study; they were all normolipidaemic and normoglycaemic (Table 1). Exclusion criteria included contraindication to aerobic exercise, pregnancy, acute or chronic illness, use of medications (including oral contraceptives) or dietary supplements, smoking, regular exercise participation (two or more times per week) and being on a special diet or having experienced weight fluctuations >2 kg during the last 6 months. The experimental protocol was approved by the Ethics Committee of Harokopio University, Athens, and all subjects signed informed consent.
Table 1 Subject characteristics (n 8)
(Mean values with their standard errors)

VO2peak, peak oxygen consumption.
Preliminary testing
During their first visit to the laboratory, subjects gave a fasting blood sample for biochemical analyses. Height was measured with a stadiometer (Holtain, UK) and body weight was measured on a medical beam scale (Seca, Germany). Body composition (fat mass and fat-free mass) was determined by dual-energy X-ray absorptiometry (model DPX-MD; Lunar, Madison, WI, USA) using software version 4.6 and a 15 min scan time. Resting energy expenditure was measured by indirect calorimetry using a ventilated hood system (Sensormedics, Vmax229; Yorba Linda, CA), after the subjects had rested for about 30 min.
In a separate visit, VO2peak was determined using a submaximal incremental brisk walking test(Reference Taylor, Buskirk and Henschel30). Subjects walked on a treadmill (Technogym Runrace; Gambettola, Italy) at constant speed (5·0–6·5 km/h), and the grade was increased by 2 % every 2 min. Heart rate was monitored continuously by a telemetric heart rate monitor (Polar Accurex Plus, Finland). Expiratory gases were measured continuously with a breath-by-breath gas analyzer system (Sensormedics, Vmax229). The test was terminated at 80 % of heart rate reserve(31) and VO2peak was predicted from the VO2–heart rate relationship.
Study design
All subjects performed two trials (control and exercise plus diet) in random order; an oral fat tolerance test (OFTT) was administered the day following each intervention. All trials were carried out in the follicular phase of the menstrual cycle(Reference Gill, Malkova and Hardman32). Subjects were asked to refrain from exercise and carry out only the activities of daily living for the 2 d prior to each intervention day.
Control
For the control trial, subjects were asked to refrain from exercise and carry out only the activities of daily living. During the afternoon of the day preceding the OFTT, all subjects remained rested at home, i.e. sat in a chair or laid in bed while reading, watching television or listening to music. They also followed a prescribed diet which provided their estimated energy needs, calculated by multiplying the measured resting energy expenditure with an activity factor of 1·3–1·5, representative of the very light to light habitual physical activity patterns of our subjects(Reference Lin, Proschan, Bray, Fernandez, Hoben, Most-Windhauser, Karanja and Obarzanek33).
Exercise plus diet
For the exercise plus diet trial, subjects were asked to refrain from exercise and carry out only the activities of daily living, with the exception of the exercise session at the laboratory on the afternoon before the OFTT. Each subject attended the laboratory during the afternoon (midway between lunch and dinner) and walked on the treadmill (Technogym Runrace) for 100 min. During exercise, oxygen consumption was measured every 15 min and the grade was adjusted, if necessary, in order for each subject to exercise at 30 % of her VO2peak. Moreover, subjects followed a prescribed diet which provided their estimated energy needs (without accounting for the exercise-induced increase in energy expenditure) minus about 1·4 MJ (diet-induced energy deficit). The restriction of energy intake occurred at the lunch meal, afternoon snack and dinner meal.
Dietary analysis
Subjects were asked to follow a prescribed diet during each intervention day that was designed to provide 50 % of energy from carbohydrate, 35 % from fat and 15 % from protein. The energy intake during the exercise plus diet trial was designed to be approximately 1·4 MJ lower than that during the control trial. This dietary energy deficit was equivalent to the estimated gross energy expenditure of the exercise session, so that approximately one half of the total energy deficit in the exercise plus diet trial (relative to the control trial) was due to reduced energy intake and the other half due to increased exercise energy expenditure. To ensure compliance to the prescribed diets subjects were asked to record their food intake. Food records for each intervention day and for the day prior to each intervention day were analysed using Nutritionist V diet analysis software (FirstDataBank, San Bruno, CA, USA).
Oral fat tolerance test
The morning following each intervention, responses to a standard high-fat meal were assessed. Subjects arrived at the laboratory after a 12 h fast, at approximately 07.30 hours. A cannula was introduced into a forearm or antecubital vein and the subject rested quietly in the seated position for 15 min, after which a baseline fasting blood sample (t 0 h) was obtained. The test meal was then consumed within 15 min. Additional blood samples were obtained at 30 and 60 min and then hourly until 6 h after completion of the test meal. The cannula was flushed with heparin-free NaCl (0·9 %) every 30 min to keep it patent. Subjects remained rested in the seated position (while reading, watching television or listening to music) throughout the 6 h postprandial period. They were able to go to the restroom at any time, with the exception of a 15 min period prior to each blood sampling. They consumed only mineral water, which was provided ad libitum during the first OFTT and replicated in the subsequent OFTT. The test meal was given as a milkshake consisting of whipping cream, vanilla ice cream and syrup (1·2 g fat, 1·1 g carbohydrate, 0·2 g protein/kg body weight)(Reference Gill, Herd, Tsetsonis and Hardman34). Oxygen uptake and carbon dioxide production during the OFTT were measured (Sensormedics, Vmax229) for 15 min before and every hour after meal consumption.
Analytical methods
Blood samples taken before and during the OFTT were collected into non-heparinized serum tubes (Sarstedt, Leicester, UK), allowed to clot, spun in a centrifuge, and then aliquoted and frozen immediately at − 80°C until analysis. Separate blood samples for plasma preparation were collected into precooled potassium-EDTA Monovettes (Sarstedt), spun immediately in a refrigerated centrifuge within 15 min, aliquoted and frozen immediately at − 80°C until analysis. One aliquot of fresh plasma was kept on ice overnight before separation of the TAG-rich lipoproteins (TRL). The TRL fraction (Sf ≥ 20) was separated as previously described(Reference Koutsari, Karpe, Humphreys, Frayn and Hardman35), by slicing the tube and collecting the upper part after preparative ultracentrifugation for 300 min at 61 000 rpm at 4°C, in an Optima TLX ultracentrifuge equipped with the fixed angle MLA-80 rotor (Beckman Instruments, Palo Alto, CA, USA).
Determination of plasma TAG, glucose, NEFA, total cholesterol and HDL-cholesterol was performed by enzymatic colorimetric methods using commercially available enzymatic kits (Alfa Wassermann Diagnostics, Woerden, The Netherlands) on an automated analyser (ACE Schiapparelli Biosystems, Fairfield, IN, USA). Serum insulin was measured with an immunoenzymetric fluorescent method using a commercially available kit (ST AIA-PACK IRI; Tosoh Medics, San Francisco, CA, USA) on an automated analyser (Tosoh AIA 600II; Tosoh Medics). All samples from each subject's two trials were analysed in the same batch.
Indirect calorimetry and energy expenditure calculations
Oxygen uptake and carbon dioxide production during exercise and OFFT were measured with a breath-by-breath gas analyser system (Sensormedics, Vmax229). Energy expenditure, fat and carbohydrate oxidation were calculated using the Weir formula(Reference Weir36) and a table for non-protein RQ(Reference Peronnet and Massicotte37), assuming that urinary nitrogen excretion was negligible. Net energy expenditure of exercise was calculated by subtracting values for energy expenditure during rest (100 min) from the corresponding gross energy expenditure values during the exercise session.
Calculations and statistics
Whole-body insulin sensitivity was assessed by the homeostasis model assessment of insulin resistance(HOMA-IR) index as: fasting serum insulin (μU/ml) × fasting plasma glucose (mmol/l)/22·5(Reference Matthews, Hosker, Rudenski, Naylor, Treacher and Turner38). The concentration of LDL-cholesterol was calculated according to the Friedewald equation as: LDL-cholesterol = total cholesterol − HDL-cholesterol − TAG/2·2(Reference Friedewald, Levy and Fredrickson39). Total and incremental (above the fasting concentration) areas under the concentration v. time curves were calculated using the trapezoidal rule, and are referred to as total and incremental response, respectively. The highest value during the postprandial period is referred to as peak response.
Normality of data was graphically explored using percentile-plots. Comparisons between trials for postprandial responses were made by using repeated measures ANOVA. Means for dietary data, fasting variables, total, incremental and peak responses were compared using Student's paired t tests if data were normally distributed, or Wilcoxon's signed rank tests if data were not normally distributed. Data are expressed as means and their standard errors. Statistical significance was set at the 5 % level (P ≤ 0·05). Results were analysed using SPSS statistical software version 13.0 (SPSS Inc., Chicago, IL, USA).
Results
Exercise session and dietary intake
Average oxygen consumption during the exercise session was 0·66 (sem 0·03) litres/min, which corresponded to 30·2 (sem 0·8) % of subjects' VO2peak. Mean RQ was 0·86 (sem 0·02) and the gross energy expenditure of exercise was 1·41 (sem 0·02) MJ, with 55 (sem 6) % originating from carbohydrate oxidation and 45 (sem 6) % from fat oxidation. The net energy expenditure of exercise was 1·04 (sem 0·01) MJ.
By design, energy intake during the exercise plus diet trial was lower than during the control intervention (5·75 (sem 0·63) and 7·14 (sem 0·65) MJ, respectively; P < 0·001), while percentages of energy derived from protein, carbohydrate and fat were not significantly different between the two trials (P>0·05).
Therefore, compared to the control trial (zero energy balance), there was an energy deficit of 2·44 (sem 0·22) MJ during the exercise plus diet trial.
Responses in the fasted state
Fasting concentrations of plasma TAG, TAG in the TAG-rich lipoproteins (TRL-TAG), total cholesterol, HDL- and LDL-cholesterol, glucose, NEFA, serum concentrations of insulin and the HOMA-IR index are shown in Table 2. Fasting plasma TAG, TRL-TAG and serum insulin concentrations were approximately 18, 34 and 30 % lower, respectively, after exercise plus diet compared with the control trial (P < 0·05). There were no significant differences between trials in fasting total cholesterol, HDL- and LDL-cholesterol, glucose and NEFA concentrations (P>0·05). Insulin resistance (HOMA-IR) was approximately 31 % lower the morning after exercise plus diet than after the control intervention (P < 0·05).
Table 2 Fasting plasma concentrations of substrates, serum insulin and homeostasis model assessment of insulin resistance (HOMA-IR) the day after the control and exercise plus diet interventions*
(Mean values with their standard errors)

TRL-TAG, TAG in the TAG-rich lipoproteins.
* For details of subjects and procedures, see Methods and Table 1.
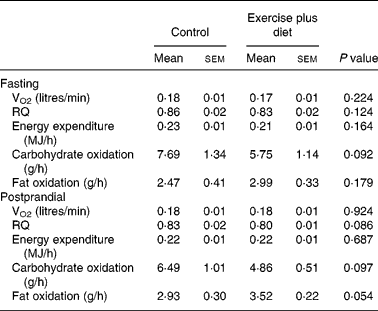
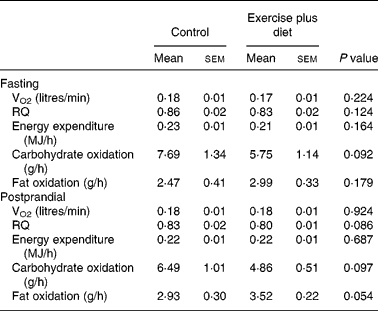
Data for oxygen consumption, resting metabolic rate and substrate utilization in the fasted state are shown in Table 3; there were no significant differences between trials (P>0·05).
Postprandial responses
Plasma TAG and TRL-TAG concentrations during the postprandial period are shown in Fig. 1. ANOVA for repeated measures revealed that postprandial plasma TAG and TRL-TAG concentrations were significantly lower the day after the exercise plus diet compared to the control intervention (P < 0·01). On average, total responses of plasma TAG and TRL-TAG to the test meal were 19 and 27 % lower (P < 0·01), respectively, the day after exercise plus diet compared to the control trial, whereas incremental responses were not different (Table 4). Peak plasma TAG and TRL-TAG responses were 18 and 22 % lower, respectively, after exercise plus diet compared to the control condition (Table 4).

Fig. 1 Concentrations of total plasma TAG (A) and TAG in the TAG-rich lipoproteins (TRL-TAG) (B) following the test meal the day after the control (●) and exercise plus diet (○) interventions. Values are means with their standard errors depicted by vertical bars. Mean values were significantly different from those after the control intervention (repeated measures ANOVA): P = 0·001.
Table 4 Summary responses of total plasma TAG, TAG in the TAG-rich lipoproteins (TRL-TAG), glucose, NEFA and serum insulin the day after the control and exercise plus diet interventions*
(Mean values with their standard errors)

* For details of subjects and procedures, see Methods and Table 1.
Serum insulin, plasma glucose and NEFA concentrations during the postprandial period are shown in Fig. 2. ANOVA for repeated measures did not reveal any significant differences between trials in these variables (P>0·05). Moreover, total, incremental and peak responses of insulin, glucose and NEFA were not significantly different between trials (Table 4).

Fig. 2 Concentrations of serum insulin (A), plasma glucose (B) and NEFA (C) following the test meal the day after the control (●) and exercise plus diet (○) interventions. Values are means with their standard errors depicted by vertical bars. Mean values were significantly different from those after the control intervention (repeated measures ANOVA): (A) P = 0·114; (B) P = 0·498; (C) P = 0·387.
There were no significant differences between trials in postprandial oxygen consumption, RQ, energy expenditure or substrate oxidation (Table 3).
Discussion
The purpose of the present study was to investigate fasting and postprandial TAG responses to acute moderate negative energy balance (approximately 2·5 MJ), induced by a combination of exercise of low energy cost and mild energy intake restriction in young, healthy, sedentary women. We observed an approximately 20 % decrease in fasting and postprandial total plasma TAG concentrations the morning after a single bout of light exercise (100 min at 30 % VO2peak) coupled with mild energy intake restriction of 1·4 MJ. This effect was entirely attributed to an approximately 30 % decrease in fasting and postprandial plasma TAG concentrations in the TRL fraction. Therefore, the present data suggest that low energy expenditure exercise along with mild hypoenergetic diet may be a practical and feasible intervention to attenuate fasting and postprandial triacylglycerolaemia.
In the present study, the total energy deficit induced by exercise plus diet compared with the control condition was approximately 2·5 MJ (i.e. about 1 MJ from exercise and about 1·5 MJ from energy intake restriction). The present results are in agreement with previous studies using similar(Reference Zhang, Ji, Fogt and Fretwell21, Reference Gill, Al-Mamari, Ferrell, Cleland, Packard, Sattar, Petrie and Caslake40) or higher(Reference Burton, Malkova, Caslake and Gill11–Reference Malkova, Evans, Frayn, Humphreys, Jones and Hardman14, Reference Tsetsonis and Hardman19) energy deficits induced solely by exercise of longer duration and/or greater intensity. On the other hand, studies in which the exercise-induced energy deficit was equivalent to the exercise-induced energy deficit in the present study (1·0–1·7 MJ), i.e. without the added diet-induced energy deficit, have consistently failed to observe a TAG-lowering effect of exercise in the fasting and postprandial states(Reference Tsetsonis and Hardman18, Reference Altena, Michaelson, Ball and Thomas20, Reference Zhang, Ji, Fogt and Fretwell21). Likewise, limited available evidence also indicates that an acute, diet-induced energy deficit equivalent to that in the present study (approximately 1·5 MJ) does not affect fasting and postprandial TAG concentrations(Reference Gill and Hardman41). Furthermore, studies that examined the effect of exercise with or without compensating for the energy expended during exercise by overfeeding, reported either an abolishment of the TAG-lowering effect of exercise(Reference Burton, Malkova, Caslake and Gill11) or not(Reference Gyntelberg, Brennan, Holloszy, Schonfeld, Rennie and Weidman42). The present findings imply that the negative energy balance is the key factor mediating the hypotriacylglycerolaemic effect, whereas the means by which this is accomplished, i.e. increased energy expenditure through exercise or decreased energy intake through hypoenergetic diet, may be of secondary importance. Hence people who find it difficult to exercise at moderate-to-high intensities or for prolonged periods of time, e.g. those who are sedentary, can make up for the lower exercise-induced energy expenditure by a mild restriction in energy intake.
We did not observe an effect of exercise plus diet on incremental TAG responses in the postprandial state, suggesting that the reduction in TAG concentrations in the fed condition was primarily due to the lowering of fasting TAG concentrations. This is consistent with the results from several other studies that examined the effects of exercise alone(Reference Burton, Malkova, Caslake and Gill11, Reference Kokalas, Petridou, Nikolaidis and Mougios43, Reference Kolifa, Petridou and Mougios44). The smaller fasting plasma TAG pool size likely results in reduced competition between endogenous (VLDL) and exogenous (chylomicrons) lipoproteins for lipoprotein lipase (LPL)-mediated hydrolysis in the fed state(Reference Brunzell, Hazzard, Porte and Bierman45–Reference Fisher, Coppack, Humphreys, Gibbons and Frayn47), thereby enhancing the clearance of postprandial TRL-TAG, which may underlie the reduction in postprandial TAG concentrations after exercise. In fact, it has been shown previously that the reduction in postprandial TAG concentrations after a single bout of exercise is predominantly, if not exclusively, due to reduced VLDL-TAG concentrations(Reference Gill, Mees, Frayn and Hardman13, Reference Malkova, Evans, Frayn, Humphreys, Jones and Hardman14, Reference Gill, Al-Mamari, Ferrell, Cleland, Sattar, Packard, Petrie and Caslake48). Likewise, we did not observe any differences in postprandial insulin concentrations. This is in accordance with studies that examined the effect of similar exercise sessions(Reference Tsetsonis and Hardman18, Reference Tsetsonis and Hardman19), suggesting that exercise-induced TAG-lowering is not mediated by an increase in insulin sensitivity(Reference Gill, Herd, Tsetsonis and Hardman34). Furthermore, many investigators have observed substantial reductions in postprandial triacylglycerolaemia without any accompanying changes in postprandial glucose(Reference Gill, Frayn, Wootton, Miller and Hardman12–Reference Malkova, Evans, Frayn, Humphreys, Jones and Hardman14, Reference Herd, Kiens, Boobis and Hardman16, Reference Tsetsonis and Hardman18) or NEFA(Reference Gill, Mees, Frayn and Hardman13, Reference Malkova, Evans, Frayn, Humphreys, Jones and Hardman14, Reference Herd, Kiens, Boobis and Hardman16, Reference Tsetsonis and Hardman18) concentrations, in agreement with the present observations.
The hypotriacylglycerolaemic effect of prolonged moderate-intensity exercise has been attributed in part to increased LPL activity(Reference Gill and Hardman7), i.e. the key enzyme responsible for circulating TAG hydrolysis. This increase is delayed and is located mainly in skeletal muscle(Reference Seip, Mair, Cole and Semenkovich49, Reference Kiens and Richter50) and not in adipose tissue(Reference Seip, Angelopoulos and Semenkovich51). Increased LPL-mediated hydrolysis likely results in augmented clearance of TRL-TAG across skeletal muscle in fasting(Reference Kiens, Lithell, Mikines and Richter52) and postprandial states(Reference Malkova, Evans, Frayn, Humphreys, Jones and Hardman14), presumably in order to replenish intramuscular TAG stores that were depleted by prior exercise(Reference Kiens and Richter50, Reference van Loon53). Studies which evaluated whole-body endogenous(Reference Tsekouras, Yanni, Bougatsas, Kavouras and Sidossis15, Reference Magkos, Wright, Patterson, Mohammed and Mittendorfer54) and exogenous(Reference Annuzzi, Jansson, Kaijser, Holmquist and Carlson55) TAG clearance the day after a prolonged (2–3 h) bout of moderate-intensity exercise found significantly augmented removal rates of plasma TAG compared with resting conditions and significantly reduced plasma TAG concentrations. However, shorter exercise bouts (60–90 min) at the same intensity, corresponding to lower energy expenditures, do not affect the clearance of either endogenous(Reference Magkos, Patterson, Mohammed and Mittendorfer56) or exogenous(Reference Gill, Mees, Frayn and Hardman13, Reference Annuzzi, Jansson, Kaijser, Holmquist and Carlson55) TAG, even though such exercise bouts may still reduce fasting and postprandial TAG concentrations(Reference Gill, Mees, Frayn and Hardman13, Reference Annuzzi, Jansson, Kaijser, Holmquist and Carlson55). It has been suggested, therefore, that exercise may also suppress TAG production by the liver(Reference Gill, Al-Mamari, Ferrell, Cleland, Sattar, Packard, Petrie and Caslake57), an assertion supported by the much higher ketone body (3-hydroxybuturate) concentrations the morning after a single bout of evening exercise(Reference Gill, Frayn, Wootton, Miller and Hardman12, Reference Malkova, Evans, Frayn, Humphreys, Jones and Hardman14, Reference Tsekouras, Yanni, Bougatsas, Kavouras and Sidossis15), implying increased hepatic fatty acid oxidation and presumably reduced availability of fatty acids for esterification and TAG synthesis. In the present study, postprandial whole-body fat oxidation tended to be higher (P = 0·054) the day after exercise plus diet. However, it is unlikely that this contributed to the observed hypotriacylglycerolaemia, since there are reports of increased whole-body fat oxidation the day after exercise without any changes in fasting and postprandial TAG concentrations(Reference Burton, Malkova, Caslake and Gill11), and also reports of reduced TAG concentrations with no changes in substrate oxidation(Reference Herd, Kiens, Boobis and Hardman16). Furthermore, although a suppressive effect of exercise on hepatic TAG secretion has indeed been observed in animals(Reference Simonelli and Eaton58, Reference Mondon, Dolkas, Tobey and Reaven59), isotope tracer kinetic studies in human subjects have consistently failed to provide support for this hypothesis(Reference Tsekouras, Yanni, Bougatsas, Kavouras and Sidossis15, Reference Magkos, Wright, Patterson, Mohammed and Mittendorfer54).
Prolonged moderate energy restriction leading to weight loss is known to reduce fasting and postprandial plasma TAG concentrations(Reference Sharman, Gomez, Kraemer and Volek60–Reference Ybarra, James, Makoundou, Bioletto and Golay63). Whether this is attributed to the negative energy balance or the accompanying weight loss is not clear; however, considerable weight loss induced by several weeks of moderate energy intake restriction does not affect fasting and postprandial plasma TAG concentrations when TAG metabolism is evaluated after a period of weight stability, i.e. when the effects of acute energy intake restriction are not present(Reference James, Watts, Barrett, Smith, Pal, Chan and Mamo64, Reference Kelley, Wing, Buonocore, Sturis, Polonsky and Fitzsimmons65). Effects on LPL activity have also been implicated in the diet-induced lowering of plasma TAG concentrations. Prolonged intense energy intake restriction (10 d on a 1674 kJ (400 kcal) diet) reduces fasting but increases postprandial LPL activity in adipose tissue(Reference Taskinen and Nikkila66), possibly in order to replenish body energy stores when dietary energy becomes available. Similarly, acute intense energy restriction (2 d on a 1674 kJ (400 kcal) diet) decreases fasting LPL activity in adipose tissue but increases fasting LPL activity in skeletal muscle, thus resulting in augmented TAG removal capacity across muscle(Reference Taskinen and Nikkila67). Studies in rodents also suggest that prolonged energy intake restriction decreases fasting but increases postprandial LPL activity in adipose tissue, and increases fasting without affecting postprandial LPL activity in skeletal muscle(Reference Quig, Layman, Bechtel and Hackler68). Though these observations suggest that energy intake restriction lowers plasma TAG concentrations by enhancing intravascular TAG hydrolysis, nonetheless, kinetic studies in man indicate that long-term hypoenergetic diet leading to weight loss lowers plasma TAG concentrations by attenuating hepatic TAG secretion without affecting TAG removal from the circulation(Reference Mittendorfer, Patterson and Klein69, Reference Ginsberg, Le and Gibson70). There are no similar studies on the effects of acute mild energy intake restriction.
Both exercise and energy intake restriction may therefore attenuate triacylglycerolaemia, though possibly via different mechanisms. Exercise-induced TAG lowering likely results from enhanced removal rate of TAG from the core of circulating TRL(Reference Tsekouras, Yanni, Bougatsas, Kavouras and Sidossis15, Reference Magkos, Wright, Patterson, Mohammed and Mittendorfer54, Reference Annuzzi, Jansson, Kaijser, Holmquist and Carlson55), whereas hypoenergetic diet-induced lowering of plasma TAG concentrations likely results from reduced secretion rate of TAG from the liver(Reference Mittendorfer, Patterson and Klein69, Reference Ginsberg, Le and Gibson70). The mechanisms leading to exercise-induced hypotriacylglycerolaemia are threshold-dependent and do not manifest after exercise of low-to-moderate energy expenditure(Reference Annuzzi, Jansson, Kaijser, Holmquist and Carlson55, Reference Magkos, Patterson, Mohammed and Mittendorfer56), whereas similar information regarding the effects of energy intake restriction is not available. The observation that a single session of light exercise in conjunction with acute mild energy intake restriction leads to decreased fasting and postprandial TAG concentrations (present study), whereas each one in itself does not(Reference Tsetsonis and Hardman18, Reference Gill and Hardman41), suggests an additive effect of diet and exercise on fasting and postprandial TAG metabolism, mediated largely by the cumulative energy deficit and the resulting negative energy balance, and is likely the result of a combination of increased TAG removal (due to exercise) and decreased TAG secretion (due to energy intake restriction). This hypothesis has never been put to test.
This is the first study to investigate the acute effects of exercise along with energy intake restriction on fasting and postprandial TAG metabolism. Previous studies have examined exercise alone(Reference Gill, Frayn, Wootton, Miller and Hardman12–Reference Malkova, Evans, Frayn, Humphreys, Jones and Hardman14, Reference Herd, Kiens, Boobis and Hardman16–Reference Tsetsonis and Hardman19), or diet alone(Reference Gill and Hardman41), or exercise in conjunction with hyperenergetic diet to maintain zero energy balance(Reference Burton, Malkova, Caslake and Gill11, Reference Gyntelberg, Brennan, Holloszy, Schonfeld, Rennie and Weidman42). A combination of low energy expenditure exercise plus mild hypoenergetic diet may be more practical and feasible than exercise alone, especially for people who cannot exercise for prolonged periods of time at moderate-to-high intensities, such as many sedentary individuals. A reduction in energy intake, such as that in the present study, can readily be achieved though a minor reduction in portion size, or a reduction of about 30 g of medium-fat meat (about 335 kJ), one slice (30 g) of bread (about 335 kJ), two tablespoons (10 g) of butter (about 300 kJ) and one cup (250 ml) of soft drink (about 420 kJ), whereas an increase in energy expenditure, such as that in the present study, can be accomplished by light walking for 100 min. Since the benefits of exercise on TAG metabolism are equal(Reference Murphy, Nevill and Hardman71, Reference Gill, Murphy and Hardman72) or greater(Reference Altena, Michaelson, Ball and Thomas20) when exercise is intermittent compared to continuous, this exercise time is easy to achieve within the day, as it may include, for example, a 40 min walk for transportation to and from work, and a 1 h walk during leisure time (e.g. a walk to the market).
In conclusion, one bout of light exercise along with mild energy intake restriction decreases fasting and postprandial total plasma and TRL-TAG concentrations in young, healthy, sedentary women. Further studies are needed to elucidate the underlying mechanisms, the duration of these effects, and the effectiveness in populations such as the obese and diabetics, i.e. those at high risk for hypertriacylglycerolaemia.
Acknowledgements
The authors would like to thank Yannis Tsekouras, Costas Anastasiou and Antigoni Tsiafitsa for technical support and all participants for their collaboration. M. M. was supported by the Greek Governmental Institute of Scholarships. This study was partially supported by the Harokopio Graduate School. M. M. conceived the study and was involved in the study design and implementation, data collection, analysis and interpretation, supervised all fieldwork and drafted the manuscript. N. C. and N. A. were involved in the study implementation and data collection and analysis. F. M. was involved in the study design, data interpretation and manuscript writing. K. P. S. supervised blood analysis. D. P. was involved in data statistical analysis and interpretation and edited the manuscript. S. A. K. was involved in the study design, co-ordinated the fieldwork and performed the venous cannulations. L. S. S. was involved in the study design, data interpretation, manuscript writing and in overall supervision of the study. All authors read and approved the final manuscript. None of the authors had a conflict of interest regarding any aspect of this research.