Post-weaning colibacillosis is a diarrhoeal disease of young pigs, strongly influenced by the drastic changes faced by the piglets immediately after weaning. Reduced feed intake, intestinal villous atrophy and reduced enzymatic and absorptive capacity of the gut may result in impaired digestion and absorption of nutrients and in the overgrowth of bacteria such as enterotoxigenic Escherichia coli (Reference Hampson1). The main aetiological agents are those enterotoxigenic E. coli expressing fimbrial antigens, which mediate adhesion to complementary carbohydrates located in the brush borders of villous enterocytes(Reference Bertschinger, Moon and Whipp2, Reference Francis, Grange and Zeman3).
So far, the most common strategy used to prevent enterotoxigenic E. coli growth in the intestine has been the addition of in-feed antimicrobial agents(Reference Verstegen and Williams4). In those situations when the use of sub-therapeutic doses of antimicrobial agents has been suppressed (i.e. in the European Union in 2006(5)), the prescription of therapeutic antimicrobial agents has considerably increased(Reference Hayes and Jenson6). In some European Union countries, pharmacological doses of ZnO (2500–3000 parts per million) are now extensively used in feed during the first 2 weeks after weaning to reduce the incidence of post-weaning E. coli diarrhoea(Reference Cardinal, D'Allaire and Fairbrother7). Besides having antimicrobial properties, some authors have proposed that high doses of Zn may increase feed intake by promoting an increase in the synthesis of ghrelin in the digestive tract(Reference Yin, Li and Li8) and may also reduce the inflammation of the intestinal mucosa(Reference Ou, Li and Cao9). Although the use of pharmacological doses of ZnO does not have the downside of selecting microbial resistances with its subsequent implications in human medicine (as opposed to the use of antibiotics), its use has raised environmental concerns about the low retention of Zn by pigs and soil toxicity.
Considerably, efforts are being made in the search of an efficient replacer of antimicrobial agents. One of the possible alternatives would be to modify the main ingredients of the feed formula to promote fermentation in the hindgut, which would protect animals from opportunistic bacterial proliferation. Several reports support that low-crude protein diets(Reference Nyachoti, Omogbenigun and Rademacher10, Reference Heo, Kim and Hansen11), diets including protein of animal origin(Reference Cardinal, D'Allaire and Fairbrother7) or diets supplemented with fermentable carbohydrates (such as high lactose levels(Reference Pierce, Callan and McCarthy12) or wheat bran (WB) and sugarbeet pulp(Reference Bikker, Dirkzwager and Fledderus13, Reference Hermes, Molist and Ywazaki14)) may also help to maintain enteric health by lowering protein fermentation and promoting the proliferation of commensal microbiota(Reference Houdijk, Hartemink and Verstegen15). In previous studies, inclusion of WB in the diet of early-weaned pigs increased intestinal fermentation(Reference Molist, Gómez de Segura and Gasa16, Reference Molist, Ywazaki and Gómez de Segura17) and diminished the incidence of diarrhoea and the attachment of E. coli K88 to the ileum mucosa(Reference Molist, Gómez de Segura and Perez18). The beneficial effects of fibrous ingredients could also be explained by additional mechanisms. Recent studies have shown, in vitro (Reference Becker and Galleti19) and in vivo (Reference Becker, van Wikselaar and Jansman20), that dietary fibres from plants, because of their carbohydrate nature and low digestibility, may act as receptor analogues, which block the attachment of bacteria to the intestinal tract. However, no information is available regarding the ability of WB to block the attachment of E. coli to the intestinal tract.
Therefore, nutritionists have two main strategies available: either using an antimicrobial approach, which tends to delay microbiota maturation to later stages, or designing diets to promote and modulate the establishment of commensal microbiota in the intestinal tract immediately after weaning. This second option, however, requires a better knowledge of the effects of dietary components on digestive physiology and on the dynamic of the microbiota ecosystem. Both these strategies appear to be contradictory in the mode of action and are difficult to combine in a comprehensive way. However, in practice, they are frequently used together, although there is little information on the formulation of starter diets containing medicines. It would be of particular interest to know about the possible influence of dietary composition on the activity of pharmacological doses of ZnO in the diet.
In the present study, we evaluate the productive performance and gut microbiota responses associated with the incorporation of ZnO, WB or their combination in the diet of early-weaned piglets (trial 1). Two in vitro trials were also designed to clarify hypotheses derived from the in vivo test: (1) the ability of WB and other fibre sources to bind E. coli in vitro (trial 2) and (2) the in vitro interactions between WB and ZnO with respect to E. coli growth (trial 3).
Materials and methods
Trial 1: in vivo experiment
Animals and diets
This experiment was performed at the Animal Facilities of the Universitat Autònoma de Barcelona and received prior approval from the Animal Protocol Review Committee of this institution. The treatment, management, housing, husbandry and slaughtering conditions conformed to the European Union Guidelines(21). A total of sixty-four commercial crossbred piglets ((Large White × Landrace) × Pietrain), which had been excluded from receiving creep feed, were weaned at 21 d of age with an average body weight of 6·7 (sem 0·37) kg. Pigs were transported from a commercial farm to the University facilities and were allocated into thirty-two pens (two animals/pen) based on the litter origin and body weight. Pens were allotted to four dietary treatments (eight replicates/treatment; Table 1) in a 2 × 2 factorial arrangement that included two levels of WB (0 v. 40 g/kg, control diet (CT) v. WB, respectively) and two levels of ZnO (0 v. 3 g/kg, CT v. ZnO diet, respectively) in the diet. The diets were formulated to be isoenergetic (averaging 16·1 MJ/kg) and isoproteic (averaging 182 g crude protein/kg) based on ground maize, barley and soyabean protein concentrate.
Table 1 Composition and chemical analysis of pre-starter diets*

CT, control diet; WB, wheat bran diet.
* Trial 1: in vivo experiment.
† Fishmeal LT, fishmeal low temperature: product obtained by removing most of the water and some or all of the oil from fish by heating at low temperature ( < 70 °C) and by pressing.
‡ Synthetic amino acids: l-Lys 0·99, dl-Met 0·99, l-Try 0·10, l-Thr 0·98.
§ Supplied per kg of feed: 13 000 IU (3900 μg) vitamin A, 1800 IU (45 μg) vitamin D3, 60·0 mg vitamin E, 3·0 mg vitamin K3, 2·0 mg vitamin B1, 6·0 mg vitamin B2, 3·0 mg vitamin B6, 0·02 mg vitamin B12, 35·0 mg niacin, 15·0 mg calcium pantothenate, 0·12 mg biotin, 1 mg folic acid, 20·0 mg Fe, 120·0 mg Cu, 110 mg Zn, 45·0 mg Mn, 0·30 mg Se, 0·10 mg Co, 1 mg I and 2·5 mg ethoxyquin as an antioxidant (Capsoquin; Itpsa, Barcelona, Spain).
Experimental procedures and sampling
Animals received the diets from day 1 to 12 of the experiment. Individual body weight and pen feed consumption were recorded on days 0, 3, 6, 9 and 12 after weaning. Physical and behavioural examination of the animals was done daily to evaluate their health status. Samples of fresh faeces were collected from the rectum of one animal/pen for microbial counts on days 3, 6, 9 and 12 after weaning. At the end of the experimental period, the faecal samples were kept in tubes and immediately frozen at − 80 °C for analysing the microbial structure, quantifying the lactobacilli counts and determining the SCFA concentration.
Analytical procedures
Chemical analyses of the diets (Table 1) were performed according to the Association of Official Analytical Chemists(22) standard procedures.
Traditional culture methods were used to determine some bacterial groups. The faecal samples were diluted 1:10 in PBS (Sigma, St Louis, MO, USA) immediately after collection, and subsequently homogenised. Viable counts of enterococci were done by plating serial tenfold dilutions onto Chromocult™ Enterococci-Agar (Merck K GaA, Darmstadt, Germany) and incubating the plates for 24 h at 37 °C. For the enumeration of E. coli and coliforms, 1 ml of solution of the corresponding dilution was pipetted onto an E. coli–coliform count plate (3M Petrifilm; Europe Laboratories 3M Santé, Cergy-Pontoise, France) with Violet Red Bile gel as an indicator of glucuronidase activity. The plates were incubated for 48 h at 35 °C, and the colonies were counted following the manufacturer's instructions. DNA from faeces was extracted and purified using the commercial QIAamp DNA Stool Mini Kit (Qiagen, West Sussex, UK), and the lactobacilli population was quantified by real-time PCR using SYBR Green dye, following Castillo et al. (Reference Castillo, Martín-Orúe and Manzanilla23). The terminal restriction fragment length polymorphism analysis of bacterial community was performed following the procedure described by Hojberg et al. (Reference Hojberg, Canibe and Poulsen24) and adapted by Castillo et al. (Reference Castillo, Martín-Orúe and Nofrarías25). Finally, SCFA concentrations were determined by GC, after an acid–base treatment followed by diethyl ether extraction and derivatisation(Reference Jensen and Jorgensen26).
Trial 2: in vitro adhesion test
Fibrous ingredients
Seven different fibrous ingredients, WB, rice hulls, soyabean hulls, oat hulls, pea hulls, sugarbeet pulp and cereal straw, were selected as test products. Bovine serum albumin (Sigma) served as the reference (negative control), following the protocol described by Becker et al. (Reference Becker, Galleti and Roubos-van den Hil27).
Bacterial strains
Two different E. coli strains were used in this experiment to elucidate the interaction between fibre substrates and bacterial fimbriae. The first one was an E. coli K88 (enterotoxigenic E. coli, strain FV12048) isolated from a colibacillosis outbreak in Spain(Reference Blanco, Blanco and Gonzalez28); the serotype (O149:K91:H10, F4+, LT1+, STb+) was provided by the E. coli Reference Laboratory, Veterinary Faculty of Santiago de Compostela (Lugo). The other strain was a non-fimbriated E. coli (F4 − , F6 − , F18 − , LT1 − , ST1 − , ST2+, Stx2e − ) isolated from the faeces of post-weaning piglets and kindly donated by the Departament de Sanitat i Anatomia Animal from the Universitat Autònoma de Barcelona.
Bacteria were cultured in unshaken Luria broth (Sigma) at 37 °C and serially passaged every 48 h, at least five times. Bacterial cells from the culture were harvested and processed as described earlier(Reference Becker, Galleti and Roubos-van den Hil27).
Adhesion test methodology
E. coli K88 and the non-fimbriated strain were allowed to adhere to different fibre components supplied as well coatings in microplates in a miniaturised adhesion test, following the protocol described by Becker et al. (Reference Becker, Galleti and Roubos-van den Hil27). Briefly, fibre ingredients were suspended in PBS to a final concentration of 4 % (w/v), and the soluble fraction was extracted for coating the flat-bottom wells of high-binding polystyrene microtitration plates. After blocking the non-specific sites with BSA, bacteria were added to a final concentration of 1·20 × 108 colony-forming units/ml. Bacteria were allowed to adhere by incubation at room temperature for 30 min. Afterwards, the wells were washed to remove non-adherent bacteria, and bacteria were allowed to grow in Luria broth media by incubation in a microplate reader (SPECTRAmax 384 Plus; Molecular Devices Corporation, Sunnyvale, CA, USA) at 37 °C. Bacterial growth was monitored as optical density (OD) at 650 nm at intervals of 10 min. The test principle, as described by Becker et al. (Reference Becker, Galleti and Roubos-van den Hil27), is based on an inverse relationship between initial cell densities and the appearance of growth defined as the duration (h) needed for the cultures to reach an OD of 0·05 at 650 nm (t OD = 0·05): the higher the adhering cell numbers, the shorter the detection times of growth.
Trial 3: in vitro wheat bran and zinc oxide interaction test
Sample preparation
In order to elucidate the interaction between WB and ZnO and the likely role of phytates, eight different samples were prepared in a 4 × 2 factorial design, which included four different buffered solutions (a negative control; 4 % WB; 4 % WB+0·02 % phytase enzyme (5000 IU/g; Ronozyme™ P500; DSM Nutritional Products Limited, Basel, Switzerland); and 4 % WB+0·02 % xylanase and glucanase enzyme mixture (22 000 visco units/g xylanase and 2000 visco units/g glucanase/g; RovabioTM Excel AP, Adisseo, France)), and two levels of ZnO (0 v. 0·3 %, w/v). Samples of buffered solutions were adjusted to a pH of 5·1 with HCl and incubated for 4 h at room temperature. Then, the suspensions were sonicated three times for 30 s each and then centrifuged at 460 g for 5 min. The supernatant obtained was adjusted to a pH of 7·0 with NaOH and ZnO added appropriate to the specific treatment.
Bacterial strains
Two different E. coli strains (E. coli K88 and a non-fimbriated E. coli strain) were used in this experiment as described earlier.
In vitro test
E. coli K88 and the non-fimbriated E. coli strains were centrifuged (1700 g) and adjusted to a final concentration of approximately 3·5–3·9 × 108 colony-forming units/ml in Luria broth. Subsequently, 750 μl of each bacterial suspension were incubated with 750 μl of each experimental treatment. Thereafter, 300 μl of each suspension were added to polystyrene microtitration plates, and the growth of the bacteria was measured in a microplate reader at 37 °C following the protocol described by Becker et al. (Reference Becker, Galleti and Roubos-van den Hil27). Bacterial growth was monitored as OD at 650 nm at intervals of 10 min for 10 h. All readings were done in two independent assays and in triplicate per assay.
Statistical analyses
The OD data from the in vitro experiments were processed by non-linear regression analysis using the non-linear P-NLIN (Gauss–Newton method) procedure(29) following the equations described by Becker et al. (Reference Becker, Galleti and Roubos-van den Hil27) in order to obtain t OD = 0·05 (h) for each treatment.
The t OD = 0·05 (h) results from the in vitro tests (trials 2 and 3), as well as all data from the in vivo trial (trial 1) were subjected to ANOVA using the generalised linear model procedure(29). Classification factors included in each model were WB, ZnO level and their interaction for trial 1, fibrous ingredient, bacterial strain and their interaction for trial 2 and buffered solution, ZnO inclusion and their interaction for trial 3. Means were calculated as least-squared means, and multiple mean comparisons were done using Tukey's correction. The α level for the determination of significance was 0·05, and tendencies for 0·05 < P < 0·15 were also presented.
Results
Trial 1: in vivo experiment
Animal performance and health status
The effects of WB and ZnO on the average daily feed intake and average daily gain of the animals as well as the incidence of diarrhoea are shown in Table 2. The inclusion of ZnO in the diet increased the average daily feed intake of the animals from day 6 to 12 (P = 0·006) and from day 0 to 12 (P = 0·035). This resulted in an increased average daily gain of the animals for the same periods (P = 0·008 and 0·036, respectively) and a higher body weight at the end of the experiment (P = 0·044) compared with the animals not receiving ZnO in the feed. The inclusion of ZnO in the diet also reduced the incidence of diarrhoea (P = 0·009).
Table 2 Body weight (BW; g), average daily feed intake (ADFI, g/animal per d), average daily gain (ADG, g/animal per d) and diarrhoea incidence (no. of animals) in early-weaned pigs*
(Mean values with their standard errors, n 8)

CT, control diet; WB, wheat bran diet; WB–ZnO, wheat bran and ZnO diet; WB × ZnO, effect of WB and ZnO inclusion in the diet.
* Trial 1: in vivo experiment.
Metabolic activity and composition of faecal microbiota
Concentrations of total and individual SCFA in the faecal samples and also the counts of major bacterial groups using culturing methods or quantitative PCR are given in Table 3. Significant differences were observed for the SCFA concentration associated with the incorporation of WB and ZnO in the diet. Moreover, the interaction between WB and ZnO was also significant for the total SCFA (P = 0·048), propionic acid (P = 0·018) and butyric acid concentrations (P = 0·007) and also tended to be significant (P = 0·120) for acetic acid. Thus, the WB diet increased the total SCFA, propionic acid and butyric acid concentrations in comparison with the CT, ZnO and WB–ZnO diets. The incorporation of WB (WB and WB–ZnO diets) increased the concentration of isoacids (P = 0·001), and the inclusion of ZnO (ZnO and WB–ZnO diets) diminished the concentrations of acetic acid (P = 0·024) and isoacids (P = 0·001).
Table 3 SCFA concentration (μmol/g fresh matter (FM)) in day 12 after weaning, enterococci and Escherichia coli counts (log colony-forming units/g FM) and lactobacilli population (log copies gen 16S rDNA/g FM) in the faeces of piglets early after weaning*
(Mean values with their standard errors, n 8)

CT, control diet; WB, wheat bran diet; WB–ZnO, wheat bran and ZnO diet; WB × ZnO, effect of WB and ZnO inclusion in the diet.
a,b Mean values within a row with unlike superscript letters were significantly different (P < 0·05).
* Trial 1: in vivo experiment.
A significant interaction was observed between the ZnO and WB supplementation on the counts of E. coli and coliform after weaning. The simultaneous incorporation of ZnO and WB in the diet increased the E. coli and coliform counts compared with the ZnO diet on day 6 after weaning (P = 0·026) and compared with the WB diet on day 9 after weaning (P = 0·024). On day 12, animals fed the WB diet showed lower counts of E. coli (P = 0·034) and coliforms (P = 0·028) than those fed the CT diet, but no significant differences were observed with the ZnO or WB–ZnO diet. On day 12, the incorporation of ZnO in the diets (ZnO and WB–ZnO) also tended to increase the enterococci population (P = 0·064) and to reduce the lactobacilli counts (P = 0·084) in the faeces of pigs compared with that in the animals that did not receive ZnO.
The terminal restriction fragment length polymorphism method was employed to evaluate global changes in the microbial ecosystem. Fig. 1 shows the analysis focused on two of the diets: the WB and ZnO diets. It shows the microbial profiles of all pens except one from which we were unable to take faecal samples. The effect of the diet on the composition of faeces was clearly observed as most of the animals were grouped into two separate clusters. Microbial profiles of pigs fed the WB diet were more similar (50–75 %) than those of pigs fed the ZnO diet, which showed more heterogeneous microbial profiles (52–90 %). In silico restriction, using Ribosomal Database Project II and MiCA software (version 3; Department of Biological Sciences, University of Idaho; http://mica.ibest.uidaho.edu/)(Reference Shyu, Soule and Bent30), was used to deduce potential ecological changes in the samples. Results are presented as potential compatible bacterial species. The WB dietary treatment showed higher diversity in compatible terminal restriction fragments (TRF) than the ZnO or the WB–ZnO treatment exposed by the following results: four animals of the WB treatment showed a TRF of 105–106 bp that was not found in any of the other three treatments (CT, ZnO and WB–ZnO). Possible bacteria compatible with this fragment size are Bacteroides fragilis (102 bp), Prevotella ruminicola (102 bp) and numerous uncultured rumen bacteria (102–104 bp). Analysing the final part of the electropherograms, animals that received the CT and WB diets showed eight and thirteen TRF of 522–582 bp, respectively, whereas only one TRF (575 bp) was found in the ZnO group, and three TRF (two of 558 bp and one of 565 bp) were found in the animals that received the WB–ZnO combination. This range of TRF is compatible with different Bacillus species (B. cereus, B. thuringiensis and B. megaterium (578–579 bp)), different Streptococcus species (S. mitis, S. bovis and S. salivarius (570–581 bp)), some Lactococcus species (L. lactis and L. garvieae (582–583 bp)) and numerous uncultured rumen bacteria.
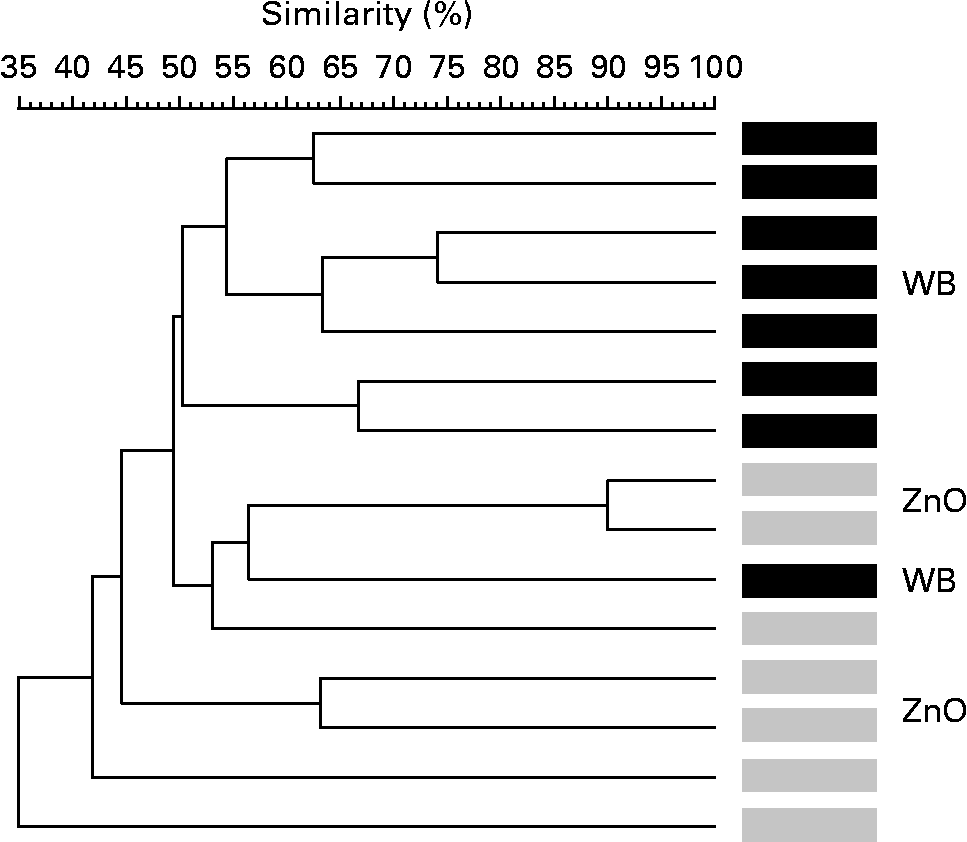
Fig. 1 Dendrogram illustrating the correlation between the experimental diets: 4 % wheat bran (WB) diet and 0·3 % ZnO diet, in terminal restriction fragment length polymorphism banding patterns of faeces of post-weaning piglets. The dendrogram distances are in percentage of similarity (trial 1: in vivo experiment).
Trial 2: in vitro adhesion test
Table 4 presents the detection times of growth for E. coli K88 and for the non-fimbriated E. coli. In the present study, an interaction was found between the E. coli strain and the fibre source (P = 0·0001). Significant differences between the fibre substrates were found related to the adhesion of the two E. coli strains. The E. coli K88 adhered more strongly (P = 0·0001) to the WB substrate compared with the other fibre substrates and the negative control treatment. Similarly, the non-fimbriated E. coli showed a higher attachment (P = 0·0001) to the WB substrate compared with soyabean hulls, sugarbeet pulp, oat hulls and the negative control treatment.
Table 4 Detection times of bacterial growth t OD=0·05 (h) for Escherichia coli K88, non-fimbriated E. coli, as a measure for adhesion in different fibre ingredients*
(Least-squared means)
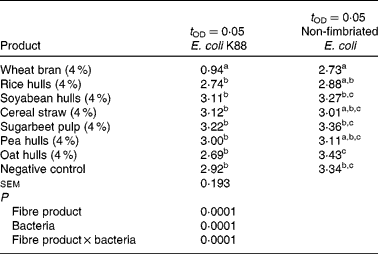
a,b,c Mean values within a column with unlike superscript letters were significantly different (P < 0·05).
* Trial 2: in vitro adhesion test.
Trial 3: in vitro analysis of interaction between wheat bran and zinc oxide
Table 5 presents the results related to trial 3 as detection times of growth for E. coli K88 and the non-fimbriated E. coli. A significant (P = 0·0001) interaction between the ZnO and buffered solutions was found related to the growth of the two E. coli strains. The incorporation of ZnO in the buffered solution inhibited (P = 0·0001) the bacterial growth for both E. coli strains in comparison with the negative control. Also, the ZnO supplementation showed antimicrobial effects when supplemented into the WB+phytase treatment. However, when it was added to the WB or WB+xylanase treatment, ZnO did not reduce the growth of E. coli.
Table 5 Detection times of bacterial growth t OD=0·05 (h) for Escherichia coli K88, non-fimbriated E. coli, as a measure of the ability of the E. coli strains to grow on different substrates*
(Least-squared means)

WB, wheat bran.
a,b,c,d Mean values within a column with unlike superscript letters were significantly different (P < 0·05).
* Trial 3: in vitro wheat bran and ZnO test.
† Negative control was based on PBS; the WB inclusion was at a level of 4 %, and the phytase and xylanase inclusion was at a level of 0·02 % (w/v).
‡ ZnO inclusion was at a level of 0·3 % (w/v).
§ Total inhibition of the bacterial growth.
Discussion
The influence of zinc oxide and wheat bran on the adaptation of piglets after weaning
The present results showed that a dietary supplementation with a high level of ZnO (3 g/kg) increased the feed intake and average daily gain of the animals and reduced the onset of diarrhoea in weanling piglets during the first days after weaning. These results are in accordance with observations from animal performance studies, in which a larger number of animals were used(Reference Hill, Cromwell and Crenshaw31, Reference Case and Carlson32). Since ZnO is known to possess antimicrobial properties, it has been usually assumed that it enhances growth by controlling pathogenic bacteria. In the present study, ZnO reduced the fermentation activity and the counts of lactobacilli and increased the counts of enterococci, as described by Hojberg et al. (Reference Hojberg, Canibe and Poulsen24). On the other hand, some studies have also demonstrated that high doses of ZnO are effective in increasing the feed intake related to modulations of gene expression(Reference Ou, Li and Cao9, Reference Martinez, Hill and Link33). Yin et al. (Reference Yin, Li and Li8) observed that ZnO supplementation increased plasma levels of ghrelin in early-weaned piglets. Ghrelin is a hormone released by the stomach, which is involved in the secretion of growth hormone and insulin-like growth factor 1 and in the stimulation of the feed intake and muscle growth.
The present results also revealed that ZnO supplementation decreased the incidence of diarrhoea and the counts of E. coli in faeces, as observed by Cardinal et al. (Reference Cardinal, D'Allaire and Fairbrother7). Diarrhoea in piglets is an important problem, which is associated, in some cases, with an overproliferation of enteropathogenic E. coli. However, animals in the present study presented diarrhoea without a pathological picture of fever, dehydration or apathy. Ou et al. (Reference Ou, Li and Cao9) demonstrated that Zn is also able to ameliorate intestinal inflammation due to inadequate feed intake after weaning by reducing the number of mast cells in the small-intestinal mucosa and submucosa and by inhibiting histamine release from mast cells.
In contrast to the results observed with the ZnO treatments, the incorporation of WB did not improve the productive performance, as was also shown in earlier studies(Reference Molist, Gómez de Segura and Gasa16, Reference Molist, Ywazaki and Gómez de Segura17), but increased the concentration of fermentation products in the faeces, especially the concentration of butyrate. These results confirm that the early-weaned pigs were able to increase carbohydrate fermentation with the inclusion of WB in the diet. It is accepted that starch and bran from wheat or oats stimulate the formation of butyrate(Reference Jensen and Jorgensen26), which is considered the principal oxidative fuel for colonocytes and may have beneficial trophic effects on the inflamed caeco-colonic mucosa(Reference Oufir, Barry and Flourie34). As observed previously with other insoluble fibre sources(Reference Molist, Ywazaki and Gómez de Segura17, Reference Kim, Mullan and Hampson35), the addition of WB in the diet decreased the E. coli and coliform bacteria counts in faeces. Previous results from our group have also indicated that the incorporation of WB in the diet also decreased the enterobacteria counts in the caecal digesta(Reference Molist, Gómez de Segura and Gasa16) and the K88 E. coli attachment to the ileum mucosa after an experimental infection(Reference Molist, Gómez de Segura and Perez18). The main mechanism involved in these changes could be an increased fermentation of carbohydrates, which may reduce protein fermentation(Reference Hermes, Molist and Ywazaki14), or likely changes in the physico-chemical properties of digesta, which can increase its water-binding capacity or the ability of some long-chain NSP to block the attachment of E. coli to the intestinal tract(Reference Becker, van Wikselaar and Jansman20).
However, a significant two-way interaction was observed between the WB and ZnO supplementation. The administration of medicated feed (ZnO) decreased the concentration of SCFA, increased coliform bacteria counts in faeces and reduced some of the microbial groups that were growth promoted when WB was included in the diet. It could be suggested that the antimicrobial effect of ZnO may have reduced the fermentation of fibre, and the main microbial changes were promoted by the WB supplementation. However, it is also intriguing that the combination of WB and ZnO also reduced the effect of therapeutic doses of ZnO in the counts of E. coli and coliform in faeces. The following two trials were designed to test hypotheses evidenced in the in vivo trial: (1) the ability of WB to bind E. coli in the intestinal digesta (trial 2) and (2) the likely mechanisms by which WB may affect the antimicrobial activity of the ZnO on the E. coli growth in vitro (trial 3).
Potential of different fibrous substrates to bind Escherichia coli
Different studies have shown the promising effects of glycoconjugates from different origins such as cranberry and blueberry extracts(Reference Ofek, Goldhar and Sharon36), mannan-oligosaccharides(Reference Spring, Wenk and Dawson37, Reference Fernandez, Hinton and Van Gils38), palm kernel extracts(Reference Allen, Fernandez and Hinton39) or soya and fermented soyabean products(Reference Kiers, Nout and Rombouts40) to inhibit the adhesion of different pathogens such as E. coli or Salmonella to the intestinal mucosa of different animal species. Dietary fibre from plants may provide an alternative adhesion matrix to enteropathogenic bacteria because of their carbohydrate nature similar to the intestinal receptors of such pathogens and low digestibility. Becker & Galleti(Reference Becker and Galleti19) tested the binding capacity of different food and feed components for E. coli K88, Salmonella enterica sv. typhimurium and Lactobacillus spp. isolated from pigs, chickens, calves and humans. They reported positive scores for sesame seed extract and soyabean products against E. coli K88 in vitro. In recent studies, Kim et al. (Reference Kim, Mullan and Hampson35) and Becker et al. (Reference Becker, van Wikselaar and Jansman20) also reported the blocking capacity of oat hulls or pea hulls against E. coli K88. In the present study, WB extracts showed the highest ability to bind E. coli K88 among the different fibre sources evaluated. The binding activity was higher in the presence of the F4-fimbriated E. coli K88 in comparison with the non-fimbriated E. coli. These results are in good agreement with those we found earlier regarding the reduction promoted by WB on enterobacteria and coliform counts in the digesta and attached E. coli K88 to the ileum mucosa(Reference Molist, Gómez de Segura and Gasa16, Reference Molist, Gómez de Segura and Perez18). WB is one of the more available fibre sources for human and animal feeding. It contains insoluble NSP(Reference Ralet, Faulds and Williamson41) mainly as arabinoxylan, cellulose and β-glucan but also minute levels of glucomannans(Reference Mares and Stone42) and arabinogalactans(Reference Fincher, Sawyer and Stone43) originating from the aleurone and endosperm cells. It might be speculated that the soluble fraction of WB may form a matrix in the gut in which fimbriated E. coli is captured. The adhesion of bacteria to the WB matrix may allow their growth, as is observed in the in vitro system, but it also provides a mechanism by which the attachment and proliferation of E. coli K88 at the intestinal epithelium is inhibited or reduced.
Possible mechanism involved in the interaction between wheat bran and zinc oxide
Negative interactions between WB and ZnO have been reported in the present study in vivo and in vitro. In the in vivo trial, the WB–ZnO combination did not reduce E. coli and the coliform counts as ZnO or WB did. In the in vitro trial, the WB–ZnO combination did not have the same antimicrobial effect on the E. coli strains as ZnO and the combination of WB–phytase and ZnO did. Therefore, it is suggested that a negative interaction between phytic acid (PA) and ZnO modifies the antimicrobial properties of therapeutic doses of ZnO in vivo and in vitro. Champagne & Fisher(Reference Champagne and Fisher44) suggested that PA, primarily found in the pericarp of cereal grains, may form a rather stable complex with bivalent cations, such as Cu2+ and Zn2+. These complexes are known not to affect Zn bioavailability in chicks(Reference O'Dell and Savage45) and also in humans(Reference Hambidge, Miller and Westcott46). Procedures that degrade phytate have been studied as a means to increase the bioavailability of Zn and other cations in the diet(Reference Bobilya, Ellersieck and Gordon47, Reference Frontela, Scarino and Ferruzza48). It is known that fermentation of feed may reduce the PA:Zn ratio, promoting a better Zn absorption(Reference Hirabayashi, Matsui and Yano49). Other authors, namely Gaetke et al. (Reference Gaetke, McClain and Toleman50), also have shown that yogurt (both active and heat-treated) protects against growth retardation in weanling rats fed high PA. In animals, feeding PA has been regarded as an anti-nutrient, which reduces P availability, and most research in this field has been aimed at eliminating PA from the animal feed by adding exogenous phytase to it. The term phytase is defined as a class of phosphatases with the in vitro capability to release at least one phosphate from PA(Reference McCollum and Hart51). Some authors(Reference Martinez, Hill and Link52, Reference Revy, Jondreville and Dourmad53) have suggested that phytase supplementation may increase the amount of Zn absorbed, even when pharmacological doses of Zn are included in the diet. Thus, Martinez et al. (Reference Martinez, Hill and Link52) suggested that present pharmacological doses of Zn (2000 mg/kg) fed to pigs could be reduced to 1000 mg/kg by adding phytase. The present study confirms a negative interaction between the WB and therapeutic doses of ZnO, which appears to be related to the high levels of PA in WB. Taking into account these results, phytase supplementation may be proposed as a good approach to increase the effectiveness or to reduce the levels of ZnO in post-weaning diets.
Conclusion
Based on the results of the present study, we conclude that the incorporation of WB in the diet of early-weaning piglets may improve their gut health by modulating the activity of the intestinal microbiota, enhancing the fermentation and blocking the attachment of E. coli K88 to the intestinal mucosa. A negative interaction observed in vivo and in vitro between WB (rich in phytate) and ZnO raises the interest of considering the inclusion of phytase enzymes to reduce the required levels of ZnO in post-weaning diets.
Acknowledgements
The present study was supported by the Spanish CICYT (project AGL2005-07438-C02-01). We thank the Ministerio de Educación, Cultura y Deporte, Spain for research fellowships. We also thank the Servei de Granges i Camps Experimentals de la UAB for their service and assistance during the experiment. There are no conflicts of interest between the authors. The contribution of each author to the study has been as follows: F. M., R. G. H., A. G. d. S., S. M. M. and J. F. P. participated in the research and in the writing of the manuscript. J. G. and E. G. M. participated in the writing of the manuscript.








