Introduction
Burkholderia pseudomallei, a saprophytic bacterium present in the soil and surface water of tropical and sub-tropical regions, is responsible for the disease melioidosis. Exposure rarely evolves to significant disease in the absence of specific comorbidities, however, in susceptible hosts, the infection can be rapidly fatal [Reference Cheng1, Reference Cheng and Currie2]. Factors responsible for the temporospatial distribution of cases are incompletely understood, although a combination of rainfall, groundwater levels and the physicochemical properties of the local soil appear important [Reference Kaestli3, Reference Hantrakun4].
Fine textured soils with a higher proportion of silt and clay particles provide conditions preferred by the bacteria. In Townsville, Australia, melioidosis cases were most prevalent on floodplain alluvial fans with poorly drained sodic and clay subsoils and less prevalent in better-drained alluvial soils and sandy beach ridges [Reference Corkeron, Norton and Nelson5]. In a smaller scale investigation with a sample size of two plots in an area with high prevalence of melioidosis with generally sandy soil, plots with a greater silt and clay content had higher B. pseudomallei counts [Reference Baker6]. In rice fields in Thailand reference negative association of B. pseudomallei with fine-textured soils has been identified [Reference Hantrakun4], suggesting that the role of soil texture in determining B. pseudomallei suitability is not uniform. Fine textured soils favour bacterial populations because of a combination of properties determined by soil porosity (proportion of pore space in soil) and pore size (size of individual pores). Fine textured soils have high porosity and small pore size compared with larger particle size soils. In more porous soils the total space available to colonisation by microbes is larger; small pore spaces create more suitable soil moisture conditions, protection from predators such as nematodes and access to necessary minerals [Reference Ruamps, Nunan and Chenu7].
Though not part of our study, a range of other soil chemical characteristics also are associated with B. pseudomallei. Field studies have identified the greater incidence of the organism in soils with a low pH and low carbon: nitrogen (C:N) ratio [Reference Ngamsang8, Reference Wang-ngarm, Chareonsudjai and Chareonsudjai9]. In controlled condition experiments, increased growth and survival of the bacterium was associated with high nitrogen, high chemical oxygen demand and pH range between five and seven [Reference Wang-ngarm, Chareonsudjai and Chareonsudjai9]. A C:N ratio of less than 40 was found necessary for survival under controlled conditions [Reference Ngamsang8]. Soil salinity limits growth [Reference Kaestli3] with salinity greater than 1% causing the death of the bacteria [Reference Baker6]. Other soil chemical properties related to B. pseudomallei occurrence include; exchangeable calcium which was hypothesised to limit growth rates [Reference Hantrakun4], iron content which promotes growth and phosphorus which promoted cell growth when added under controlled conditions [Reference Kaestli3].
Soil water status plays an important role in the distribution of B. pseudomallei and the incidence of cases of melioidosis. In melioidosis-endemic areas, the majority of clinical cases was present during the wet season [Reference Stewart10, Reference Currie, Ward and Cheng11]. Severe rainfall events, or rainfall early in the wet season, correlate with an increased number of cases [Reference Liu12, Reference Kaestli13]. In central Australia, unusually heavy rain in an arid area resulted in an outbreak of melioidosis, leading to the hypothesis of bacterial survival during dry conditions with activation and dispersion after rainfall [Reference Yip14]. In regions where soils contain greater populations of B. pseudomallei, adjacent rivers and rice fields have been found to contain a high density of the bacteria [Reference Vongphayloth15]. B. pseudomallei contaminated water has been identified as a stronger predictor of the spatial distribution of melioidosis cases than soil [Reference Dai16], indicating that although the soil is a reservoir for the bacteria, exposure to contaminated water also influences transmission.
Melioidosis is endemic in Far North Queensland (FNQ), Australia, the area having one of the highest rates of the disease in the world [Reference Stewart10]. For this study, it is hypothesised that in Cairns (FNQ's largest city) cases of melioidosis are spatially clustered, independent of spatial clustering of the population. The study aimed to investigate if observed clusters can be explained by soil characteristics which we expected to affect suitability for B. pseudomallei.
Methods
Cases of melioidosis were determined by examining the electronic database of Cairns Hospital laboratory, the sole microbiology provider for public health services in the Cairns metropolitan area, which had a population of 145 374 in 2011. Patients were included if they had a positive culture for B. pseudomallei between 1 January 1998 and 31 July 2016 and if they acquired the infection while living within the Cairns metropolitan area based on their home address on pathology request form at the time of sample collection. Cases with recurrent infection were excluded to prevent the dataset containing cases caused by a relapse of the infection. Cases with ambiguous addresses were excluded, as were cases with case notes indicating recent travel allowing that transmission may have occurred outside the study area.
The geographic bounds for spatial analysis were the Cairns residential area, which was digitised using Google Earth aerial photographs. Only areas with residential housing were included in the polygons; areas with no or very little residential housing were omitted. The distribution of the Cairns population was mapped by placing representative points per suburb in proportion to population records from the Australian Bureau of Statistics (ABS) 2011 census [17]. To identify spatial clustering, the Ripley's K statistical analysis was performed on the melioidosis cases and Cairns population datasets using ArcMap 10.2 (ESRI Inc., USA). The analysis used 20 distance bands ranging from 0 to 10 000 m. A simulation of outer bound values correction was used to compensate for edge effects. Confidence intervals were generated from 99 permutations. The point density function in ArcMap was used on the Cairns population dataset to produce the population density surface (Fig. 3). Soils of the Babinda–Cairns Area Map 1: 50 000 [Reference Murtha, Cannon and Smith18] provided the soil types and properties relevant to this study as presented in Table 1, with porosity calculated by the Saxton method [Reference Saxton19].
Table 1. Prominent soil types and characteristics [Reference Murtha, Cannon and Smith18] and occurrence of melioidosis cases in Cairns

Porosity calculated by Saxton method [Reference Saxton19], – indicates no data.
The Far North Queensland Human Research Ethics Committee granted approval for the study and waived the requirement for individual patient consent (HREC/15/QCH/46–977).
Results
There were 40 culture-confirmed cases during the study period. Twelve of these cases occurred in 2016, the other cases were evenly distributed across the study period indicating that outbreak like the situation was not responsible for the resultant spatial distribution. Of the 40 cases, three were excluded due to case notes that indicated the disease may have been contracted away from the patients home address.
The K values (from Ripley's K analysis) for melioidosis cases were higher than an expected normal distribution (Fig. 1) and outside the 99% confidence interval at distances of between 1.5 and 2.5 km, indicating statistically significant clustering in the distribution of melioidosis cases at this scale. The observed K value for the population was within the 99% confidence interval at all distances, indicating no significant clustering of general population distribution. The distribution of both melioidosis cases and population were dispersed at distances of greater than 5 km, as indicated by K values less than the expected normal distribution.
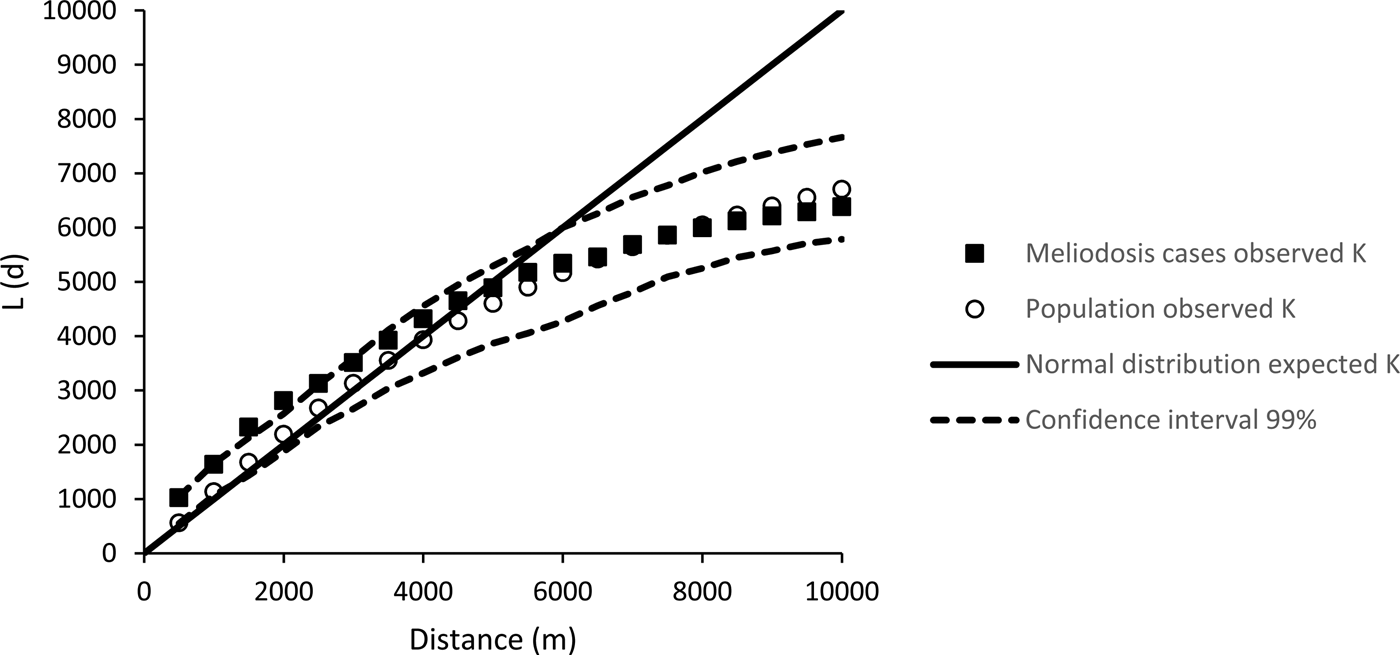
Fig. 1. Result of Ripley's K analysis of cases of distribution of melioidosis cases in Cairns (1998 to 2016) and the distribution of Cairns population in 2011.
The Ripley's K analysis identifies the presence of clustering, but not its location. Visual observation of the distribution of cases shows clusters around two main areas; in the inner western suburbs of Cairns, around the suburb Manoora (twelve cases) and the northern beach suburbs around Kewarra Beach (eight cases) (Fig. 2). These clusters appear associated with the Buchan and Clifton soil types, respectively. Both clusters occur on alluvial fan landforms and appear independent of population distribution, particularly the Kewarra Beach cluster (Fig. 3). The central, western and eastern suburbs did not record a substantial number of cases and the southern suburbs recorded some cases, but there was no clustering. The population density in the area around the Manoora cluster was high (Fig. 3), while the population density around the Kewarra Beach cluster was comparatively low. This suggests that the Kewarra Beach cluster results from environmental factors, as it occurs despite the low population density.
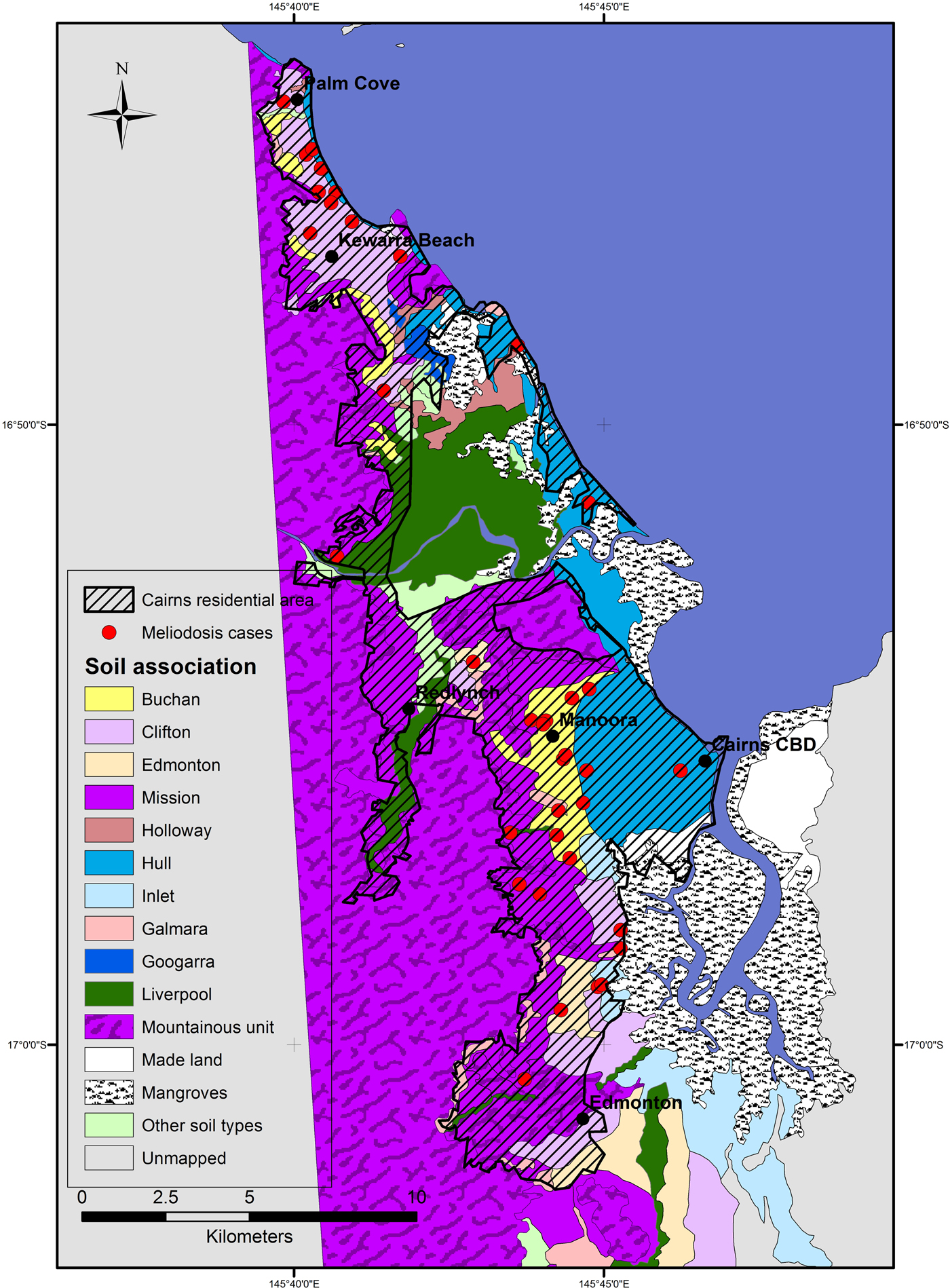
Fig. 2. Soil types and distribution of melioidosis cases in Cairns between 1998 and 2016. Cairns residential area is extent of residential housing as observed from aerial photography.
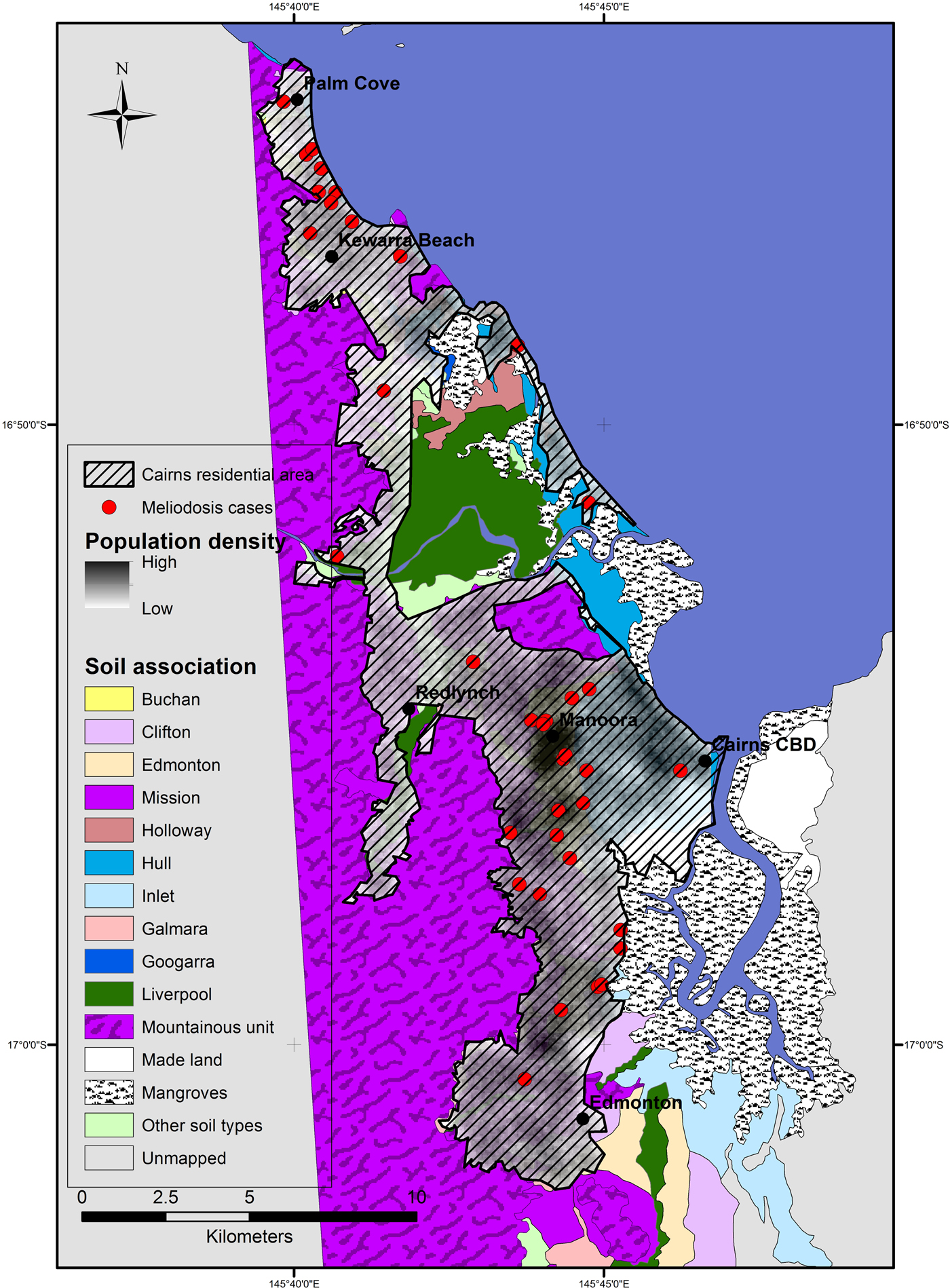
Fig. 3. Distribution of Cairns population in 2011 derived from population data per suburb and suburb geographical boundaries. Cairns residential area is the extent of residential housing as observed from aerial photography.
Discussion
The incidence of melioidosis in metropolitan Cairns was lower than observed for other metropolitan regions where melioidosis is endemic. For comparison, a study of spatial distribution of melioidosis cases in Townsville (with a similar population to Cairns) returned 67 cases over a 12-year period [Reference Corkeron, Norton and Nelson5]. Significant clustering in the distribution of cases, independent of population clustering, was identified in Cairns (Fig. 2) and this clustering can be related to the distribution of soil types.
The soil type around the Kewarra Beach area was predominantly Clifton; strongly bleached gradational textured soils on alluvial fans from metamorphic rocks. The Kewarra Beach cluster was bounded by pale loams occurring on steep hillsides to the west. The Hull soil type occupies the area between the mapped Clifton soil type and the ocean and occurs on beach ridge landforms (Fig. 1). The Clifton soil type occupies only 13% of the Cairns metropolitan area but had 30% of all the cases which indicates the soil type has characteristics which make it suitable for B. pseudomallei. Clay content was moderate for the Clifton soil and pH and EC were also comparable with other soils in the area. The soil exhibits evidence of poor drainage, mottling is described from 16 cm depth in the profile [17], which has potential to retain conditions suitable for the growth of B. pseudomallei. Similar conditions; poorly drained soils adjacent to slopes which drain perched water tables have been shown to create habitat suitable to B. pseudomallei [Reference Corkeron, Norton and Nelson5, Reference Baker and Warner20]. Clifton soils share other characteristics with soils considered suitable for B. pseudomallei growth [Reference Limmathurotsakul21]. Acrisols have been described as highly suitable for B. pseudomallei, this type of soil has strong bleaching and base saturation of less than 50%, consistent with the Clifton soil type.
The soil surrounding the Manoora cluster was largely the Buchan soil type. The Buchan soil type is a yellow gradational textured soil formed on alluvial fans, is silty clay loam with moderate clay content and porosity. Electrical conductivity and pH were within expected ideal range for B. pseudomallei and comparable with other soils in the area. The clay content indicates that Buchan soil type is likely to have slower drainage characteristics and higher retention of soil moisture compared with the adjacent much sandier and well-drained Hull soil type.
The Hull soil type, despite occupying similarly densely populated area of Cairns, had low melioidosis incidence, this difference is likely controlled by the large differences in soil characteristics between the two soil types and is indicative of soil less suitable for B. pseudomallei. The majority of cases recorded on the Hull soil type occur along boundaries with other soils, within the error margin for the precision of the maps.
Soil porosity and pore size is a determinant of total microbial activity and has been correlated with microbial biomass [Reference Strong22] and enzymatic activity [Reference Pagliai and De Nobili23]. The role of porosity in determining soil moisture conditions is likely to be the main controlling factor controlling soil microbial activity. The decrease of microbial activity after reduction of soil pore size through compaction has been demonstrated experimentally [Reference De Neve and Hofman24] and pore size probably controls suitability for B. pseudomallei in several ways. Fine textured soils typically have smaller pore sizes which have greater matric potential and store water in spaces accessible to smaller microbes. Experimentation of organisms that measure 0.97 µm has shown they are more responsive than larger soil biota to introduction of substrate in small pore spaces [Reference Ruamps, Nunan and Chenu7], B. pseudomallei cells range in size from 0.5 to 1 µm [Reference Inglis and Sagripanti25] and are therefore likely to be similarly dependent on small pore spaces Therefore it is expected that fine-textured soils with high porosity and smaller pore spaces in the environment will influence distribution.
Few melioidosis cases were recorded 10 km south of the Cairns central area despite the dense population. The soils in this area were predominantly mapped as soil types ‘mountainous unit’, Mission and Galmarra. The mountainous unit and Galmarra soils occur on sloping land; these landforms may not produce soil moisture conditions required for the development of large communities of B. pseudomallei. The Clifton soil type which was associated with a melioidosis case cluster in the north of the city was also present in this area, along with the Edmonton Soil type which recorded a high melioidosis incidence rate. The Edmonton area has grown in population relatively recently compared with the other suburbs of Cairns. In the initial years of the study period, Edmonton had a proportionally smaller population, reducing the chance of melioidosis cases occurring here, possibly offsetting the likelihood of a cluster of cases developing. The Inlet soil type, described as alluvial plain landform, occurs to the south-east of the Buchan case cluster and has soil physical properties similar to both Buchan and Clifton. However, the parent material for Inlet is described as partially of marine origin [Reference Murtha, Cannon and Smith18] and the location of the soil type next to mangrove areas indicates potential acid sulfate sediments which is likely limiting for B. pseudomallei growth.
Sociodemographic characteristics of the suburb of Manoora case cluster are likely to contribute to the incidence of melioidosis in this area. Manoora has the highest percentage (25.9%) Aboriginal and Torres Strait Islander (ATSI) population, of all Cairns suburbs [26]. The FNQ ATSI population has a higher rate of risk factors for melioidosis than non-indigenous Australians with a Rate Ratio (RR) of diabetes of 3.3 [27] and an RR 3.7 for chronic kidney disease [28, 29]. Diabetes and renal disease are two key risk factors for melioidosis contraction. Kewarra Beach has a lower ATSI population (2.5%) [30], further supporting the contention that environmental factors are contributing to the local incidence of the disease.
Confirmation of a link between clinical case clusters and B. pseudomallei soil population in these areas could be established through core sampling. The investigation into the soil characteristics and micro-ecology in the case cluster areas could further elucidate important environmental factors for the Far North region [Reference Rac and McLaughlin31].
Case mapping is essential for disease surveillance and aids healthcare professionals to identify high-risk zones and populations. Identifying areas at risk of melioidosis is important as the disease can be rapidly fatal and patients presenting with sepsis require early and specific antibiotic therapy to improve survival [Reference Wang-ngarm, Chareonsudjai and Chareonsudjai9]. It could also further encourage local healthcare professionals to aggressively manage chronic medical conditions known to predispose people to melioidosis. Meanwhile, identifying low-prevalence areas facilitates antimicrobial stewardship.
Conclusion
The clustering of cases of melioidosis in the Cairns metropolitan area, suggests local variations in the environmental suitability for B. pseudomallei. The association between cases and soils with a combination of high-clay content and porosity and low position in the landscape support the hypothesis that these conditions provide the ideal milieu for bacterial growth. Sociodemographic characteristics are likely to have also contributed to the presence of melioidosis case clusters and further soil investigation of the presence of environmental B. pseudomallei would be useful to understand factors controlling melioidosis in the area. Understanding meliodosis distribution might be used to identify high-risk locations for the disease and inform strategies to reduce melioidosis related morbidity and mortality.
Acknowledgements
Many thanks to Dr Joshua Hanson and Dr Simon Smith at Cairns Hospital for guidance and advice.






