Introduction
New Zealand has a high proportion of rare, endangered, or extinct species of birds (King, Reference King1984; Pryde & Cocklin, Reference Pryde and Cocklin1998), the loss or decline of many of which has been attributed to the introduction of mammalian predators and pressures from hunting and deforestation (King, Reference King1984; Duncan & Blackburn, Reference Duncan and Blackburn2004). Intensive predator control on offshore islands and in mainland sanctuaries enables reintroduction of native birds where predation was a cause of decline (Armstrong & McLean, Reference Armstrong and McLean1995; Pryde & Cocklin, Reference Pryde and Cocklin1998).
New Zealand has a long history of using reintroductions as a management tool, defined as ‘an attempt to establish a species in an area that was once part of its historical range, but from which it has been extirpated or become extinct’ (IUCN, 1998). As a result, many endangered bird populations have been re-established in new or restored island communities (Craig, Reference Craig1990; Armstrong & McLean, Reference Armstrong and McLean1995; Pryde & Cocklin, Reference Pryde and Cocklin1998). Reintroductions have averted extinction of little spotted kiwi Apteryx owenii (Jolly & Colbourne, Reference Jolly and Colbourne1991), North Island saddleback Philesturnus carunculatus rufusater (Saunders, Reference Saunders and Serena1994), South Island saddleback P. c. carunculatus (Armstrong & McLean, Reference Armstrong and McLean1995) and black robins Petroica traversi (Flack, Reference Flack and Temple1978; Craig, Reference Craig1990). Passerine birds have been successfully reintroduced in New Zealand (e.g. Armstrong et al., Reference Armstrong, Castro, Alley, Feenstra and Perrott1999; Armstrong & Ewen, Reference Armstrong and Ewen2002; Oppel & Beaven, Reference Oppel and Beaven2004; Taylor et al., Reference Taylor, Jamieson and Armstrong2005).
The endemic rifleman, or titipounamu Acanthisitta chloris, is one of the smallest (5-8 g) passerine birds in the world (Sherley, Reference Sherley1985). Rifleman are a locally common member of the wren family (Acanthisittidae; Heather & Robertson, Reference Heather and Robertson2000). In contrast, the rock wren Xenicus gilviventris is nationally Vulnerable (Hitchmough, Reference Hitchmough2002), while the Stephens Island wren Traversia lyalli and the bush wren X. longipes have become extinct (Gill & Martinson, Reference Gill and Martinson1991). Although neither North Island rifleman A. c. granti nor South Island rifleman A. c. chloris are in danger of global extinction, they have fragmented distributions, and in some areas have become regionally threatened or extinct (Heather & Robertson, Reference Heather and Robertson2000).
Until recently, South Island rifleman were present in the northern sector of Stewart Island/Rakiura (Heather & Robertson, Reference Heather and Robertson2000), just south of the South Island (Fig. 1). Vertebrate pests may have caused the rifleman’s extinction here as recently as the 1990s (Beaven, Reference Beaven2002), leaving only one known population in the Rakiura region, on Codfish Island/Whenua Hou. Rifleman from this location are most closely related to the now extinct Stewart Island population. In 2002 it was decided to establish a second population by reintroducing birds from Codfish Island to Ulva Island. This was part of the long-term goal of reintroducing rifleman back to Stewart Island, ultimately using a new self-sustaining Ulva Island population as a source of founders. Only one known reintroduction of wrens had been carried out prior to the rifleman reintroduction, with a small-scale reintroduction of six bush wren attempted in New Zealand (Blackburn, Reference Blackburn1965). Here we report on the techniques, shortcomings and successes of the reintroduction of rifleman to Ulva Island.
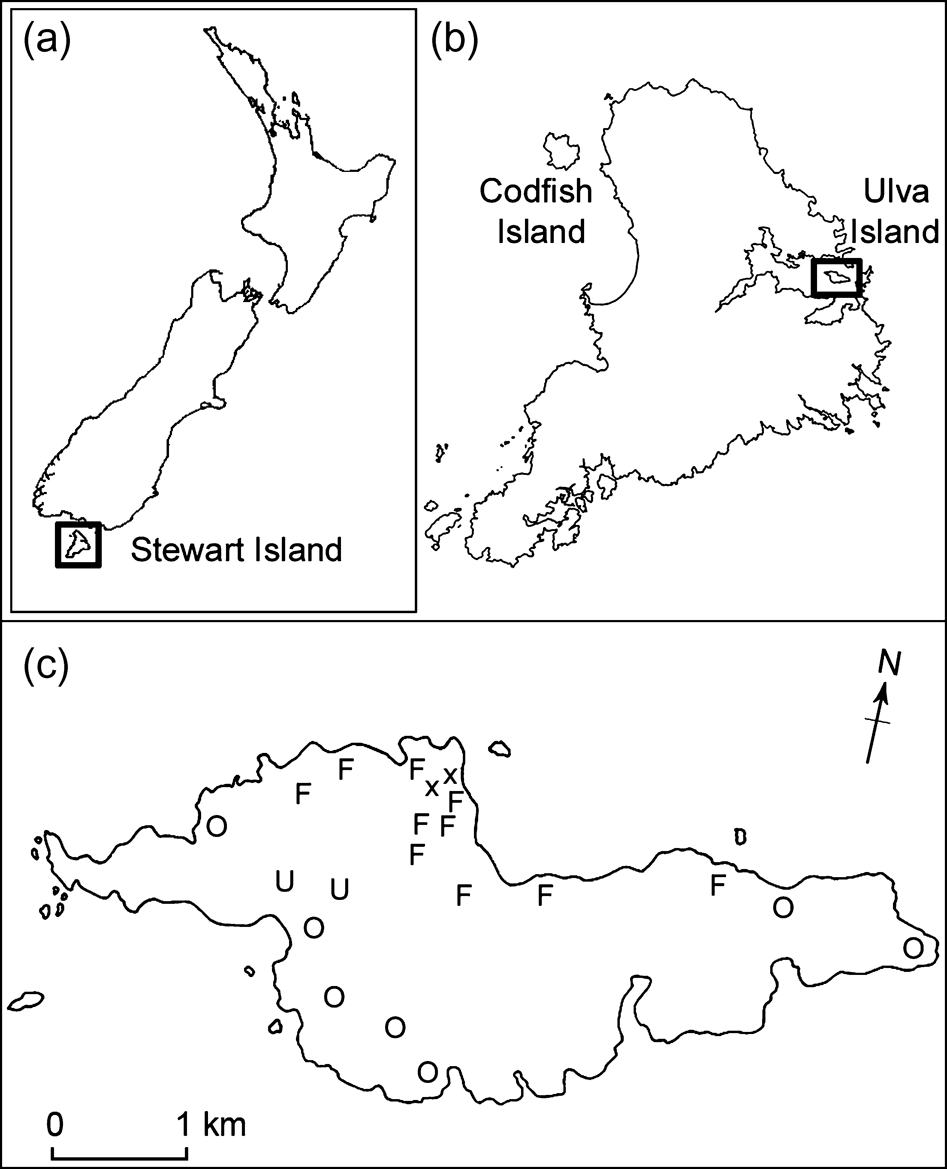
Fig. 1 (a) New Zealand (b) Stewart Island and (c) Ulva Island. The map of Ulva Island illustrates rifleman territory establishment in the 2004/2005 breeding season (x, release sites; F, territories established by at least one founder; O, territories established by offspring pairs; U, unidentified occupants).
Study sites
Codfish Island, a 1,396 ha island sanctuary managed by the Department of Conservation, is located c. 3 km north-west of Stewart Island (Fig. 1). Pacific rats Rattus exulans were eradicated from Codfish Island by 2000 (McClelland, Reference McClelland, Veitch and Clout2001). Ulva Island is a 267 ha predator free open sanctuary located 1.5 km inside Paterson Inlet of Stewart Island (Fig. 1). Ulva has a typical southern New Zealand mixed podocarp forest similar to that on Codfish Island. Ulva is the nearest predator-free island to the source of Codfish founders. Norway rats R. norvegicus were eradicated from Ulva by 1995.
Methods
Capture and holding
Techniques were developed and refined based on previous literature on rifleman (Gray, Reference Gray1969; Gaze, Reference Gaze1978; Moeed & Fitzgerald, Reference Moeed and Fitzgerald1982; Sherley, Reference Sherley1993, Reference Sherley1994), and consultation with professionals experienced in small passerine captures and/or transfers. The rifleman population on Codfish Island was assessed as widespread and abundant. Capture of rifleman took place during 6-14 February 2003 and followed the methods outlined in Sherley (Reference Sherley1985). Mist-net rigs and digital recordings of local rifleman calls were used during 07.00-14.00 and 18.00-21.00. Capture rates were highest during the first half-hour of the operation. Once caught, birds were held in dark cloth bags for up to 2 h, banded, and then moved to aviaries or transfer boxes.
Four aviaries were constructed of wooden beams and plywood covered in a double layer of chicken wire and shade cloth, spaced approximately 100 m apart. The cages were approximately 14*4.5*2 m and enclosed vegetation similar to that outside the aviaries. Water and mealworms Tenebrio molitor were provided ad libitum, and supplemented the rifleman’s natural insectivorous diet within the aviaries. Previous work confirmed that rifleman fed on mealworms and drank from the pools in the aviary during a 4-day trial. Aggression, particularly between males, required territorial groups to be separated. Individual birds were held for up to 5 days depending on the date of capture with respect to release date. Birds exhibiting stress after capture were fed glucose-enriched water, or given subcutaneous injections of 0.2 ml Hartmanns solution. Rifleman released the same day as capture were held only in transfer boxes constructed of plywood, each 40*30*30 cm. One side was netting for ventilation, with dark breathable cloth covering the netting to reduce light.
Transfer and release
Transfers took place during 10-14 February 2003. On the day of release rifleman were placed in transfer boxes. Transfer from Codfish to the two release sites took c. 4 hours, involving fixed-wing aircraft or helicopter, car, boat and foot. Release sites were chosen based on ease of access, both at the north-eastern extent of the island (Fig. 1). Rifleman were hard-released, i.e. with no additional management. Quick capture and hard release are most suitable to small territorial insectivorous passerines (Lovegrove & Veitch, Reference Lovegrove and Veitch1994; Lovegrove, Reference Lovegrove1996).
Post-release monitoring
Rifleman were monitored for 34 consecutive days from the day of the first release to assess survival and dispersal during the 1-month establishment phase. One month after release a full survey of Ulva was conducted using playback of recorded contact and alarm calls to detect all surviving rifleman. On days with no wind or rain rifleman calls could be heard up to 50 m from the speaker. Full surveys of Ulva were repeated during the first (2003/2004) and second (2004/2005) breeding seasons.
Population Viability Analysis
We developed a simple deterministic matrix model for rifleman to estimate the annual population growth rate, lambda (λ). Environmental and genetic stochasticity, catastrophes and density dependence were not modeled because of the uncertainty of these parameters for rifleman. A three-stage model following a post-breeding census format (Fig. 2) was created in the software Microsoft Excel using the PopTools add-on (Hood, Reference Hood2005). Because assessment of egg survival to hatching, fledging, and overwintering could not be separated between surveys, the survival of juveniles (stage 1) was estimated as combined survival from egg laying to the beginning of their first breeding season. Rifleman breed at the end of their first year (Sherley, Reference Sherley1985). Stage 2 represented early adults from the beginning of their first to the beginning of their second breeding season. Stage 3 included all adults from the beginning of their second breeding season and older.
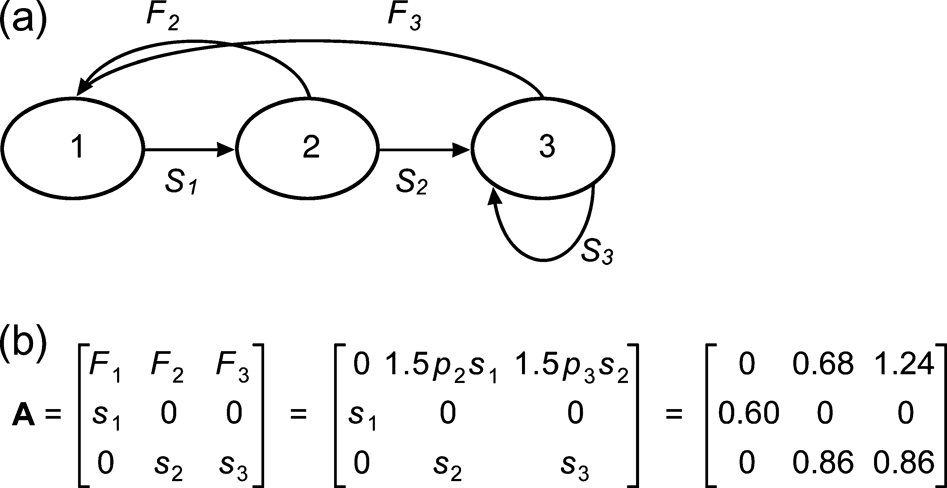
Fig. 2 (a) Post-breeding life-cycle diagram and (b) three-stage Leslie Matrix model developed for the reintroduced rifleman population on Ulva Island, New Zealand. Stages are the same as those defined in the text. F, s and p represent stage-specific fecundity, survival probability and proportion breeding, respectively.
Stage-specific survival and reproductive rates (Fig. 2) were estimated from field data collected on Ulva and from studies conducted at Kowhai Bush, near Kaikoura, South Island (Sherley, Reference Sherley1993). Stage-specific fertility was based on the average number of female chicks produced per female per year, survival from the previous stage, and proportion of rifleman breeding. A sex ratio of 1:1 was assumed for chicks (Sherley, Reference Sherley1993). Each female breeder was assumed to have 1.5 female chicks per year, based on observations from the 2003/2004 breeding season on Ulva. Only 75% of rifleman breed in their first year (Sherley, Reference Sherley1993) and therefore this proportion of breeding early-adults (stage 2) was assumed. As not all founders had confirmed mates in the 2003/2004 breeding season, the proportion of breeding adults (stage 3) was 95%.
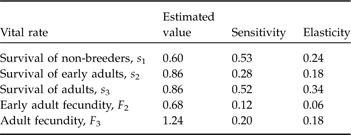
Survival rates were estimated from the survivorship of juveniles and adults from the 2003/2004 to 2004/2005 breeding seasons. Four unidentified rifleman were allocated equally to juvenile and adult groups for calculation of survival rates. As longevity was unknown for rifleman on a predator-free offshore island, a maximum age of survival was not defined. Sensitivity and elasticity analyses were undertaken for the matrix model; these are perturbation measures in matrix modeling that determine the impact of individual population parameters on population growth rates (for descriptions see Morris & Doak, Reference Morris and Doak2002).
Results
Pre-release activities
A total of 58 rifleman were captured on Codfish Island, from 29 different territories. Only 30 (52%) survived to be released on Ulva (Table 1); 14 died within the aviaries, accounting for the majority of mortality. The glucose-enriched water and Hartmanns solution treatments administered to birds exhibiting stress within the aviaries were ineffective. Six rifleman due to be released on the day of capture died in transfer boxes. Deaths occurred when birds were confined in transfer boxes for long periods of time (i.e. 6-8 hours), or when transfer boxes were in close proximity and birds attempted to attack each other. Six rifleman died during transfer from Codfish to Ulva Island. The sex ratio of the 32 released birds was 1:1, with two known juvenile and 30 adult birds from 20 different family groups. However, two birds were found dead at the release site a few hours later; their deaths are attributed to the transfer.
Table 1 Fates of rifleman caught on Codfish Island/Whenua Hou for reintroduction to Ulva Island.

Post-release survival
Of the 30 rifleman successfully released onto Ulva, 19 (63%) were positively identified 1 month post-release. Monitoring during the first breeding season (October-December 2003) confirmed the presence of 22 birds (12 males, 10 females) of the 30 released (73%). Surveys undertaken during the second breeding season (October-November 2004) estimated a minimum annual survival rate of founders of 77-95% (17 confirmed identities, 4 unconfirmed; 9 males, 8 females). The cohorts of the four unconfirmed birds could not be verified. Survival rates are considered a minimum as it is possible that birds may have been alive but undetected despite intensive surveys of the entire island.
Approximately 30 offspring were produced by founders in the 2003/2004 breeding season, assuming an average clutch size of three eggs for 10 confirmed breeding pairs, based on breeding observations. Here offspring refers to the first group of unbanded fledglings from the 2003/2004 breeding season. The precautionary estimate of 30 offspring was based on the assumption that breeding pairs had one clutch. First clutches are often laid in late September to early October, with some second or replacement clutches laid at the end of December (Heather & Robertson, Reference Heather and Robertson2000). As the first fledgling was seen mid-December, we believe that pairs had only one clutch because of their relatively late timing of breeding. The exact number of offspring hatched and fledged was unknown as they were not banded. Of the 30 estimated offspring a minimum of 53-80% (16 confirmed, 4 unconfirmed; minimum 8 males, 8 females) survived to the 2004/2005 season. The survival rate of offspring was calculated as the total survival of hatching, fledging, plus survival over the winter period.
Dispersal and territory establishment
During the first breeding season 10 pairs established breeding territories and two solitary males remained without known mates. Territories were denoted breeding territories if confirmed by the location of nests. For birds with unconfirmed nests, territories represented the locations where they were frequently sighted. Defined territories held by all pairs and solitary individuals in the 2003/2004 breeding season were established close to release sites with the exception of three pairs that dispersed across the island (Fig. 1).
There were 19 rifleman territories on Ulva during the 2004/2005 breeding season (Fig. 1). All founders occupied the same areas as those of the previous breeding season, and breeding pairs from the 2003/2004 season remained together for the 2004/2005 breeding period when both members of the pair survived. Offspring pairs dispersed more widely across the island than the founders. Territories were almost always held by pairs, with one exception in which a mate may have been present but was undetected. Nine rifleman nests were located in the 2004/2005 season, indicating at least nine pairs were actively breeding. Both founding and offspring pairs were breeding, as six nests with founding birds and three nests with breeding offspring were confirmed.
Population viability analysis
The simple deterministic matrix model projected the population growth rate (λ) of the current rifleman population on Ulva Island to be 1.33, indicating the likelihood of rifleman population persistence in the short- to medium-term. The sensitivity analysis showed that juvenile survival had the greatest influence on the growth rate of the rifleman population, followed closely by adult survival. Adult survival demonstrated the greatest elasticity (Table 2).
Table 2 Sensitivity and elasticity analyses of vital rates on population growth rate for a three-stage matrix model for rifleman on Ulva Island, New Zealand.
Discussion
Pre-release activities
Highest mortality was observed during the holding/transport stage. Transfers should minimize holding periods where possible, although other New Zealand and Australian wild-caught birds have survived holding in temporary captivity for up to several weeks with low mortality (Castro et al., Reference Castro, Alley, Empson, Minot and Serena1995; Danks, Reference Danks and Serena1995). Mortality of rifleman both before and during transfer may have been due to stress induced by aggression and metabolic requirements. Future translocations should separate territorial groups during holding, allow sufficient time for natural feeding prior to recapture, and increase supplemental feeding during holding and transport.
The small size of rifleman may have also increased the likelihood of stress and mortality caused by capture and confinement (Flack, Reference Flack and Temple1978). Stress-related mortality has been observed in other relocated birds (Lovegrove & Veitch, Reference Lovegrove and Veitch1994; Castro et al., Reference Castro, Alley, Empson, Minot and Serena1995; Gerlach & Wanless, Reference Gerlach, Wanless, Soorae and Seddon2000; Fancy et al., Reference Fancy, Nelson, Harrity, Kuhn, Kuhn, Kuehler and Giffin2001) and elevated stress levels have resulted from longer transport times (Groombridge et al., Reference Groombridge, Massey, Bruch, Malcolm, Brosius, Okada and Sparlin2004). If some mortality due to stress is unavoidable during all stages of the reintroduction it will be important to weigh the ethical considerations of capturing more birds than required on the assumption that some will die.
Post-release survival
As reintroductions often rely on small numbers of founders (Taylor et al., Reference Taylor, Jamieson and Armstrong2005), high survival of released rifleman is necessary to prevent population decline and local extinction. Reintroduced rifleman had a survival rate of 73% 8 months after release, with no mortality between 1 month post-release and the first breeding season. Armstrong et al. (Reference Armstrong, Castro, Alley, Feenstra and Perrott1999) reviewed a number of reintroductions that showed a period of high mortality immediately following release. Survival rates of the stitchbird Notiomystis cincta ranged from 42 to 68% shortly after reintroduction, with the majority disappearing within the first month after release (Castro et al., Reference Castro, Alley, Empson, Minot and Serena1995; Armstrong et al., Reference Armstrong, Davidson, Dimond, Perrott, Castro, Ewen, Griffiths and Taylor2002). Birds moved to predator-free offshore islands tend to show high survival rates in the first 6 months post-release (Armstrong et al., Reference Armstrong, Davidson, Dimond, Perrott, Castro, Ewen, Griffiths and Taylor2002). Although Sherley (Reference Sherley1985) found that mortality of rifleman >1 year old is low, some founders may have been older adults at the time of release and thus natural mortality occurred. The shorter life-span and smaller size of rifleman may have contributed to lower survival rates in comparison to other reintroduced birds on predator-free offshore islands.
Between 77-95% of founders and 53-80% of offspring survived from the first breeding season to the second. Previous research found that rifleman in their first year have lower survival rates than adult birds (Sherley, Reference Sherley1985). Survival of adult males was slightly higher than that of females, and was similar for each sex for fledged rifleman offspring, similar to survival rates reported by Sherley (Reference Sherley1985). The precautionary estimate of 3 eggs per nest was possibly too low compared to other rifleman studies with average first clutch sizes in nest boxes of 3.8 - 4.5 (Gray, Reference Gray1969; Gaze, Reference Gaze1978; Sherley, Reference Sherley1985). Average second clutch sizes may be 3.0 - 3.8 (Gray, Reference Gray1969; Sherley, Reference Sherley1985), and third clutches are sometimes successful in years with low or absent predation (G. Sherley, pers. comm.). Rifleman on Ulva nested in natural tree cavities, which may have led to lower productivity than in nest boxes. Conservative estimates were used because of the uncertainty of rifleman productivity on the island. If a greater number of fledglings than estimated resulted from the 2003/2004 breeding season, our juvenile survival rates may be overestimates. Predation by mammals is the main factor influencing the survival rates of rifleman offspring on mainland New Zealand, with only 14-23% of offspring surviving at Kowhai Bush (Sherley, Reference Sherley1985; Cameron, Reference Cameron1990).
Dispersal and territory establishment
Rifleman exhibited fidelity to the release sites during the first breeding season post-release, as territories were primarily established close to the release sites. Pair bonds were maintained between the first and second breeding season, and the same territories were occupied when one or both members of a pair survived. Site fidelity and long-term bonds were also observed in rifleman at Kowhai Bush (Cameron, Reference Cameron1990).
Future persistence
The PVA indicated positive population growth in the short- to medium-term. Because the sensitivity and elasticity analyses indicated survival had a greater influence on population growth rate than did fertility, future rifleman research should focus on estimation of stage-structured survival to improve model accuracy. The results of the rifleman model should be applied cautiously, however. Whereas sparse data may result in imprecise population parameters for long-term population growth (Morris & Doak, Reference Morris and Doak2002), a smaller data set for a qualitative analysis may be sufficient for modeling positive growth in the short- to medium-term. Our population model could provide a foundation on which future monitoring and modeling of rifleman populations can be based.
In a small population with no immigration, inbreeding may reduce reproductive fitness and survival (Frankham et al., Reference Frankham, Ballou and Briscoe2002). Hatching failure was found to be widespread among New Zealand birds passing through bottlenecks of <150 individuals (Briskie & Mackintosh, Reference Briskie and Mackintosh2004). The rifleman population on Ulva had an initial breeding population of 20 known individuals in the first breeding season. Although 58 riflemen were originally captured from 29 family groups, the rifleman that bred in the first breeding season represented only 15 family groups. It is possible that the rifleman population may undergo a slight founder effect.
Suggestions for future reintroductions
The rifleman reintroduction to Ulva Island can be viewed as a model for future reintroductions of similar small passerine birds, such as the more threatened rock wren. Because there is an element of risk involved when dealing with threatened birds, the successes and failures of previously reintroduced species having similar biological and ecological characteristics can be used to predict the outcome of future reintroductions. Saunders (Reference Saunders and Serena1994) suggested that the availability of a suitable reintroduction technique may have avoided an unsuccessful translocation attempt of Stead’s bush wren X. l. variabilis and prevented extinction of the species.
Future translocation efforts of rifleman and other small passerines may be advised to consider temporary portable aviaries erected in birds’ territories. An alternative is that individuals from different territories could be placed in separate holding aviaries to avoid aggression. Re-capture of rifleman from the aviaries for transfer may best take place later in the morning after birds have fed. Ideally, the immediate transfer of birds would avoid holding altogether. Minimizing the period of captivity may decrease stress and starvation related mortality observed when transferring birds.
Long-term post-release monitoring of animal reintroductions is essential to gain a full understanding of the probability of persistence of reintroduced populations. Vital rates that should be monitored annually include age- or stage- specific survival, reproductive success (i.e. hatching and fledging success), demographic stochasticity (i.e. sex ratio, age distribution) and the degree of inbreeding. Inbreeding assessment is particularly important for reintroductions of a small number of founders, although it is generally accepted in New Zealand that at least 30 individuals are required for a bird translocation (Armstrong & McLean, Reference Armstrong and McLean1995). However, Taylor et al. (Reference Taylor, Jamieson and Armstrong2005) reported that releasing only small numbers of saddleback and New Zealand robins P. australis did not prevent population growth to carrying capacity, providing introduced mammalian pests were absent or controlled. Taken together, the population model for rifleman supports the suggestion that a small population of released birds can increase quickly, at least in the initial stages when the negative influences of density dependence and inbreeding depression may be absent. In time, a self-sustaining population of rifleman on Ulva Island may provide a source of founders for a future reintroduction of rifleman to Stewart Island.
Acknowledgements
This work was made possible by the combined support of the Southland Conservancy of the Department of Conservation, the University of Otago, the Ulva Island Trust and the New Zealand National Parks and Conservation Foundation. Individual recognition is merited for Ros Cole, Murray Willans, Megan Willans, Jessyca Bernard, Andrew King, Kari Beaven, Jo Wright, Diedre Vercoe, Wally Hockly, Ian Jamieson, Paul Igag, Greg Sherley, Peter Dilks, Pete McClelland, Sue Heath, Peter Gaze, Rachel Johnston, Karin Ludwig, Ken Miller and Phred Dobbins. We also thank Greg Sherley and Brian Bell for comments on an earlier version of the manuscript.
Biographical sketches
Tara Leech’s research interests include the spatial ecology and population dynamics of native forest birds and conservation priority-setting.
Emma Craig is currently involved in the large-scale conservation management and research of kiwi.
Brent Beaven is conducting and overseeing a wide range of conservation research and management projects in the Rakiura region of New Zealand.
David Mitchell is currently working on the inventory and conservation of a wide range of biodiversity of the East Papuan Islands.
Philip Seddon is currently director of the Wildlife Management programme at the University of Otago, where his research interests include the restoration and conservation management of native species in New Zealand. He is the current Chair of the Bird Section of the IUCN/SSC Reintroduction Specialist Group.






