Introduction
The family Anacardiaceae presents some of the most remarkable examples of seed protection by means of a hard woody endocarp, as well as some of the most ingenious devices to allow of the escape of the germinating embryos (Hill, Reference Hill1933).
The large cashew family Anacardiaceae (Magnoliidae, Sapindales) consists of 82 genera and 950 species. It includes two subfamilies and five tribes: Anacardioideae [tribes Anacardieae (Mangiferae sensu Engler, Reference Engler, Engler and Prantl1892), Dobineeae, Rhoeae and Semecarpeae] and Spondioideae (tribe Spondiadeae). The family is monophyletic and sister to the Burseraceae. The Anacardiaceae consists mostly of trees, shrubs and lianas, and its geographical distribution primarily is pantropical, with some members, for example Cotinus, Rhus and Toxicodendron, extending into the temperate zone. It occurs on all of the continents except Antarctica. The fruit type of most members (c. 80% of genera) of the Anacardiaceae is a drupe, and its highly variable endocarp anatomy is a central issue in determining the kind of seed dormancy (or non-dormancy). The family has a fossil fruit record that extends back to the Palaeogene. Regarding germination, some members of subfamily Spondioideae have an operculum (‘germination lid’) in the endocarp, while this structure is absent in subfamily Anacardioideae (Engler, Reference Engler, Engler and Prantl1892; Hill, Reference Hill1933, Reference Hill1937, Reference Hill1939; Mitchell and Mori, Reference Mitchell and Mori1987; Wannan and Quinn, Reference Wannan and Quinn1990, Reference Wannan and Quinn1991; Gadek et al., Reference Gadek, Fernando, Quinn, Hoot, Terrazas, Sheahan and Chase1996; Pell, Reference Pell2004; Mitchell et al., Reference Mitchell, Daly, Pell and Randrianasola2006; Wannan, Reference Wannan2006; Pell et al., Reference Pell, Mitchell, Lowry, Randrianasolo and Urbatsch2008, Reference Pell, Mitchell, Miller, Lobova and Kubitzki2011; Weeks et al., Reference Weeks, Zapata, Pell, Daly, Mitchell and Fine2014; APG IV, 2016; Mabberley, Reference Mabberley2017; Wheeler and Madeira, Reference Wheeler and Madeira2017; Herrera et al., Reference Herrera, Mitchell, Pell, Collinson, Daly and Manchester2018).
The germination unit of most species of Anacardiaceae is the drupe (or more specifically the true seed + endocarp; hereafter seed). Furthermore, the seed coat does not contain a mechanical layer. Thus, the functional roles of the seed coat, namely embryo protection and regulation of germination, are performed by the endocarp. In a few species (e.g. Pleiogynium), the mesocarp also is stony and helps to protect the seed. In a few genera, the endocarp of the mature fruit is water-impermeable, and thus, the seed has physical dormancy (PY), which may be combined with physiological dormancy (PD), that is combinational dormancy (PY + PD). However, in most genera, the endocarp is water-permeable, and the seed either is non-dormant (ND) or has PD. The embryo is fully developed, and the endosperm is scant or absent in mature seeds. Thus, the seed cannot have morphological dormancy or morphophysiological dormancy (Hill, Reference Hill1939; Corner, Reference Corner1976; von Teichman, Reference von Teichman1988b, Reference von Teichman1991; Baskin and Baskin, Reference Baskin and Baskin2014 and references cited therein).
There is considerable variation in endocarp anatomy within Anacardiaceae. Wannan and Quinn (Reference Wannan and Quinn1990) recognized two distinct basic types of endocarps within the family: the Spondias-type and the Anacardium-type (Table 1). The Spondias-type occurs throughout the tribe Spondiadeae, and the Anacardium-type occurs in the other four tribes of the family. However, the endocarps of Buchanania of tribe Anacardieae and of Campnosperma and Pentaspadon of tribe Rhoeae are similar to those of the Spondias-type (Wannan and Quinn, Reference Wannan and Quinn1990). Furthermore, within the tribe Rhoeae, Wannan and Quinn (Reference Wannan and Quinn1990) defined three groups (subtypes) of endocarps (A, B and C), and von Teichman (Reference von Teichman1991, Reference von Teichman1998) and von Teichman and van Wyk (Reference von Teichman and van Wyk1996) added a fourth one (i.e. Group D) (Table 1).
Table 1. Classification of endocarp structure (anatomy) in Anacardiaceae (primarily from Wannan and Quinn, Reference Wannan and Quinn1990, except Anacardium-type tribe Rhoeae Group D from von Teichman, Reference von Teichman1991, Reference von Teichman1998; von Teichman and van Wyk, Reference von Teichman and van Wyk1996)
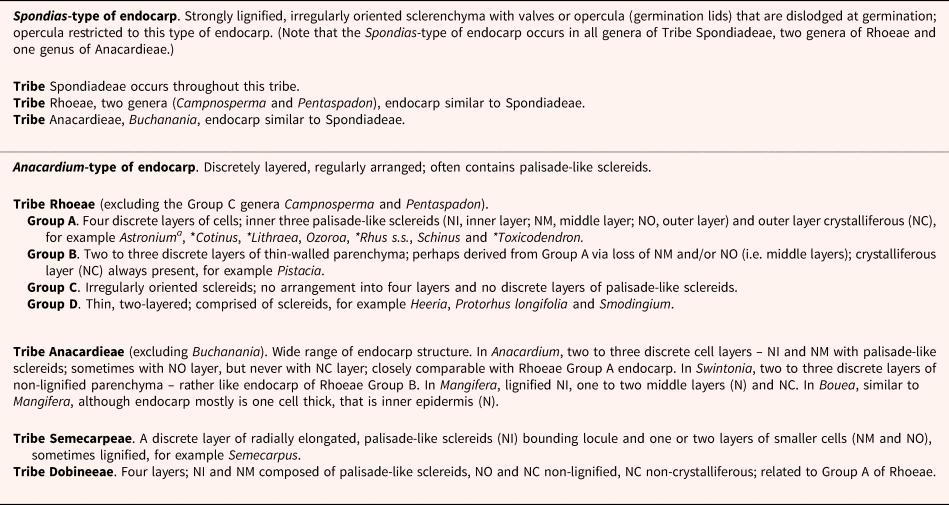
Toxicodendron endocarp anatomy is from Copeland and Doyel (Reference Copeland and Doyel1940). Only those genera for which we have information on germination are included as examples of groups and subgroups. An asterisk (*) indicates a water-impermeable endocarp, that is physical dormancy and/or physical + physiological dormancy in the genus.
a Wannan and Quinn (Reference Wannan and Quinn1990) classified the Astronium endocarp as Anacardium-type Rhoeae Group A based on Astronium urundeuva fruit morphology. However, this species has been synonymized as Myracrodruon urundeuva (see de Lima et al., Reference de Lima, Tölke, da Silva-Luz, Demarco and Carmello-Guerreiro2022). Carmello-Guerreiro and Paoli (Reference Carmello-Guerreiro and Paoli2000) demonstrated that the pericarp structure of Astronium graveolens lacks the four-layered Rhoeae Group A endocarp as defined by Wannan and Quinn (Reference Wannan and Quinn1990) for Astronium. Carmello-Guerreiro and Paoli (Reference Carmello-Guerreiro and Paoli2000) concluded that the two-layered endocarp of A. graveolens is Anacardium-type B of Wannan and Quinn. de Lima et al. (Reference de Lima, Tölke, da Silva-Luz, Demarco and Carmello-Guerreiro2022) studied the fruit morphology of seven of the eight species of Astronium and the two species of Myracrodruon, and they observed the same pattern in all Astronium species except one, in agreement with Carmello-Guerreiro and Paoli (Reference Carmello-Guerreiro and Paoli2000). de Lima et al. (Reference de Lima, Tölke, da Silva-Luz, Demarco and Carmello-Guerreiro2022) concluded that Anacardium-type Rhoeae Group A endocarp was present in Myracrodruon but not in Astronium.
The primary aim of this review was to determine the relationship between kind (class, sensu Baskin and Baskin, Reference Baskin and Baskin2021) of seed dormancy (or non-dormancy), taxonomic position and endocarp anatomy within the family Anacardiaceae. In particular, we wanted to know if PY and PY + PD are associated only with the Anacardium-type Rhoeae Group A endocarp, such as it is in the Rhus s.s. species (Li et al., Reference Li, Baskin and Baskin1999a,Reference Li, Baskin and Baskinb,Reference Li, Baskin and Baskinc,Reference Li, Baskin and Baskind,Reference Li, Baskin and Baskine). Furthermore, do all taxa with this endocarp subtype have PY?
Methods
An extensive literature search was conducted for information on seed dormancy in Anacardiaceae. In a number of cases, the type of seed (i.e. true seed + endocarp) dormancy was inferred from seed germination phenology studies and/or from what already was known about dormancy and how to break it in that particular taxon (genus, tribe). Seeds that germinated without pretreatment within about 30 d of sowing in either phenology or laboratory studies were considered to be ND, whereas those that required longer periods to germinate were judged to have either PY, PD or PY + PD (Baskin and Baskin, Reference Baskin and Baskin2014). Seeds with PY or PY + PD were distinguished from those with PD based on treatment(s) required to break dormancy/imbibe water, such as treatment with GA3 or warm and/or cold stratification for PD, mechanical scarification for PY and mechanical scarification plus cold stratification for PY + PD, endocarp anatomy and/or information on other species in the genus (see Baskin and Baskin, Reference Baskin and Baskin2014, Reference Baskin and Baskin2021). The kind (class) of seed dormancy of each species was examined in relation to its endocarp type or subtype and taxonomic position (subfamily, tribe) in the Anacardiaceae.
Results and discussion
We found information on both seed dormancy/germination and endocarp anatomy [i.e. Anacardium-type Rhoeae Groups A, B, C or D sensu Wannan and Quinn, Reference Wannan and Quinn1990; von Teichman, Reference von Teichman1991, Reference von Teichman1998; von Teichman and van Wyk, Reference von Teichman and van Wyk1996 and/or Anacardium (A1), Anacardium (A2) or Spondias (B) sensu Wannan and Quinn, Reference Wannan and Quinn1991] for taxa in all five tribes of Anacardiaceae (Table 2). The 55 genera listed in Table 2 include 67.1% of the 82 genera and 742 (78.1%) of the 950 species in the family (sensu Mabberley, Reference Mabberley2017). Seeds of the 15 genera of tribe Spondiadeae (Spondias-type endocarp) are ND or have PD, those of the six genera of Anacardieae (Anacardium-type endocarp) are ND or have PD, those of the 30 genera of Rhoeae (Anacardium-type Rhoeae Groups A, B, C and D) are ND or have PY, PD or PY + PD and those of the three genera in tribe Semecarpeae and the one genus in the tribe Dobineeae (Anacardium-type endocarp) have ND and PD and PD, respectively. Recalcitrant seeds have been documented in 16 species and 9 genera of tribes Anacardieae (Bouea, Gluta and Mangifera), Rhoeae (Heeria, Protorhus and Sorindeia) and Spondiadeae (Lannea, Spondia and Tapirira) (Subbiah et al., Reference Subbiah, Ramdhani, Pammenter, Macdonald and Sershen2019).
Table 2. Types of endocarp and kinds of seed dormancy (or non-dormancy) in Anacardiaceae

Endocarp types Spondias (B), Anacardium (A1) and Anacardium (A2) are from Wannan and Quinn (Reference Wannan and Quinn1991), Rhoeae Group D from von Teichman (Reference von Teichman1991, Reference von Teichman1998), von Teichman and van Wyk (Reference von Teichman and van Wyk1996) and all others from Wannan and Quinn (Reference Wannan and Quinn1990). ND, non-dormancy; PD, physiological dormancy; PY, physical dormancy; PY + PD, combinational dormancy. Regarding ND versus PD, some species have a mixture of ND and PD, and others are wholly or primarily ND or PD.
a See footnote to Table 1.
In the five tribes of Anacardiaceae, PY and PY + PD occur only in the Rhoeae. Furthermore, within Rhoeae, PY is restricted to those taxa with Group A endocarp anatomy (Table 1). However, although seeds of Myracrodruon, for example, have this type of endocarp anatomy, they do not have PY; in fact, they are ND. de Melo et al. (Reference de Melo, Ribeiro and de Fritas Lima1979) reported that fresh seeds of M. urundeuva germinated to 35.5% in 14 d. In another study, Rizzini (Reference Rizzini1965) reported that seeds of M. urundeuva germinated to 90% after 19 d when planted under a cerrado (Brazilian savanna) climate in Brazil. Lorenzi (Reference Lorenzi1992) reported that seeds of M. urundeuva planted on sand enriched with organic matter germinated to 80% in 8–12 d, indicating that a high percentage of the seeds of the species are ND.
For seeds of three of the genera in tribe Rhoeae, namely Actinocheita, Haplorhus and Rhodosphaera (Table 2), information in the literature is not sufficient for us to have high confidence in assigning a dormancy class to them. Thus, we have placed a question mark beside the dormancy class. For example, Montoya Maquin (Reference Montoya Maquin1972) suggested that the pericarp (endocarp?) of Haplorhus peuviana might be impermeable to water. However, no studies were done to compare water uptake in intact versus scarified seeds to verify (or not) the impermeability of the endocarp.
Neither seeds of species in tribe Rhoeae with Group B (Pistacia), Group C (Pentospadon) nor Group D (Heeria and Smodingium) endocarp anatomy have PY. Seeds of Heeria, Pentaspadon and Smodingium are ND (Table 2). However, it appears that while seeds of some species of Pistacia have PD, those of others are ND. For example, seeds of Pistacia lentiscus germinated to ≥75% without any treatment (Piotto, Reference Piotto1995; Garcia-Fayos and Verdú, Reference Garcia-Fayos and Verdú1998). Neither mechanical scarification, prechilling nor scarification + prechilling significantly increased germination percentages over that of the control. The only effect of these treatments was to cause a small, but significant, increase in germination rate (speed). Furthermore, Morrero (Reference Morrero1949) reported that average time after sowing to germination for three sowings of P. chinensis seeds was 12 d.
Based primarily on the number of carpel lobes of female flowers and on endocarp anatomy (see Table 3), Wannan and Quinn (Reference Wannan and Quinn1991) provided a tentative taxonomic arrangement of genera within Anacardiaceae. They used these various characters for placement of genera in this family into either Group A or Group B (not to be confused with Rhoeae endocarp Group A and Rhoeae endocarp Group B sensu Wannan and Quinn, Reference Wannan and Quinn1990), which were subdivided into two subgroups (Table 3). Wannan and Quinn's (Reference Wannan and Quinn1991) scheme uses endocarp anatomy as one of the characters to subdivide Group A into Subgroups A1 and A2, but it uses only characters of the gynoecium to subdivide Group B into Subgroups B1 and B2. Since they did not use endocarp anatomy to subdivide genera in Group B into Subgroups B1 and B2, genera in this group included in our study are indicated as ‘Spondias (B)’ in Table 2. With regard to endocarp anatomy, it is shown in Table 2 how the genera for which we have information on both seed dormancy/germination and endocarp anatomy fit into both of Wannan and Quinn's (Reference Wannan and Quinn1990, Reference Wannan and Quinn1991) arrangements.
Table 3. Endocarp structure included (along with characters of gynoecium, see text) in taxonomic arrangement of Anacardiaceae into two groups (from Wannan and Quinn, Reference Wannan and Quinn1991; Wannan, Reference Wannan2006)

Only those genera for which we have information on germination are included as examples of groups and subgroups. An asterisk (*) indicates physical dormancy and/or physical + physiological dormancy in the genus.
a See footnote to Table 1.
Applying Wannan and Quinn's (Reference Wannan and Quinn1991) taxonomic arrangement to the 55 genera in Table 2, the following can be seen. (1) Four of the six genera in Anacardieae are in Subgroup A1, one (Anacardium) in Anacardium Subgroup A2 and one (Buchanania) in Spondias Group B. (2) With only one exception (Anacardium), all genera in Subgroup A2 are in tribe Rhoeae. (3) All genera in Group B belong to Spondiadeae, except Buchanania (Anacardieae), Campnosperma (Rhoeae) and Pentaspadon (Rhoeae). (4) All genera with PY or PY + PD are in Subgroup A2. However, this Subgroup also includes genera with ND seeds, PD seeds and both ND and PD seeds.
The fossil fruit record shows that species of Anacardiaceae with PY (Rhus s.s.) and ND/PD (Anacardium and the extinct genus Pentoperculum, Spondiadeae) extend at least as far back as the Eocene (Manchester, Reference Manchester1994; Manchester et al., Reference Manchester, Wilde and Collinson2007; Herrera et al., Reference Herrera, Mitchell, Pell, Collinson, Daly and Manchester2018 and references cited for fossil endocarps of subfamily Spondioideae; Flynn et al., Reference Flynn, DeVore and Pigg2019; Manchester and Judd, Reference Manchester and Judd2022). Baskin et al. (Reference Baskin, Baskin and Li2000) suggested that PY + PD may have originated in the Oligocene, in conjunction with climatic cooling. According to Wannan and Quinn (Reference Wannan and Quinn1990), ‘The occurrence of the Spondias-type of endocarp in a genus [Conarium, Burseraceae] of the sister group [of Anacardiaceae] suggests that this type of endocarp is plesiomorphic and that the Anacardium-type is derived.’ In this case, a water-impermeable endocarp and thus PY and PY + PD are derived in subfamily Anacardioideae. Furthermore, the diaspores of Burseraceae do not have PY or PY + PD, and the embryo is fully developed; they are either ND or have PD (e.g. Ng, Reference Ng1991; Sautu et al., Reference Sautu, Baskin, Baskin, Deago and Condit2007; Baskin and Baskin, Reference Baskin and Baskin2014; Rodríguez-Arévalo et al., Reference Rodríguez-Arévalo, Mattana, García, Liu, Lira, Dávila, Hudson, Pritchard and Ulian2017). However, PY/PY + PD does occur in two other families of Sapindales (sensu APG IV, 2016), namely Biebersteinaceae (Boesewinkel, Reference Boesewinkel1997; Koutsovoulou et al., Reference Koutsovoulou, Vassiliades, Yannitsaros and Thanos2005) and Sapindaceae (Baskin et al., Reference Baskin, Davis, Baskin, Gleason and Cordell2004; Turner et al., Reference Turner, Merritt, Baskin, Baskin and Dixon2006, Reference Turner, Cook, Baskin, Baskin, Tuckett, Steadman and Dixon2009; Cook et al., Reference Cook, Turner, Baskin, Baskin, Steadman and Dixon2008). In Anacardiaceae and Biebersteinaceae, the water-impermeable layer in diaspores with PY/PY + PD is the endocarp of the fruit, but in the Sapindaceae, the water-impermeable layer is in the seed coat.
Concluding remarks
Seeds with PY or PY + PD in Anacardiaceae have evolved only in tribe Rhoeae and only in genera with the Anacardium-type Rhoeae Group A endocarp anatomy. However, neither PY nor PY + PD is present in seeds of all genera that have this specialized anatomical endocarp type. Two distinct morphoanatomical features of the seed/fruit (endocarp) of diaspores with PY are the water-impermeable palisade (macrosclereid) layer(s) with a linea lucida (light line) and a water gap (or water gap complex) that opens (permanently) in response to an environmental signal, thereby serving as the initial predetermined pathway for the entrance of water into the seed (e.g. Hamly, Reference Hamly1932; Christiansen and Moore, Reference Christiansen and Moore1959; Egley and Paul, Reference Egley and Paul1981; Manning and Van Staden, Reference Manning and Van Staden1987; Gama-Arachchige et al., Reference Gama-Arachchige, Baskin, Geneve and Baskin2013; Baskin and Baskin, Reference Baskin and Baskin2014; Burrows et al., Reference Burrows, Alden and Robinson2018; Geneve et al., Reference Geneve, Baskin, Baskin, Jayasuriya and Gama-Arachchige2018). In the case of diaspores, such as those of the anacardiaceous genera Lithraea and Rhus s.s. with PY, the endocarp is composed of four layers of cells; three of these are palisade macrosclereids, only one of which (osteosclereid layer) has a light line. Thus, it seems that a water-impermeable macrosclereid palisade layer with a linea lucida and a water gap together constitute the ‘PY syndrome’ in diaspores of angiosperms with a water-impermeable seed/fruit coat. In this case, the presence/absence of these morphoanatomical features can be used to determine which genera with Anacardium-type Rhoeae Group A endocarp have PY (or PY + PD) and which do not. A water gap (‘carpellary micropyle’) in Anacardiaceae has been identified/characterized only in Rhus s.s. species (Li et al., Reference Li, Baskin and Baskin1999b), which have an Anacardium-type Rhoeae Group A endocarp and PY or PY + PD (Table 2). Overall, then, a detailed anatomical comparison of water-permeable and water-impermeable Anacardium-type Rhoeae Group A endocarps should provide insight on why the diaspores of some genera in tribe Rhoeae have PY or PY + PD and others are ND or have PD.
With regard to determining if a seed or other diaspore is water-permeable or impermeable, it is important to keep in mind that impermeability (i.e. PY) develops either during maturation drying on the mother plant or even during post-dispersal drying (Baskin and Baskin, Reference Baskin and Baskin2014; Jaganathan, Reference Jaganathan2016, Reference Jaganathan2022; Thusithana et al., Reference Thusithana, Amarasekara, Jayasuriya, Gama-Arachchige, Baskin and Baskin2021). Seeds or fruits with PY become impermeable to water only when the moisture content (MC) of the diaspore falls below a species-specific MC threshold (see Table 6.2 in Baskin and Baskin, Reference Baskin and Baskin2014); otherwise, the diaspore will remain water-permeable. Gallará et al. (Reference Gallará, López Tapia, Zeballos, Brailovsky and Maggi2021) showed that the drupes of Lithraea molleoides (Anacardiaceae) needed post-dispersal drying for the induction of PY. However, in fact, the diaspore MC at which PY is induced also can vary even between populations of the same taxon growing under different environmental conditions (Thusithana et al., Reference Thusithana, Amarasekara, Jayasuriya, Gama-Arachchige, Baskin and Baskin2021). And furthermore, the development of PY does not occur at the same time for all seeds in the same days-after-pollination cohort and thus not at the same MC [see discussion in Qu et al. (Reference Qu, Baskin and Baskin2010) and references cited therein]. Therefore, it is essential for the MC of diaspores be considered in addition to morphoanatomical traits (see above) in assigning PY or PY + PD to diaspores. In sum, then, the presence of the PY syndrome is an indicator of the potential of a species to develop PY, if, and only if, they reach the threshold MC during maturation or post-dispersal drying.
Acknowledgements
We thank Dr. Xiaojie Li for translating the papers on Rhus chinensis (by Huang and Qia) and Toxicodendrom verniciflua (by Xu and Xu) from Chinese to English, Dr. Zhenying Huang for sending us seeds of Dobinea from China and Dr. Fernando Gallará for providing information that allowed us to write footnote ‘a’ of Table 1.
Conflicts of interest
None declared.






