Alzheimer's disease (AD) is a progressive neurodegenerative disorder pathologically characterised by the presence of senile amyloid-β (Aβ) plaques and neurofibrillary tangles generated by γ-secretase-induced amyloid precursor protein proteolysis and tau hyperphosphorylation, respectively(Reference Ittner and Götz1). Recent increases in the number of patients with dementia involving AD are a grave public health problem, and medical costs will be commensurate(Reference Allegri, Butman and Arizaga2). Alzheimer's Disease International has recently reported that 35·6 million people worldwide live with dementia; the number has doubled every 20 years and is expected to reach an estimated 65·7 million by 2030, and 115·4 million by 2050(Reference Prince and Jackson3).
Polyphenols, such as catechin and resveratrol, are a large group of natural antioxidants that are found in fruits and vegetables, and in beverages such as tea, coffee and wine. Current studies have strongly suggested that polyphenols reduce the risk of CVD, cancers, neurodegenerative diseases including AD, diabetes and osteoporosis(Reference Arts and Hollman4, Reference Scalbert, Manach and Morand5). Indeed, there is growing evidence that intake of polyphenols is effective for AD risk reduction and the improvement of cognitive impairment, which is the predominant symptom of dementia involving AD(Reference Ramassamy6, Reference Singh, Arseneault and Sanderson7). An epidemiological study has indicated that consumption of red wine, in which polyphenols exist in abundance, is associated with a lower incidence of dementia and AD(Reference Luchsinger and Mayeux8). Furthermore, improvement of cognitive performance due to the long-term (almost 5–6 months) oral intake of polyphenols, such as green tea epigallocatechin-3-gallate, has been observed in studies using an AD mouse model(Reference Li, Zhao and Zhang9–Reference Wang, Ho and Zhao12).
The senescence-accelerated mouse, a model mouse for accelerated senescence, was established by Takeda et al. (Reference Miyamoto, Kiyota and Yamazaki13, Reference Takeda14). This senescence-accelerated mouse consists of senescence-prone (SAMP) and senescence-resistant (SAMR) inbred strains(Reference Miyamoto, Kiyota and Yamazaki13). The SAMP8 strain, one of the substrains in SAMP, shows early deficits of learning and memory and early development of lesions in the brain that are similar to those seen in aged animals(Reference Miyamoto, Kiyota and Yamazaki13, Reference Takeda14). For instance, passive avoidance testing shows that the learning memory of SAMP8 mice has already declined at 2 months of age(Reference Flood and Morley15). Moreover, spontaneous overproduction of Aβ and hyperphosphorylated tau is found in the hippocampus of SAMP8 mice(Reference Canudas, Gutierrez Cuesta and Rodríguez16–Reference Manich, Mercader and del Valle19); therefore, SAMP8 mice are proposed as a model mouse for AD.
To avoid increases in the number of patients having dementia and AD, retarding the development of such diseases by the attenuation of early and mild cognitive impairment (MCI) is thought to be very important. Therefore, we hypothesised that the short-term administration of polyphenol could possibly be effective for the attenuation of early cognitive impairment and MCI of SAPM8 mice. In the present study, we used oligomerised lychee fruit-derived polyphenol (OLFP), which is an optimised phenolic product derived from lychee fruit-derived polyphenols and contains catechin-type monomers and low-molecular-weight oligomers(Reference Fujii, Nishioka and Wakame20). Polyphenols reportedly exert beneficial effects that reduce the risk of AD through the modification of intracellular molecules, such as secretase, AMP-activated protein kinase and cyclic AMP response element-binding protein, in the hippocampus(Reference Li, Zhao and Zhang9, Reference Lee, Lee and Ban21, Reference Vingtdeux, Giliberto and Zhao22). OLFP has also exhibited various biological effects via the regulation of intracellular molecules. For example, OLFP enhances lipolysis in primary rat adipocytes through the activation of the extracellular signal-regulated kinase (ERK) 1/2 pathway, and OLFP-induced attenuation of hepatic damage in type 2 diabetic obese mice relates to the regulation of transcription factor nuclear factor-κB expression in the liver(Reference Ogasawara, Kitadate and Nishioka23, Reference Noh, Park and Yokozawa24). Thus, we further hypothesised that OLFP target molecules must exist in the hippocampus given the attenuating effects on hippocampus-dependent memory impairment in SAMP8 mice, and therefore we performed a DNA array analysis to search for OLFP target molecules in the hippocampus. Of the possible candidate factors, we chose Wolfram syndrome 1 (Wfs1), the expression of which is up-regulated in the hippocampus of SAMP8 mice by OLFP intake, because it is believed that Wfs1 impairs endoplasmic reticulum (ER) stress, which is involved in the pathology of AD(Reference Fonseca, Ishigaki and Oslowski25–Reference Ueda, Kawano and Takeda27), and little is known about the effect of polyphenols on ER stress in neuronal cells. In the present study, a culture of NG108-15 neuronal cells was used to investigate whether OLFP could directly enhance the expression of Wfs1, and attenuate ER stress in neuronal cells by evaluating the expression of immunoglobulin heavy chain-binding protein (BiP), which is an ER stress-related marker(Reference Pincus and Walter28).
Materials and methods
Oligomerised lychee fruit-derived polyphenol
OLFP was obtained by oligomerising polyphenol polymers from lychee fruit-derived polyphenol using a modification of a patented technology described previously(Reference Fujii, Nishioka and Wakame20). The process involves mixing proanthocyanidins with tea extract but not with l-cysteine and purifying the mixture using a column. OLFP contains 15·7 % polyphenol monomer ((+)-catechin and ( − )-epicatechin, etc.) and 13·3 % polyphenol dimer (procyanidin B2, etc.), while lychee fruit-derived polyphenol contains 6·4 % polyphenol monomer and 9·9 % polyphenol dimer. The safety of OLFP as a food or dietary supplement and as a pharmaceutical additive has already been confirmed, as described previously(Reference Fujii, Nishioka and Wakame20). OLFP is commercially available (Amino Up Chemical Company Limited).
Animal care
Male SAMP8 (n 12) and SAMR1 (n 6) mice (4 months old) were obtained from the Council for SAM Research via Japan SLC, Inc. Mice were housed individually in cages in a temperature-controlled room at 23°C under a 12 h light–12 h dark cycle, with food and water available ad libitum. SAMP8 mice were randomly divided into two groups: a control group (SAMP8 mice: n 6) and an OLFP intake group (OLFP-SAMP8: n 6). OLFP-SAMP8 mice were given OLFP (100 mg/kg per d each) added to their drinking water for 2 months. All experiments conducted in the present study were approved by the Experimental Animal Ethics Committee of Kyorin University, School of Medicine, Mitaka.
Conditioned fear memory
Mice were tested for contextual fear conditioning and extinction using modified methods described previously by Fujiwara et al. (Reference Fujiwara, Mishima and Kofuji29) and Ohta et al. (Reference Ohta, Akiguchi and Seriu30). For this purpose, two conditioning chambers with different shapes (context A, square; context B, triangle) were used for the conditioned fear memory test. Context A had a shock grid floor made of twenty-six stainless rods with a length of 18·0 cm (0·2 cm in diameter) spaced 0·7 cm apart (centre to centre), and context B had stainless rods with a length from 0·8 to 15·5 cm. Each mouse experienced ten incidents of tone-shock (conditioned stimulus: 20 dB)–electrical foot-shock (unconditioned stimulus: 1·0 mA, 2 s) pairings in context A. After the conditioned stimulus–unconditioned stimulus pairing, mice were allowed to remain in the conditioning chamber for 30 s before being returned to their home cages. At approximately 24 h after pairing, each mouse was placed in context A to study contextual fear memory, and was scored for the freezing response for 3 min. Moreover, to study cued fear memory, mice were returned to context B and scored for the freezing response to the conditioned stimulus for 57 s without the unconditioned stimulus.
DNA array analysis
Total RNA from the hippocampus of each mouse and neuronal cell lines was prepared using Isogen (311-02 501; Nippon Gene). The mRNA was converted to single-stranded DNA (complementary DNA), labelled and hybridised to the Sentrix® Mouse-6 Expression BeadChip (BD-26-101; Illumina, Inc.), according to the manufacturer's instructions, and with the assistance of GP BioSciences Limited.
Quantitative real-time PCR
Complementary DNA was amplified using a TaqMan Universal PCR Master Mix (4304437; Applied Biosystems) in triplicate with a 7500 Real-Time PCR System (Applied Biosystems). PCR conditions were 50°C for 2 min and 95°C for 10 min followed by forty-five cycles of 95°C for 15 s and 60°C for 1 min. Each PCR was conducted in 25 μl TaqMan Gene Expression Assays for Wfs1, cerebellin 4 (Cbln4) and BiP/GRP78 (Mm00495979, Mm00558663 and Mm00517691; Applied Biosystems). TaqMan Gene Expression Assays consist of a pair of unlabelled PCR primers and a TaqMan probe with a fluorescent dye label on the 5′ end, and minor groove binder non-fluorescent quencher on the 3′ end. 18S Ribosomal RNA was used as an internal control (TaqMan Ribosomal RNA Control Reagent: 4308329; Applied Biosystems).
Western blot analysis
Total protein was extracted from mouse hippocampal tissues or neuronal cells with T-PER Tissue Protein Extraction Reagent (78 510; Pierce) containing protease inhibitors. Each protein sample was subjected to 10–12 % NuPAGE (NP0321BOX; Invitrogen) gradient gel electrophoresis and transferred onto a nylon membrane. The membrane was blocked using Tris-buffered saline with Tween-20 (TBS-T; 20 mm-Tris–HCl, pH 7·5, 137 mm-NaCl and 0·1 % Tween-20) containing 5 % non-fat dry milk, and was then probed with an anti-Wfs1 (11 558-AP; Proteintech Group), anti-BiP (SPC-107B; StressMarq Biosciences), anti-phosphorylated ERK 1/2 (sc-16 982-R; Santa Cruz Biotechnology), anti-ERK1/2 (V1141; Promega), anti-phosphorylated c-Jun N-terminal kinase (JNK, 4668; Cell Signaling Technology), anti-JNK (sc-571; Santa Cruz Biotechnology) or β-actin antibody (sc-1616; Santa Cruz Biotechnology). After washing with TBS-T, the bound antibody was detected using an enhanced chemiluminescence (ECL) system (RPN2106; GE Healthcare Life Sciences). The intensities of bands from Western blot analyses were quantified using National Institute of Health Image software.
Cell culture
Neuroblastoma × glioma hybrid NG108-15 cells (ECACC 88112302) were cultured in Dulbecco's modified Eagle's medium supplemented with 10 % fetal bovine serum. For neuronal differentiation, NG108-15 cells were cultured in Dulbecco's modified Eagle's medium supplemented with 1 % fetal bovine serum and 1 mm-N6-2′-O-dibutyryladenosine 3′,5′-cyclic monophosphate (D0627; Sigma-Aldrich) for 4 d. Differentiated NG108-15 cells were treated with OLFP (2 μg/ml) for 30 min, 2 or 6 h, and then the expression level of Wfs1 and the phosphorylation state of ERK or JNK were assessed. Moreover, to clarify whether the activation of ERK or JNK associates with the up-regulation of Wfs1 expression in NG108-15 cells by the treatment with OLFP, differentiated NG108-15 cells were pretreated with 5 μm-U0126 (an ERK inhibitor) or 50 μm-SP600125 (a JNK inhibitor) (U120 and S5567; Sigma-Aldrich) for 30 min before the treatment with OLFP. In addition, NG108-15 cells were cultured in the presence of tunicamycin (0·5 μg/ml, T7765, Sigma-Aldrich), which is an inducer of ER stress, with or without OLFP for 12 h, and the mRNA and protein expression of BiP was examined.
Data expression and statistical analysis
Values are presented as means with their standard errors. The significance of multiple comparisons was determined using ANOVA followed by the Bonferroni post hoc test. We considered P< 0·05 to be statistically significant. Moreover, as for the results of conditioned fear memory, the effect size (Cohen's d) was calculated using mean changes between the SAMP8 and SAMP8-OLFP mice and the pooled standard deviation. A conventional rule is to consider a Cohen's d of ≥ 0·8 or ≤ − 0·8 to be a large effect(Reference Cohen31).
Results
Effects of oligomerised lychee fruit-derived polyphenol intake on the conditioned fear memory of senescence-accelerated mouse prone 8 mice
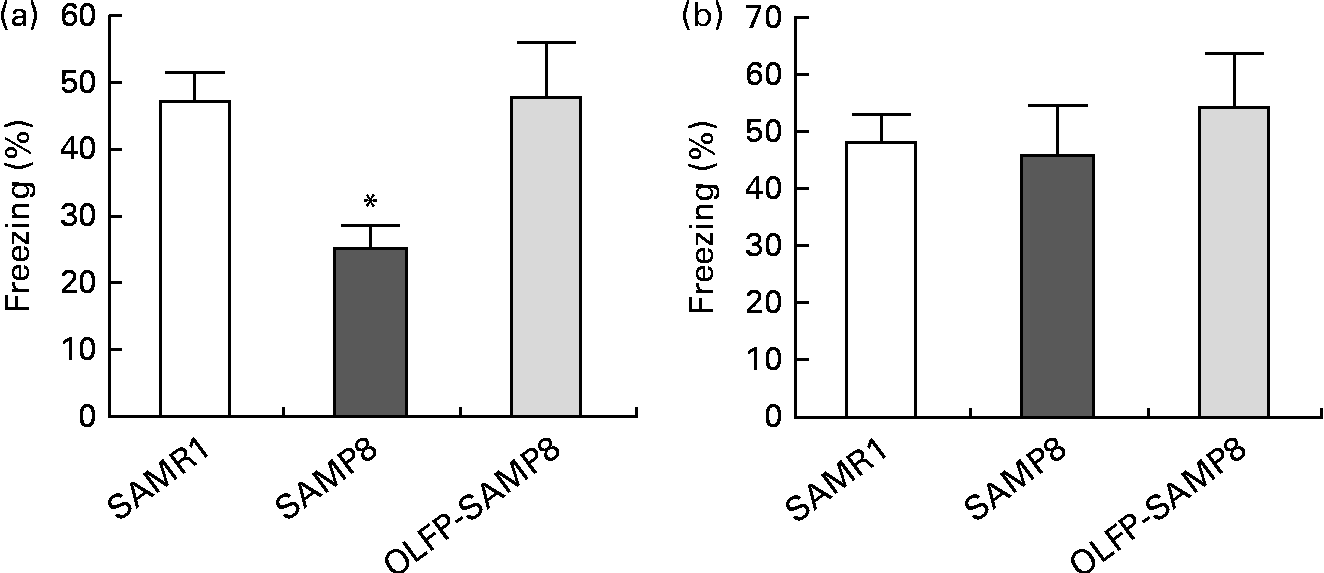
In order to examine the effects of OLFP intake on the memory impairment of SAMP8 mice, we first conducted a contextual fear conditioning test, since contextual fear memory of 4month-old SAMP8 mice reportedly deteriorates(Reference Ohta, Akiguchi and Seriu30). The ratio of freezing responses to training in context A (contextual fear memory) of SAMP8 mice at 24 h after the conditioned stimulus–unconditioned stimulus pairing was significantly decreased compared with that of SAMR1 mice, but no significant difference in freezing responses was observed between the SAMR1 and OLFP-SAMP8 mice (Fig. 1(a)). On the other hand, no significant differences in cued fear memory were observed between the SAMR1 and SAMP8 mice, as reported previously(Reference Ohta, Akiguchi and Seriu30), and OLFP intake did not significantly affect the cued fear memory of SAMP8 mice (Fig. 1(b)). With respect to contextual fear memories, comparing OLFP-SAMP8 mice with SAMP8 mice revealed a large effect (Cohen's d= 1·76), although the size of the effect in cued fear memories was small (Cohen's d= 0·37).

Fig. 1 Effects of oligomerised lychee fruit-derived polyphenol (OLFP) on the conditioned fear memory of senescence-accelerated mouse prone 8 (SAMP8) mice. Each mouse was trained with ten tone-shock pairings and tested for (a) contextual fear memory and (b) cued fear memory 24 h after training. Freezing responses to contextual and cued fear conditioning are shown. Values are means (n 6 per group), with their standard errors represented by vertical bars. * Mean value was significantly different from that of senescence-accelerated mouse resistant 1 (SAMR1) mice (P< 0·05).
Effects of oligomerised lychee fruit-derived polyphenol intake on mRNA levels in the hippocampus of senescence-accelerated mouse prone 8 mice
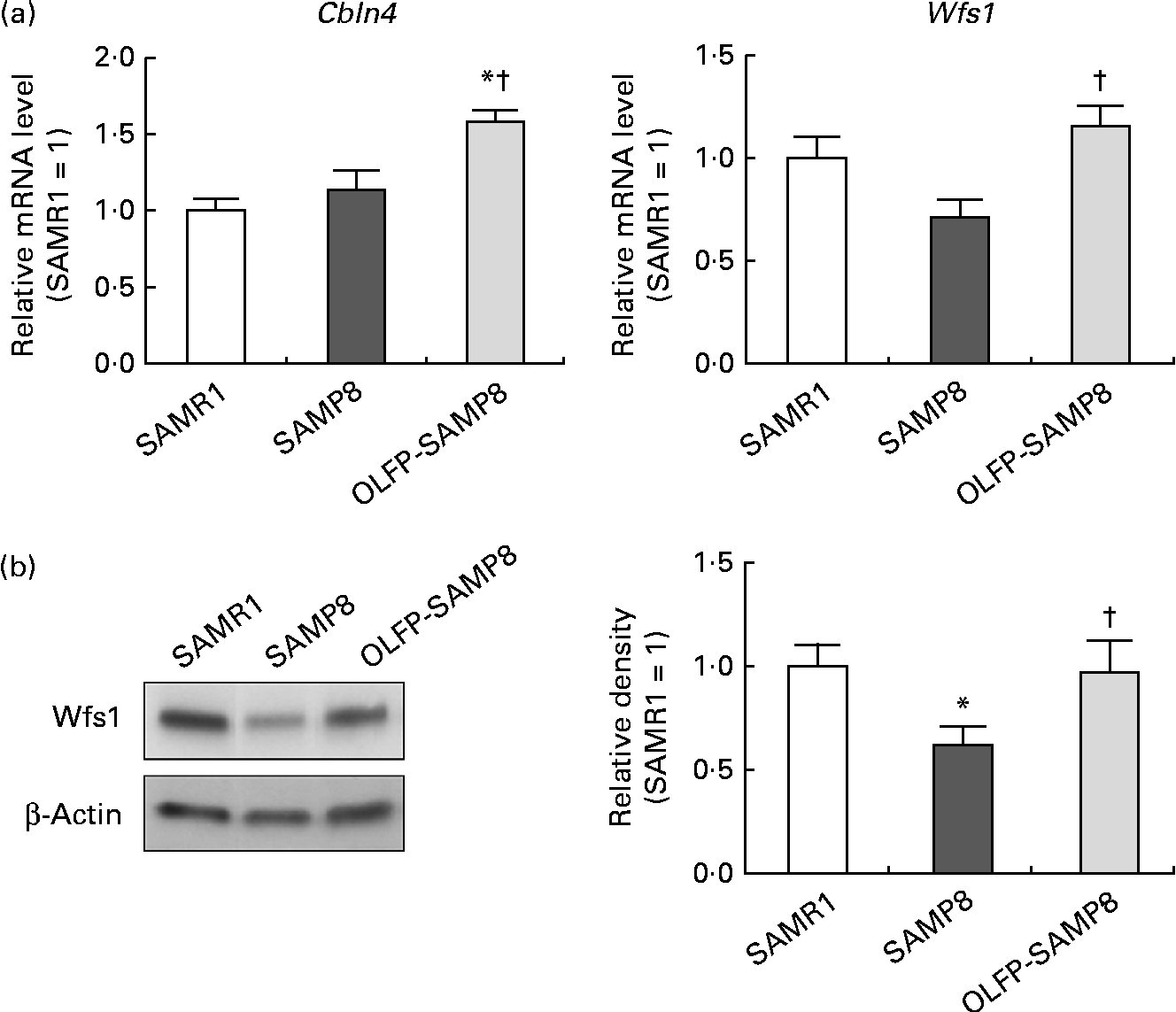
Contextual fear memory is considered hippocampus- and amygdala-dependent memory, whereas cued fear memory is considered amygdala-dependent memory(Reference Rogan and LeDoux32). Therefore, based on the results of Fig. 1, OLFP could rescue the impaired consolidation of contextual memory in SAMP8 mice in part through the enhancement of hippocampal function. To search for OLFP target molecules, which may relate to the OLFP-induced attenuation of cognitive impairment in the hippocampus, we next performed a DNA array analysis. The representative genes picked out in the analysis are listed in Table 1. The mRNA expression levels of Wfs1, Cbln4, lamin B1 and matrix Gla protein in the hippocampus of OLFP-SAMP8 mice were higher than those of SAMP8 mice; on the contrary, ankyrin repeat domain 11 and guanine nucleotide-binding protein, α inhibiting activity polypeptide 1 were down-regulated in the hippocampus of OLFP-SAMP8 mice. The results of real-time PCR analysis revealed that the mRNA level of Cbln4 in the hippocampus was significantly higher in OLFP-SAMP8 mice than in either SAMR1 or SAMP8 mice (Fig. 2(a), left). Moreover, compared with SAMP8 mice, the mRNA expression of Wfs1 was significantly increased in the hippocampus of OLFP-SAMP8 mice (Fig. 2(a), right). In addition, the expression level of the Wfs1 protein in the hippocampus of SAMP8 mice was lower than that of SAMR1 mice, and the intake of OLFP significantly enhanced the expression of the Wfs1 protein in the hippocampus of SAMP8 mice (Fig. 2(b)).
Table 1 Representative genes with expression changes in the hippocampus of oligomerised lychee fruit-derived polyphenol-administered senescence-accelerated mouse prone 8 (OLFP-SAMP8) mice compared with those of SAMP8 mice*
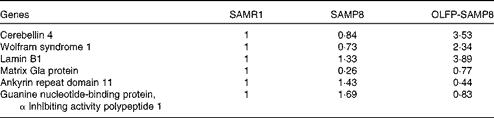
SAMR1, senescence-accelerated mouse resistant 1.
* The expression intensities for the indicated genes are the values that are relative to the expression levels in SAMR1 mice (set to 1).

Fig. 2 Effects of oligomerised lychee fruit-derived polyphenol (OLFP) on the expression of cerebellin 4 (Cbln4) and Wolfram syndrome 1 (Wfs1) in the hippocampus. (a) mRNA expression levels of Cbln4 and Wfs1 in the hippocampus. Total RNA were extracted from the hippocampus of each mouse and then subjected to quantitative real-time PCR. The expression level of each mRNA was normalised using the 18S ribosomal RNA level. Values are related to an arbitrary unit of senescence-accelerated mouse resistant 1 (SAMR1) mice (set to 1). (b) The expression level of the Wfs1 protein in the hippocampus. Total protein was extracted from the hippocampus of each mouse and then subjected to Western blot analysis. Representative data from the Western blot analysis are shown (left). The expression level of the Wfs1 protein was normalised to that of the β-actin protein. Values are related to the optical density of SAMR1 mice (set to 1). Values are means (n 6 per group), with their standard errors represented by vertical bars. * Mean values were significantly different from those of SAMR1 mice (P< 0·05). † Mean values were significantly different from those of senescence-accelerated mouse prone 8 (SAMP8) mice (P< 0·05).
Effects of oligomerised lychee fruit-derived polyphenol on the Wolfram syndrome 1 level in NG108-15 neuronal cells
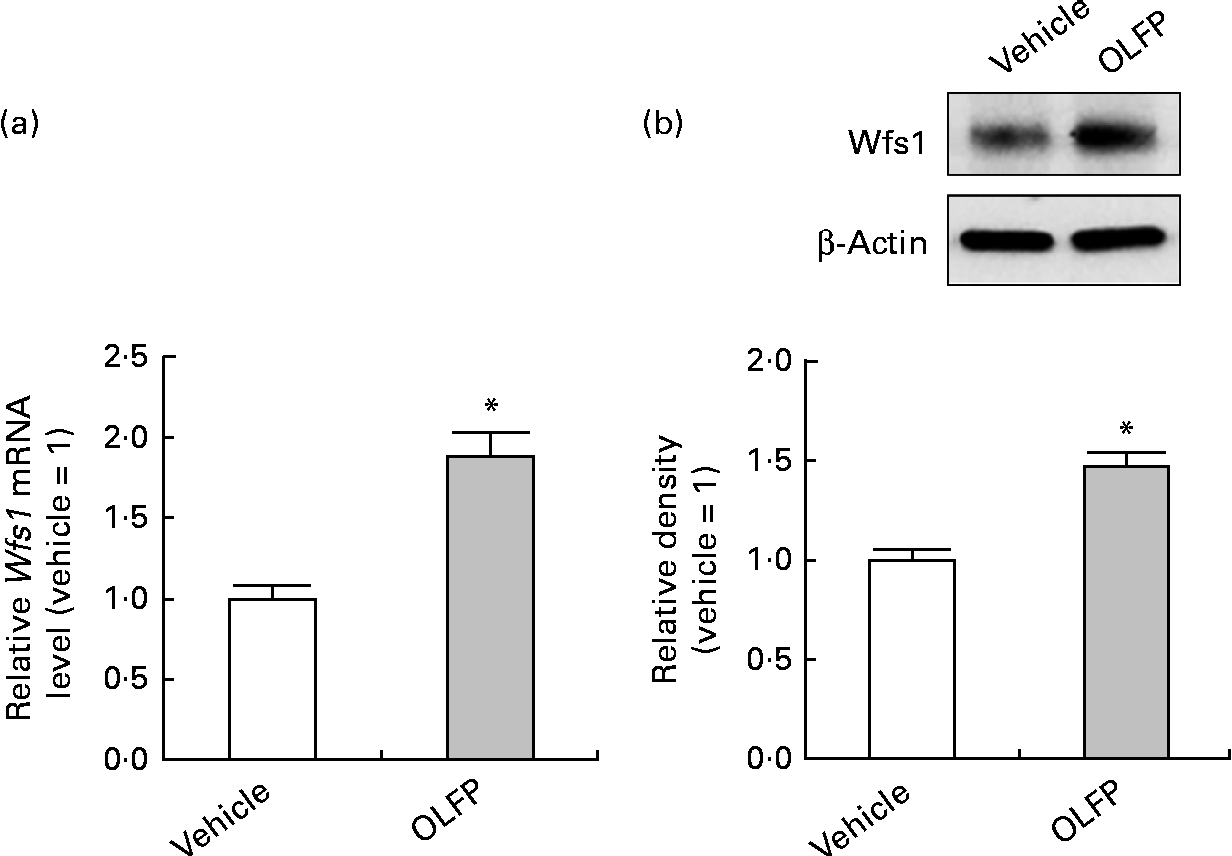
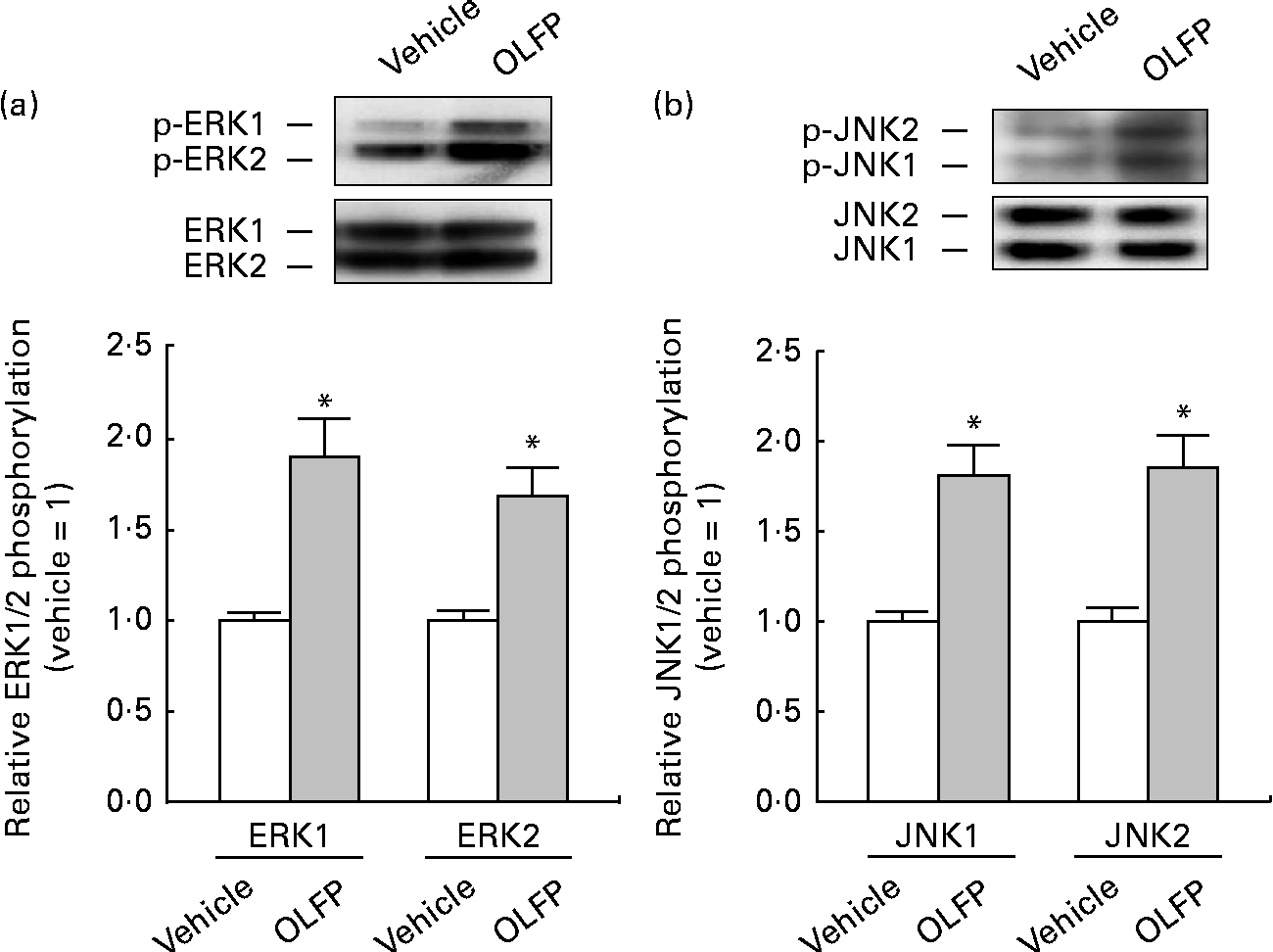
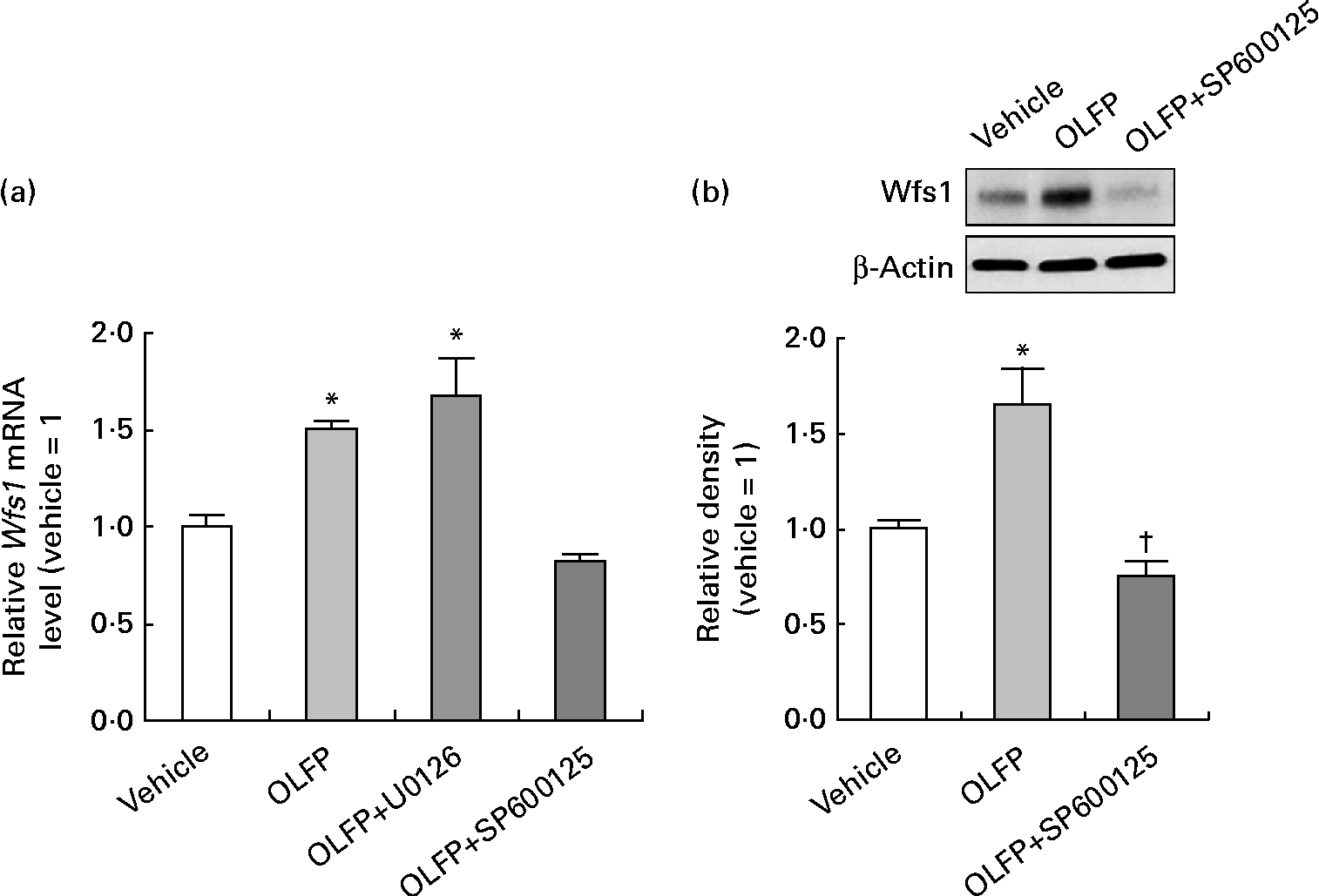
The Wfs1 protein localises primarily to the ER membrane, and is observed in the Cornet d'Ammon 1 (CA1) region of the rodent hippocampus(Reference Rigoli, Lombardo and Di Bella33, Reference Takeda, Inoue and Tanizawa34). Moreover, Wfs1 is suggested to have protective effects against ER stress that is involved in the pathology of AD(Reference Fonseca, Ishigaki and Oslowski25–Reference Ueda, Kawano and Takeda27). Therefore, in the present study, we focused on Wfs1, and investigated whether OLFP could directly enhance the expression of Wfs1 in neuronal cells. As shown in Fig. 3, the expression of gene and protein for Wfs1 in NG108-15 cells was significantly increased by the treatment with OLFP. Polyphenols have reportedly enhanced the activation of mitogen-activated protein kinases, such as ERK and JNK, in various cell types(Reference Ogasawara, Kitadate and Nishioka23, Reference Briviba, Pan and Rechkemmer35, Reference Wu, Qi and Iwasaki36). In the present study, the enhanced phosphorylation of ERK and JNK in NG108-15 cells was also observed due to the treatment with OLFP (Fig. 4). Furthermore, OLFP-induced enhanced expression of the Wfs1 gene and protein was inhibited by the treatment of the cells with SP600125 (a JNK inhibitor), but the treatment with U0126 (an ERK inhibitor) had no effect on the mRNA expression of Wfs1 (Fig. 5(a) and (b)). Thus, it is thought that the enhanced expression of Wfs1 in NG108-15 cells by OLFP is mediated through the activation of JNK.

Fig. 3 Expression changes of Wolfram syndrome 1 (Wfs1) in NG108-15 neuronal cells by oligomerised lychee fruit-derived polyphenol (OLFP). Total RNA and protein were extracted from the NG108-15 cells treated with OLFP (2 μg/ml) for 2 and 6 h, respectively. (a) mRNA expression level of Wfs1 was examined using quantitative real-time PCR, and normalised using the 18S ribosomal RNA level. Values are related to the arbitrary unit of vehicle-treated NG108-15 cells (set to 1). (b) Expression of the Wfs1 protein was examined by Western blot analysis. Representative data from the Western blot analysis are shown (top). Expression level of the Wfs1 protein was normalised to that of the β-actin protein. Values are related to the optical density of the vehicle-treated cells (set to 1). Values are means, with their standard errors represented by vertical bars, n 3. * Mean values were significantly different from those of the vehicle-treated cells (P< 0·05).

Fig. 4 Phosphorylation of extracellular signal-regulated kinase (p-ERK) and c-Jun N-terminal kinase (p-JNK) in NG108-15 neuronal cells by oligomerised lychee fruit-derived polyphenol (OLFP). Total protein was extracted from the NG108-15 cells treated with OLFP (2 μg/ml) for 30 min, and subjected to Western blot analysis. Representative data from Western blot analyses are shown (top). Phosphorylation levels of (a) ERK1/2 and (b) JNK1/2 were normalised to the expression levels of ERK1/2 and JNK1/2, respectively. Data were expressed as ratios, with the value of the vehicle-treated cells being set to 1. Values are means (n 3), with their standard errors represented by vertical bars. * Mean values were significantly different from those of the vehicle-treated cells (P< 0·05).

Fig. 5 Effects of mitogen-activated protein kinase inhibitor on the oligomerised lychee fruit-derived polyphenol (OLFP)-induced enhanced expression of Wolfram syndrome 1 (Wfs1) in neuronal cells. NG108-15 cells were incubated in culture medium with 5 μm-U0126 (an extracellular signal-regulated kinase inhibitor) or 50 μm-SP600125 (a c-Jun N-terminal kinase inhibitor) for 30 min before treatment with OLFP, and the expression of Wfs1 was examined. (a) The mRNA expression level of Wfs1 was examined using quantitative real-time PCR, and was normalised using the 18S ribosomal RNA level. Values are related to the arbitrary unit of the vehicle-treated cells (set to 1). (b) Expression of the Wfs1 protein was examined by Western blot analysis. Representative data from the Western blot analysis are shown (top). Expression level of the Wfs1 protein was normalised to that of the β-actin protein. Values are related to the optical density of the vehicle-treated cells (set to 1). Values are means, with their standard errors represented by vertical bars, n 3. * Mean values were significantly different from those of the vehicle-treated cells (P< 0·05). † Mean value was significantly different from that of the OLFP-treated cells (P< 0·05).
Effects of oligomerised lychee fruit-derived polyphenol on tunicamycin-induced endoplasmic reticulum stress in NG108-15 neuronal cells
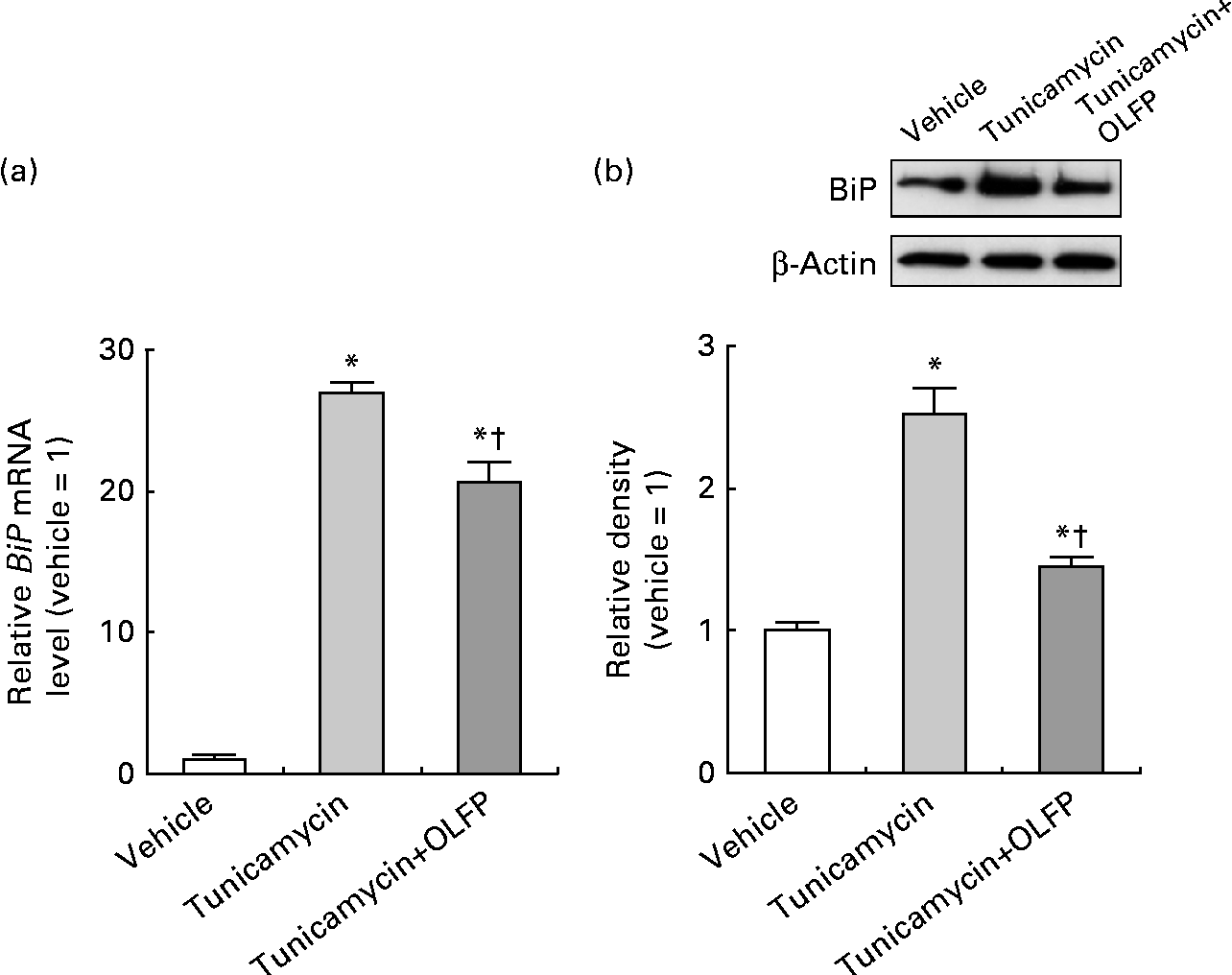
We finally investigated whether OLFP diminishes ER stress by evaluating the expression of BiP, a well-recognised marker of ER stress(Reference Pincus and Walter28), in neuronal cells. As shown in Fig. 6, the mRNA and protein expression of BiP was markedly up-regulated by the treatment with the ER stress inducer tunicamycin in NG108-15 cells, but OLFP significantly diminished such tunicamycin-induced BiP expression. These results suggest that OLFP has protective effects against ER stress in neuronal cells.

Fig. 6 Effects of oligomerised lychee fruit-derived polyphenol (OLFP) on the expression of the tunicamycin-induced endoplasmic reticulum (ER) stress marker BiP (immunoglobulin heavy chain-binding protein) in NG108-15 neuronal cells. NG108-15 cells were incubated in culture medium with OLFP for 1 h before the treatment with the ER stress inducer tunicamycin (0·5 μg/ml) for 12 h, and the expression of BiP was examined. (a) The mRNA expression level of BiP was examined using quantitative real-time PCR, and normalised using the 18S ribosomal RNA level. Values are related to the arbitrary unit of the vehicle-treated cells (set to 1). (b) Expression of the BiP protein was examined by Western blot analysis. Representative data from the Western blot analysis are shown (top). Expression level of the BiP protein was normalised to that of the β-actin protein. Values are related to the optical density of the vehicle-treated cells (set to 1). Values are means (n 3), with their standard errors represented by vertical bars. * Mean values were significantly different from those of the vehicle-treated cells (P< 0·05). † Mean values were significantly different from those of the tunicamycin-treated cells (P< 0·05).
Discussion
At present, for most patients with dementia or AD, a full recovery from cognitive impairment is extremely difficult using existing medications and other approaches, such as physical exercise and intake of supplements. Therefore, a reduction in the risk of dementia involving AD, as well as by developing a medication specifically for AD, is thought to be effective methods to avoid an increase in the number of patients with such disorders. The concept of ‘MCI’ has been proposed as a diagnosis for the risk of developing dementia and AD(Reference Petersen37). MCI is categorised as an intermediate state of cognitive function between changes seen in normal ageing individuals and those with dementia or AD(Reference Petersen37). Thus, the establishment of risk-reducing strategies for early cognitive impairment, such as MCI, is thought to lead to a reduction in the risk of dementia involving AD. SAMP8 mice are a strain of mice with accelerated senescence, and have recently been the focus of attention, as they show several alterations that have also been described in AD patients(Reference Morley, Farr and Kumar38). SAMP8 mice overproduce a compound similar to Aβ, and Aβ granules in the hippocampus of SAMP8 mice are observed from 6 months of age(Reference Del Valle, Duran-Vilaregut and Manich17). The formation of Aβ granules in the hippocampus increases with age. As a result, amyloid plaques occur late in life (approximately 20 months)(Reference Morley, Kumar and Bernardo18). Therefore, SAMP8 mice used in the present experiment (4 months old) are thought to correspond to the MCI state in humans, which is supported by previous experimental results that, when 4-month-old SAMP8 mice were used, spatial learning and memory was not impaired in a Morris water maze test, although memory deficiency was observed when mice were subjected to passive avoidance and conditioned fear memory tests(Reference Li, Zhao and Zhang9, Reference Ohta, Akiguchi and Seriu30). In the present study, OLFP intake seemed to have attenuating effects on early cognitive impairment and MCI in SAMP8 mice, which corresponds to MCI in humans – a result that is expected to lead to a risk reduction of AD.
Polyphenols are a large group of powerful antioxidants that are found in fruits and vegetables, such as grapes and lychee fruits, and in beverages including tea and wine. Usually, the bioactivity and absorption coefficients of polyphenols in the body seem to be implicated in their components – the polymerisation degree of polyphenols. For example, Fujii et al. (Reference Fujii, Sun and Nishioka39) found that oligomerisation of purified grape seed polyphenols is more conducive to higher absorption rates in vivo than the non-oligomerised form. We have also previously found that antioxidative effects of OLFP on adipocytes are stronger than those of non-OLFP, and that the intensity of the antioxidative effects of these polyphenols in adipocytes is clearly reflected in the expression of genes for adipokines(Reference Sakurai, Nishioka and Fujii40). Moreover, the inhibitory effects of procyanidins on pancreatic lipase activity increased with a rise in the degree of polymerisation from dimer to pentamer(Reference Sugiyama, Akazome and Shoji41). Recently, interesting evidence about the relationship between the degree of polymerisation in polyphenols and its effects on cognitive impairment in an AD model of amyloid precursor protein transgenic mice has been published. Wang et al. (Reference Wang, Ferruzzi and Ho42) fractionated a grape-derived polyphenolic mixer to monomer and polymer-enriched proanthocyanidin components, and gave each fraction to amyloid precursor protein transgenic mice and rats. As a result, only the monomeric fraction improved the cognitive function of amyloid precursor protein transgenic mice, and metabolites from the monomeric fraction were able to selectively reach and accumulate in the brain of rats. Therefore, OLFP might have the attenuating effects on the memory deficiency of SAMP8 mice due to the presence of catechin-type monomers and oligomers derived from the lychee fruit(Reference Fujii, Nishioka and Wakame20). However, as for an inhibitory effect on angiotensin I in the conversion of enzyme activities, among procyanidins consisting of dimers, tetramers or hexamers, since procyanidin tetramers have the lowest half-maximal inhibitory concentration value(Reference Ottaviani, Actis-Goretta and Villordo43), proper polymeric forms of polyphenols are considered to differ according to specific biological effects.
Mechanisms responsible for the polyphenol-induced attenuating effects on cognitive impairment have been found by several research groups. Mitochondrial dysfunction and production of Aβ in the brains of patients with AD result in the generation of reactive oxygen species and subsequent oxidative stress in neuronal cells(Reference Ferreiro, Baldeiras and Ferreira44, Reference Massaad45). Oxidative stress-induced neuronal cell damage in the brain involving the hippocampus is considered to be associated with AD-related cognitive dysfunction(Reference Ferreiro, Baldeiras and Ferreira44, Reference Massaad45). Dietary supplementation with antioxidant molecules, such as polyphenols, reportedly brings about an improvement in memory deficiency with a reduction in the level of oxidative stress in the hippocampus of old rats and rats injected with Aβ(Reference Haque, Hashimoto and Katakura46, Reference Haque, Hashimoto and Katakura47). Furthermore, the intake of green tea catechin has diminished Aβ through a modification of the enzyme activity of secretase, and has activated the cyclic AMP response element binding protein, which is considered to be necessary in the formation of long-term memory(Reference Sakamoto, Karelina and Obrietan48), in the hippocampus of a mouse(Reference Li, Zhao and Zhang9, Reference Lee, Lee and Ban21). In the present study, we performed DNA array analysis to search novel OLFP target molecules in the hippocampus. Consequently, Wfs1 was identified. Moreover, we found that OLFP directly enhanced the expression of Wfs1 in neuronal cells via the activation of JNK. Inoue et al. (Reference Inoue, Tanizawa and Wasson49) identified human WFS1 as the gene responsible for the Wolfram syndrome (OMIM 222300), which is a neurodegenerative disorder defined by insulin-dependent diabetes mellitus and progressive optic atrophy. OLFP could significantly enhance the expression of Wfs1 in the hippocampus of SAMP8 mice, which appears to be related to the OLFP-induced attenuation of memory deficiency, as shown in conditioned fear memory tests, because abundant expression of the Wfs1 transcript has been confirmed in the CA1 region of the rodent hippocampus(Reference Takeda, Inoue and Tanizawa34). Moreover, it has been suggested that the CA1 region is implicated in contextual fear conditioning and extinction(Reference Matsuo, Reijmers and Mayford50–Reference Tronson, Schrick and Guzman52). However, it is difficult to draw conclusions since there were no differences between Wfs1-deficient mice and wild types in conditioned fear memory testing(Reference Luuk, Plaas and Raud53). There is no denying that the role of Wfs1 in memory formation in the hippocampus of the adult brain may be different from that during postnatal brain development. Thus, the relationship between Wsf1 and memory function remains unclear, and further studies are required.
Recent interesting results have indicated that the Wfs1 protein localises primarily to the ER membrane, and has protective effects against ER stress(Reference Fonseca, Ishigaki and Oslowski25, Reference Ueda, Kawano and Takeda27, Reference Rigoli, Lombardo and Di Bella33). In fact, Wfs1 deficiency in mice leads to a progressive loss of pancreatic β-cells, impaired glucose tolerance and cell-cycle progression, accompanied by the activation of ER stress/unfolded protein response pathways(Reference Fonseca, Ishigaki and Oslowski25, Reference Ueda, Kawano and Takeda27). The accumulation of unfolded proteins following disruptions of proper protein folding and modification in the ER causes ER stress and activates a signalling network called the unfolded protein response(Reference Lai, Teodoro and Volchuk54). When cellular protective changes mediated by the unfolded protein response fail to restore folding capacity, ER stress leads to a maladaptive response and apoptosis(Reference Lindholm, Wootz and Korhonen26). Several neurodegenerative diseases including AD have been suggested to have an accumulation of misfolded proteins, which leads to altered neuronal connectivity, neuronal death and a dysfunctional process of ER-associated degradation machinery(Reference Lindholm, Wootz and Korhonen26, Reference Bence, Sampat and Kopito55, Reference Soto56), which shows that ER stress is involved in the pathology of AD and in other neurodegenerative diseases. Indeed, the administration of Aβ induced the activation of ER stress in cultured neurons and in rabbits in vivo (Reference Ferreiro, Resende and Costa57, Reference Ghribi, Herman and DeWitt58). Also, caspase-12 mediates the apoptosis of ER stress-specific neuronal cells via Aβ(Reference Nakagawa, Zhu and Morishima59). OLFP significantly attenuated the tunicamycin-induced up-regulation of BiP expression in NG108-15 cells, indicating that OLFP possesses the potential for protective effects against ER stress in neuronal cells in part due to an up-regulation of Wfs1. Thus, it is conceivable that the administration of OLFP brings about the risk reduction of AD due to the attenuation of ER stress in the hippocampus. Nevertheless, since no significant differences in the expression levels of the BiP protein in the hippocampus were observed among the SAMR1, SAMR8 and SAMP8-OLFP mice (data not shown), although the expression level of the Wfs1 protein in the hippocampus of SAMP8 mice was lower than that of SAMR1, and OLFP significantly enhanced the expression of the Wfs1 protein, additional investigations are needed to reveal the relationship between the anti-ER stress effect of OLFP and the risk reduction of AD.
In conclusion, the results obtained from the present study suggest that the administration of OLFP attenuates contextual fear memory deficiency in SAMP8 mice. Moreover, we identified Wfs1 as a novel factor, which may relate to the OLFP-induced attenuation of cognitive impairment in the hippocampus.
Acknowledgements
The present study was partially supported by Grants-in-Aid for Specific Project Research from the Ministry of Education, Culture, Sports, Science and Technology of Japan. T. S., S. S. and J. O. collected and analysed the data. T. S., K. K., H. N. and H. F. prepared the materials for the experiment. T. F. and K. A. provided the experimental guidance and contributed to the experimental design. T. K., T. I. and H. O. contributed to the interpretation of the results and gave critical comments. T. S. and H. O. wrote the manuscript. All authors read and approved the final manuscript. There are no conflicts of interest.