Over the past few decades, the incidence of oesophageal adenocarcinoma (OAC) and gastro-oesophageal junctional adenocarcinoma (JAC) has increased rapidly in many Western countries( Reference Lagergren and Lagergren 1 , Reference Buas and Vaughan 2 ), while that of oesophageal squamous-cell carcinoma (OSCC) has declined( Reference Lagergren and Lagergren 3 ). Although surveillance, diagnostic measures and therapy have been developed and improved for these tumours, the overall 5-year survival remains less than 15 % in Western societies( Reference Lagergren and Lagergren 3 ). This highlights the need to identify preventive measures. Dietary factors play an important role in the aetiology of oesophageal cancer, and epidemiological studies have demonstrated the protective effects of certain food groups, particularly fruits and vegetables( Reference Terry, Lagergren and Hansen 4 ) and possibly also cereals( Reference Terry, Lagergren and Ye 5 ), coffee( Reference Tavani, Bertuzzi and Talamini 6 ) and tea( Reference Tavani, Bertuzzi and Talamini 6 ). All these food groups are rich sources of not only several vitamins, but also dietary phytochemicals, which have anti-carcinogenic properties( Reference Arts and Hollman 7 ). Lignans, quercetin and resveratrol are the three most widely occurring phytochemicals with oestrogenic properties within the Western diet( Reference Adlercreutz 8 – Reference Lamuelaraventos, Romeroperez and Waterhouse 11 ). Due to having chemical structures similar to those of female hormones, these phytochemicals have been found to have the ability to bind to oestrogen receptors and thus exert oestrogenic effects( Reference Penttinen, Jaehrling and Damdimopoulos 12 – Reference Gehm, McAndrews and Chien 14 ). Furthermore, the presence of oestrogen receptors in oesophageal tissue has been observed in in vitro studies( Reference Tiffin, Suvarna and Trudgill 15 – Reference Joubert and Marais 17 ). This is interesting as oestrogen has been suggested to be an important factor for the low incidence of OAC and JAC in women in comparison with men, with a male:female sex ratio of up to 9:1( Reference Lagergren and Lagergren 1 ). High intake of lignans has been found to possibly decrease the risk of OAC and JAC in humans( Reference Lin, Yngve and Lagergren 18 ); quercetin might increase lipolysis in adipocytes and induce apoptosis( Reference Kuppusamy and Das 19 ); and resveratrol can reduce the synthesis of lipids in the liver and suppress proliferation and induce apoptosis in vitro ( Reference Ferry-Dumazet, Garnier and Mamani-Matsuda 20 ). Interestingly, a combination of lignans, quercetin and resveratrol has been found to synergistically suppress adipogenesis and induce apoptosis in an in vitro study( Reference Park, Yang and Ambati 21 ). Dietary pattern analysis captures the variation in overall dietary intake and is useful for reflecting the interaction among foods or nutrients. Recently, a hypothesis-based reduced-rank regression (RRR) analysis has been used to better identify dietary patterns( Reference Hoffmann, Schulze and Schienkiewitz 22 ). In the present study, data from a Swedish nationwide case–control study using RRR were analysed with the aim of assessing whether a dietary pattern rich in lignans, quercetin and resveratrol decreases the risk of OAC, JAC and OSCC.
Methods
Study subjects and study design
Data were derived from a Swedish nationwide and population-based case–control study, which has been described in detail previously( Reference Lagergren, Bergstrom and Lindgren 23 ). In brief, individuals aged < 80 years and born and living in Sweden from 1995 to 1997 were enrolled in the study. All newly diagnosed patients with OAC or JAC and patients with OSCC born on even-numbered days were eligible to participate in the study as cases. A comprehensive organisation for rapid ascertainment of cases ensured that every potential patient throughout the country was identified shortly after the confirmation of diagnosis. This organisation included contact persons at all the 195 relevant hospital departments in Sweden, as well as the six regional tumour registries. Age (within 10 years)- and sex-matched controls were randomly selected from among the individuals in the Swedish population identified in the Swedish Register of the Total Population. Professional interviewers, who were trained to treat cases and controls in a strictly similar manner, personally interviewed all the cases and controls. The participants were asked to provide details regarding their background and relevant exposures, which have been studied in separate publications, e.g. addressing the role of gastro-oesophageal reflux( Reference Lagergren, Bergstrom and Lindgren 23 ), obesity( Reference Lagergren, Bergstrom and Nyren 24 ), tobacco smoking status( Reference Lagergren, Bergstrom and Lindgren 25 ), alcohol consumption( Reference Lagergren, Bergstrom and Lindgren 25 ), educational level( Reference Jansson, Johansson and Nyren 26 ), physical activity( Reference Lagergren, Bergstrom and Nyren 24 ) and Helicobacter pylori infection in the aetiology of OAC, JAC and OSCC( Reference Ye, Held and Lagergren 27 ). The reflux symptoms considered in the present study referred to symptoms during the entire lifetime until 5 years before the interview. The cut-off used to define reflux was well in line with the present guidelines( Reference Vakil, van Zanten and Kahrilas 28 ). BMI 20 years before the interview was used to classify obesity.
Ethical approval
All six Regional Ethics Review Boards in Sweden approved the present study.
Dietary intake assessment
Dietary intake was assessed using a written validated FFQ concerning the habitual intake of sixty-three foods and beverages as recalled from 20 years before the interview. This time period was considered for dietary intake assessment to obtain a plausible induction time point between exposure and the onset of the invasive cancer( Reference Wolk, Bergstrom and Hansson 29 ). The questionnaires were distributed before the interview, and the written answers were completed and other uncertainties clarified during the interview. The consumption frequency of each food item was assessed on the basis of open answers, i.e. number of times of consumption per d, week, month or year. The intakes of lignans, quercetin and resveratrol were the exposure variables in the present study. A specific database was created with information from published analytical data for the contents of these three phytochemicals. Lignans are mainly present in whole-grain bread, berries, fruits, vegetables and flaxseed( Reference Adlercreutz 8 ). The intake of lignans was calculated by taking six precursors of mammalian lignans into account: matairesinol; secoisolariciresinol; lariciresinol; pinoresinol; syringaresinol; medioresinol( Reference Adlercreutz 8 , Reference Adlercreutz and Mazur 30 , Reference Valsta, Kilkkinen and Mazur 31 – Reference Thompson, Boucher and Liu 38 ). Data on quercetin contents in onions, broccoli, lettuce, tomatoes, strawberries, apples, wine, tea and fruit juice were accumulated( Reference Hertog, Hollman and Katan 9 , Reference Hertog, Hollman and Vandeputte 39 – Reference Stecher, Huck and Popp 41 ). The major sources of resveratrol were wine, tea and berries( Reference Lamuelaraventos, Romeroperez and Waterhouse 11 , Reference Stecher, Huck and Popp 41 – Reference Jandera, Skerikova and Rehova 43 ). As green tea was effectively non-existent in the Swedish market during the exposure period of interest (mid-1970s), the intake of tea was calculated by assuming all teas consumed to be black tea. The intake of wine was estimated by assuming the wine consumed to be two-thirds red wine and one-third white wine, based on typical Swedish alcohol consumption data. Energy contents were obtained from the Swedish National Food Administration database. To calculate the intake of each food item, the consumption frequency was multiplied by sex-specific portion sizes, using data from the Swedish National Dietary Survey( Reference Becker and Riksmaten 44 ).
Statistical analyses
To derive a dietary pattern defining the intake of lignans, quercetin and resveratrol, the statistical method RRR was employed using the partial least-squares procedure in SAS( Reference Hoffmann, Schulze and Schienkiewitz 22 ). A factor score was derived using RRR – a linear combination of predictor variables (food groups or food items) that maximised the variation in response variables (lignans, quercetin and resveratrol). The number of extracted dietary pattern scores was equal to the number of response variables, i.e. three dietary pattern scores were extracted, one for each single phytochemical-related dietary pattern. The first dietary pattern score was retained for subsequent analyses because it explained most of the variation in the intake of the three phytochemicals. The total intake of all the phytochemicals was analysed as energy-adjusted densities, i.e. per 4184 kJ/d (1000 kcal/d). To analyse less population-dependent dietary pattern variables, the original dietary pattern scores were shortened and simplified( Reference Schulze, Hoffmann and Kroke 45 ). The simplified dietary pattern scores were obtained by summing unweighted standardised scores of intake for each food group, which explained most of the inter-individual variation in the original dietary pattern scores. The commonly used simplified approach was used by selecting only food items with high factor loadings (i.e. ≥ |0·2|)( Reference Schulze, Hoffmann and Kroke 45 ). Factor loadings represent the correlations of the intake of each food item with the dietary pattern score. The simplified dietary pattern scores were used to evaluate the association between the dietary pattern and the risk of oesophageal cancer subtypes in logistic regression models. Dietary pattern scores were categorised into quintiles on the basis of the distribution among the control subjects. To test for linear trends across dietary pattern scores, the median scores within each quintile were used and these values were treated as a continuous variable.
Unconditional logistic regression was used to estimate OR and 95 % CI. The dietary pattern score was categorised into five levels based on the quintiles of scores among the control subjects. The lowest quintile of dietary pattern score was set as the reference. Possible confounding or effect modification by aetiological factors was considered in a multivariable logistic regression model: age (categorised into < 55, 55–64, 65–74, or ≥ 75 years); sex (male or female); gastro-oesophageal reflux symptoms (yes or no); BMI ( < 18·5, 18·5–24·9, 25–29·9, or ≥ 30·0 kg/m2); tobacco smoking status (never smoker, previous smoker, or current smoker of any tobacco 2 years before the interview); alcohol consumption (no alcohol, >0–15, 16–70, or >70 g/week of any alcoholic beverage); educational level ( ≤ 9, 10–12, or ≥ 13 years of formal education); physical activity (in quartiles); H. pylori infection (yes or no, assessed from blood samples); total energy intake (in quartiles, based on the distribution among the control subjects). A basic model included adjustment for only age and sex, while the fully adjusted multivariable model included all the variables listed above, selected depending on the specific subtype of oesophageal cancer analysed. The reason for this tailored adjustment is that the aetiological factors differ substantially between these cancer subtypes( Reference Lagergren and Lagergren 3 ). Especially, reflux and obesity are the main risk factors for OAC and JAC and alcohol consumption and tobacco smoking status are the main risk exposure factors for OSCC( Reference Lagergren and Lagergren 3 ). Participants with more than 10 % missing values in the FFQ were excluded from the analyses. The SAS statistical package (version 9.0, SAS Institute, Inc.) was used for all analyses.
Results
Study participants
Among all the 618 included cases, 189 had OAC (88 % participation rate), 167 had OSCC (73 %) and 262 had JAC (84 %). The number of control subjects was 820 (73 % participation rate). Participants with more than 10 % missing values in the FFQ were excluded, leaving 181 cases of OAC, 158 cases of OSCC, 255 cases of JAC and 806 controls for the final analysis. The demographics and characteristics of the study participants are summarised in Table 1. Owing to frequency matching, age and sex distributions were similar among the cases and controls. OAC and JAC cases had more reflux and higher BMI than controls, while OSCC cases consumed more tobacco and alcohol. All case groups had fewer years of education and a lower percentage of never-tobacco-smoking participants compared with the control group. The control group consumed more tea, lettuce, whole-grain bread and mixed vegetables and less milk when compared with the case groups (P< 0·05; t test). No differences were found in the intake of tomatoes and wine. The intake of quercetin and lignans was higher in the control group than in each of the case groups (P< 0·05; t test, data not shown), while the intake of resveratrol was similar in the control and case groups (P= 0·68; t test). The simplified dietary pattern scores were positive in the control group, but negative in all the three case groups (Table 1). All cases had a lower dietary pattern score than their matched controls (P< 0·0001; t test, data not shown).
Table 1 Characteristics of oesophageal cancer cases and control subjects in a Swedish population-based case–control study (Number of subjects and percentages)

* Including secoisolariciresinol, matairesinol, lariciresinol, isolariciresinol, pinoresinol, syringaresinol and medioresinol.
† A simplified dietary pattern score was obtained by summing standardised intake values of tea, wine, lettuce, mixed vegetables, tomatoes, milk and whole-grain bread as energy-adjusted densities.
Food groups contributing to the lignan, quercetin and resveratrol pattern scores
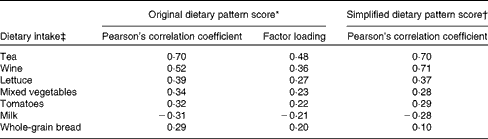
Tea, wine, lettuce, mixed vegetables, tomatoes, milk and whole-grain bread, with a factor loading ≥ |0·2|, were the most important food items contributing to the intake of dietary lignans, quercetin and resveratrol, based on the estimation using the RRR analysis, and retained in the simplified dietary pattern (Table 2). Pearson's correlation coefficients of the dietary pattern scores were slightly lower for the simplified dietary pattern than for the original dietary pattern. Both the original and simplified dietary pattern scores were strongly correlated with the intake of tea and wine, which explained most of the variation in the original dietary pattern scores. Only the intake of milk was negatively associated with the studied dietary pattern scores (Table 2).
Table 2 Food groups mainly contributing to a high phytochemical (resveratrol, quercetin and lignans) dietary pattern score
* The original dietary pattern score was estimated using reduced-rank regression analysis.
† A simplified dietary pattern score was obtained by summing standardised intake values of tea, wine, lettuce, mixed vegetables, tomatoes, milk and whole-grain bread as energy-adjusted densities.
‡ Dietary intake is expressed as energy-adjusted densities, i.e. per 4184 kJ/d (1000 kcal/d).
Dietary pattern characterised by the intake of lignans, quercetin and resveratrol and the risk of oesophageal cancer by histological type
The identified dietary pattern characterised by the intake of lignans, quercetin and resveratrol was strongly associated with a decreased risk of OAC, JAC and OSCC (Table 3). Dose-dependent associations were found between higher simplified dietary pattern scores and a decreased risk of each of the studied cancer subtypes (all P for trend < 0·05) (Table 3). Comparison of the extreme quintiles (quintile 5 v. quintile 1) of scores in the fully adjusted model revealed decreased risk estimates of OAC (OR 0·24, 95 % CI 0·12, 0·49), OSCC (OR 0·31, 95 % CI 0·15, 0·65), JAC (OR 0·49, 95 % CI 0·28, 0·84), and OAC and JAC combined (OR 0·42, 95 % CI 0·26, 0·67).
Table 3 Quintiles of simplified dietary pattern scores based on the intake of food items that were derived to explain variation in the intake of the phytochemicals resveratrol, quercetin and lignans (Odds ratios and 95 % confidence intervals; number of subjects and percentages)

* Two-sided P values for trend were calculated by the Wald statistics, using the median values for each category of dietary pattern score as a continuous variable.
† Adjusted for age, sex, energy, educational level, tobacco smoking status, alcohol consumption, BMI, physical activity, reflux and Helicobacter pylori infection.
‡ Adjusted for age, sex, energy, educational level, tobacco smoking status, alcohol consumption and physical activity.
Discussion
This is the first study to define a dietary pattern characterised by the intake of the phytochemicals lignans, quercetin and resveratrol and to examine its potential association with the risk of oesophageal cancer. Strong negative associations were found between a dietary pattern rich in lignans, quercetin and resveratrol and the risk of all histological types of oesophageal cancer.
High dietary pattern scores for these three phytochemicals were mainly derived from a high intake of tea, wine, lettuce, mixed vegetables, tomatoes, and whole-grain bread and a low intake of milk.
Tea, a rich food source of quercetin and lignans in Western populations, had the highest loading factor in the dietary pattern characterised by the intake of phytochemicals, and black tea has been indicated as a factor that can prevent the development of oesophageal cancer( Reference Tavani, Bertuzzi and Talamini 6 ). Moreover, animal research has identified black tea to have anti-carcinogenic properties, which could be synergistically enhanced by the intake of resveratrol( Reference George, Singh and Srivastava 46 ). Tea consumption has been shown to be a significant risk factor for OSCC in Iran, which may be mainly due to the habit of drinking tea at very high temperatures, which could cause injury to the oesophageal mucosa and increase the risk of OSCC( Reference Islami, Pourshams and Nasrollahzadeh 47 ). In Sweden, where tea is not consumed at high temperatures, no associations between tea consumption and the risk of any of the main histological types of oesophageal cancer have been found( Reference Terry, Lagergren and Wolk 48 ). Wine, which is rich in resveratrol, was another key component in the defined dietary pattern in the present study. It is possible that resveratrol might have contributed to the decreased risk of OAC and OSCC among wine drinkers observed in some studies( Reference Wu, Wan and Bernstein 49 ). Furthermore, high intake of fruits and vegetables has been found to decrease the risk of oesophageal cancer( Reference Terry, Lagergren and Hansen 4 ). Fruits and vegetables are the main sources of the three studied phytochemicals( Reference Brown, Swanson and Gridley 50 ). Particularly, tomatoes and lettuce, the main contributors of quercetin intake, were highlighted in the defined dietary pattern. This finding parallels that of a previous study, in which tomatoes were a key component in a ‘healthy diet’ pattern for preventing the development of oesophageal cancer( Reference Hajizadeh, Rashidkhani and Rad 51 ). Whole-grain bread is the most important dietary source of lignans in Sweden and other European countries( Reference Lin, Wolk and Hakansson 52 ). High intake of whole-grain bread has been reported to be associated with a decreased risk of OAC and has been found to be a component of a ‘healthy pattern’ for OAC, together with a low intake of milk( Reference Chen, Ward and Graubard 53 ). A high intake of energy from milk is usually associated with a low intake of energy from fruits and vegetables, which often results in a low intake of these three phytochemicals, and a high intake of milk might increase the risk of oesophageal cancer( Reference Bosetti, La Vecchia and Talamini 54 ). However, we cannot confirm that the beneficial effect of these three phytochemicals on the risk of oesophageal cancer observed in the present study is different from any accumulated or synergistic effect of phytochemicals in the plant-based diet.
Epidemiological studies examining lignans, quercetin and resveratrol separately in relation to the risk of oesophageal cancer are scarce. The oestrogenic and anti-obesity properties of lignans might have contributed to the decreased risk of oesophageal cancer found in the present study( Reference Adlercreutz 8 ). Animal research has found that quercetin can induce apoptosis( Reference Chen, Corteling and Stevanato 55 ), but no epidemiological studies have addressed the role of quercetin intake in the development of oesophageal cancer. However, a strong inverse association between quercetin intake and distal gastric cancer risk has been reported( Reference Ekstrom, Serafini and Nyren 56 ). Resveratrol can exert anti-carcinogenic effects and modulate lipid and lipoprotein metabolism and has oestrogenic properties( Reference Signorelli and Ghidoni 57 ). A dietary pattern characterised by a high combined intake of these three phytochemicals might exert an anti-carcinogenic effect based on the synergistic effect on the suppression of adipogenesis and induction of apoptosis, as indicated in animal research( Reference Park, Yang and Ambati 21 ).
The strengths of the present study include the population-based design, including most newly diagnosed cases of the three oesophageal cancer subtypes in Sweden over 3 years, and the rigorous tumour classification of site and histological types. The random sampling of controls from the entire Swedish population reduced the possibility of biased selection. Another strength was the ability to adjust the results for all the established risk factors, counteracting bias due to confounding. Finally, the adopted RRR approach is a novel and powerful tool for describing overall dietary habits, with a focus on the variation in the intake of the interested disease-related nutrients. Weaknesses include information bias of exposures, as dietary intake estimations based on FFQ are typically associated with some level of measurement errors. The requirement to recall past dietary habits could result in misclassification of the exposure factors, but significant differences in the recall of dietary habits between cases and controls are not likely to occur because of their case–control (recall bias) status. In fact, we have shown in a previous validation study that cases and controls have equal reliability regarding recalling remote dietary habits( Reference Wolk, Bergstrom and Hansson 29 ). Moreover, previous dietary studies based on the same case–control study on which the present study is based have reported clear differences in associations depending on the histological type of oesophageal cancer, further indicating that recall bias is not a major concern( Reference Terry, Lagergren and Ye 58 , Reference Terry, Lagergren and Wolk 59 ). The phytochemical exposure was based on the dietary intake 20 years before the interview, resulting in an increased risk of misclassification due to difficulties in recalling previous dietary habits. Furthermore, the phytochemical concentration values in the food database used in the present study were derived from a recently published paper, which might not be a good representative of foods consumed in the 1970s because of changes in industrial technology and cooking procedures. However, the cooking procedures and dietary habits have apparently not changed since the 1970s in the Swedish population, according to the Swedish National Food Administration. Moreover, this method of dietary intake assessment of food items 20 years earlier has been validated with good results and shown to capture a combination of previous and current dietary habits( Reference Wolk, Bergstrom and Hansson 29 ). Non-participation was limited, but existed, which introduces a risk of selection bias. However, an association between participation and the dietary pattern studied was not likely, and thus non-participation cannot explain the findings of the present study. Finally, although the sample size was comparatively large, there were a limited number of cases in each quintile of dietary pattern scores, leaving a risk of chance errors. However, the strongly decreased risk estimates obtained in the present study were clearly statistically significant, and the dose–response trends further indicate the robustness of the findings.
In conclusion, this comprehensive and population-based case–control study indicates that a high combined dietary intake of foods rich in lignans, quercetin and resveratrol (represented by a high intake of tea, wine, tomatoes, lettuce, mixed vegetables, and whole-grain bread and a low intake of milk) could prevent the development of OAC, OSCC and JAC.
Acknowledgements
The authors thank all the 227 doctors who acted as contact persons at the participating hospital departments throughout Sweden.
The present study was supported by the Faculty Funds for Partial Financing of New Doctoral Students from Karolinska Institutet (12059012/KID-medel 2010), the Swedish Cancer Society, and the Swedish Research Council (SIMSAM).
The authors’ contributions are as follows: J. L., Y. Lu and Y. Lin were responsible for the conception and design of the study; J. L. and Y. Lu obtained financial support to conduct the study; J. L. was responsible for the provision of study materials; J. L. collected and assembled the data; J. L., Y. Lu and Y. Lin analysed and interpreted the data. All authors contributed to manuscript preparation.
None of the authors has any conflicts of interest to declare.





