With its global expanding prevalence(Reference Zimmet, Alberti and Shaw1), especially in Japan(Reference Eshak, Iso and Maruyama2), type 2 diabetes mellitus (T2DM) ranks as one of the top health problems. Previous epidemiological studies reported that improving diet quality should be targeted for the prevention of T2DM (Reference Eshak, Iso and Maruyama2–Reference Martini, Catania and Ferreira5). A growing body of evidence suggested a considerable role of micronutrients in the pathogenesis and complications of T2DM(Reference Eshak, Iso and Muraki3–Reference Martini, Catania and Ferreira5).
The antioxidant properties of vitamin C have been suggested as a plausible mechanism for the reduced risk of T2DM with high vitamin C intakes or plasma concentrations(Reference Zhou, Na and Shan6,Reference Song, Xu and Park7) . However, a review of observational studies indicated that these associations were evident in some but not in all previous studies(Reference Christie-David, Girgis and Gunton8). Moreover, no association was reported in a large randomised controlled trial(Reference Song, Cook and Albert9).
On the other hand, the plasma levels in almost all the B-group vitamins have been found lower in diabetic than in non-diabetic individuals(Reference Valdés-Ramos, Guadarrama-López and Martínez-Carrillo4,Reference Martini, Catania and Ferreira5) as a consequence of diabetes, either because of behavioural changes or changes in metabolism. However, most of the available research on B-group vitamins/T2DM associations has concentrated on vitamins B6, folate and vitamin B12, and has shown inconsistent findings(Reference Song, Cook and Albert10–Reference Gargari, Aghamohammadi and Aliasgharzadeh12). Similar large studies to investigate the associations of other B vitamin intakes, such as vitamin B1 (thiamine), B2 (riboflavin), B3 (niacin) and B5 (pantothenic acid) are scarce(Reference Al-Attas, Al-Daghri and Alfadda13–Reference Demirci, Demir and Dost18). Moreover, the previous observations were either for the vitamin B-group status(Reference Ahn, Min and Cho11,Reference Al-Attas, Al-Daghri and Alfadda13,Reference Zhou, Li and Sun17) or supplemental(Reference Christie-David, Girgis and Gunton8,Reference Song, Cook and Albert9,Reference Gargari, Aghamohammadi and Aliasgharzadeh12,Reference González-Ortiz, Martínez-Abundis and Robles-Cervantes14–Reference Sazonov, Maccubbin and Sisk16,Reference Demirci, Demir and Dost18) rather than dietary intakes(Reference Song, Xu and Park7). Therefore, in the present analysis, we aimed to investigate the association between dietary consumptions of the whole set of water-soluble vitamins (B1, B2, B3, B5, B6, B9, B12 and C) and the risk of 5-year cumulative incidence of T2DM among a large cohort of Japanese men and women, hypothesising that intakes of at least some of these water-soluble vitamins could associate inversely with the risk of T2DM.
Methodology
Study population and baseline covariates
A total of 52 658 diabetes-free residents of forty-five Japanese communities aged 40–79 years participated in the Japan Collaborative Cohort Study for Evaluation of Cancer Risk and completed a self-administered questionnaire after subjects or community leaders had given informed consents. The questionnaire inquired about subjects’ demographic data; medical histories of chronic diseases, diabetes mellitus and hypertension; drinking habits; smoking; exercise; supplement use and others. The details of the Japan Collaborative Cohort Study for Evaluation of Cancer Risk protocol were described previously(Reference Tamakoshi, Ozasa and Fujino19). The protocol of this investigation was conducted according to the Declaration of Helsinki and had been approved by the ethics committees of Hokkaido and Osaka universities. The final study subjects (n 19 168) are those without missing information for history of diabetes at two time points: the baseline in 1988–1990 and 5 years later (see online Supplementary Flow Chart). Participants’ characteristics of included and excluded subjects were given in online Supplementary Table S1.
Table 1. Baseline characteristics of men according to quartiles of water-soluble vitamins intakes† (Mean values and standard deviations; numbers of participants; percentages)
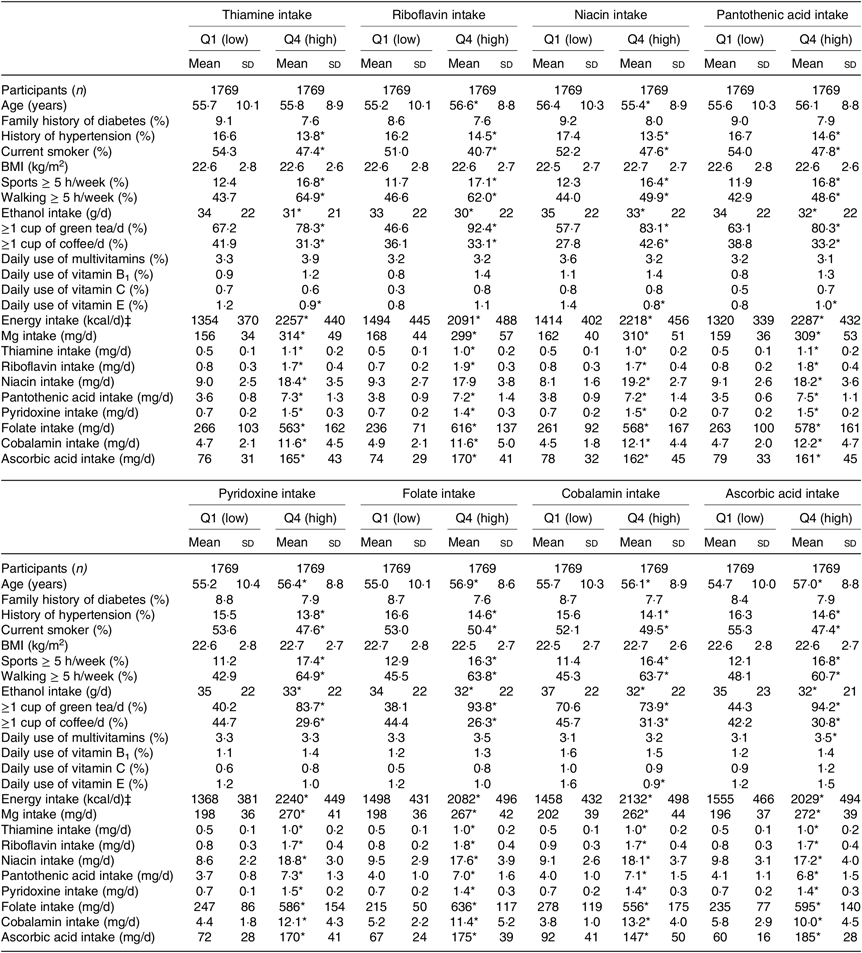
* P for trend < 0·05.
† Linear regression was used for continuous variables, and logistic regression was used for categorical variables.
‡ To convert kcal to kJ, multiply by 4·184.
Dietary assessment
The dietary intakes of foods and drinks, without specifying portion size, depending on the habitual consumption pattern within the past year were collected by a validated forty-item FFQ with five possible responses for the frequency of consumption: rarely, 1–2 times/month, 1–2 times/week, 3–4 times/week and almost every day(Reference Date, Fukui and Yamamoto20).These corresponding frequencies were transformed into a daily intake score of 0, 0·38, 1·5, 3·5 and 7·0 respectively. The intake score of each food item was multiplied by its specific portion size that was estimated from a validation study(Reference Date, Fukui and Yamamoto20). Then, we calculated the nutrients’ intakes from each item based on the content in 100 g of each food item according to the fifth edition of the Japan Food Tables(21). Total intakes from diet only of each nutrient including water-soluble vitamins were calculated by adding nutrient intakes from all food items. The validation study, conducted among eighty-five subjects to estimate the 1-year period consumption levels measured by four seasonal 3-d weighted dietary records(Reference Date, Fukui and Yamamoto20), showed the FFQ valid for assessing the water-soluble vitamin intakes, with moderate correlations as indicated by the Spearman’s rank correlation coefficients between the FFQ and dietary records which ranged from 0·24 for vitamin B12 to 0·44 for vitamin B2. Regarding the test–retest reliability of the FFQ, the Spearman’s rank correlation coefficients between two applications of the same FFQ 1 year apart ranged from 0·32 for niacin to 0·51 for ascorbic acid.
Assessment of type 2 diabetes mellitus
Individuals without a history of diabetes at baseline and reported newly diagnosed diabetes on the 5-year survey were considered to have incident diabetes. The comparison between the self-report of diabetes and diabetes diagnosed by laboratory findings and/or therapy data in a sample of 1837 women and 1230 men showed sensitivity and specificity of 75 and 98 %, respectively, in women, and 70 and 95 %, respectively, in men. Details of used diagnostic criteria and validity of self-reported data were given previously(Reference Eshak, Iso and Maruyama2,Reference Eshak, Iso and Muraki3) .
Statistical analysis
We used the 5-year cumulative incidence of T2DM without calculating person-years, because we do not have data on the precise dates of diabetes onset. Water-soluble vitamins were modelled as four quartiles, and the linear (for continuous variables) and logistic (for categorical variables) regressions modelling were used to assess the significance of the trend in the distribution of the age-adjusted participants’ baseline characteristics across the increasing quartiles of the vitamin intakes. The basic assumptions of these regression analyses were tested and for some non-normally distributed continuous variables the log transformation was applied to normalise the residuals. Because the P-interaction with sex was <0·05, the sex-specific associations between intakes of water-soluble vitamins with the risk of T2DM and the respective OR and 95 % CI in each quartile of intake were assessed using multiple logistic regression modelling that adjusted for age, family history of diabetes (yes, no); past history of hypertension (yes, no); smoking status (never, former smoker, current smoker of 1–19 and ≥20 cigarettes/d); BMI calculated as weight (kg)/(height (m))2 (quartiles); walking hours (almost no, daily 0·5, 0·6–0·9, and ≥1 h); exercise hours (almost no, weekly 1–2, 3–4 and ≥5 h); and supplement use of vitamins E, C, B1 and multivitamins (yes, no), alcohol intake (never, former and current daily drinker of 0·1–22·9, 23·0–45·9, 46·0–68·9 and ≥69·0 g ethanol); coffee intake in cups (<once/week, 1–6/week, 1–2/d, and ≥3/d); green tea intake in cups (<once/week, 1–6/week, 1–2/d, 3–5/d, and ≥6/d); total energy intake (quartiles) and energy-adjusted intakes of Mg and fat-soluble vitamins A, K, E and D (quartiles). Tests for trends were conducted using the median intake value (mg/d) for each quartile as a continuous variable.
Due to the large sample size of our study, an 80 % statistical power for testing was guaranteed and several stratified analyses were feasible. We tested if the associations will vary by different levels of a group of variables, including younger, 40–54 years v. older 55–65 years of age; having a BMI <25 v. ≥25 kg/m2; current smokers v. non-current smokers; having a family history of diabetes v. no such a family history. The P for interaction was computed for interaction terms using dichotomous stratifying variables and the median value of dietary intakes of each water-soluble vitamin (mg/d).
Two-tailed statistical tests were performed, and a P-value <0·05 was considered statistically significant. SAS 9.4 software (SAS Institute Inc.) was used.
Results
A total of 494 subjects developed T2DM (2·6 %); 249 (3·5 %) among men and 245 (2·0 %) among women (comparing women with men, P < 0·001).
When compared with men and women in the lowest quartiles of water-soluble vitamin intakes, those in the highest quartiles were less likely to be hypertensive and to smoke, but more likely to practise sports. While alcohol intake was lower, Mg intake was higher in the highest v. lowest quartiles of water-soluble vitamins for both men and women (Tables 1 and 2).
Table 2. Baseline characteristics of women according to quartiles of water-soluble vitamins intakes† (Mean values and standard deviations; numbers of participants; percentages)
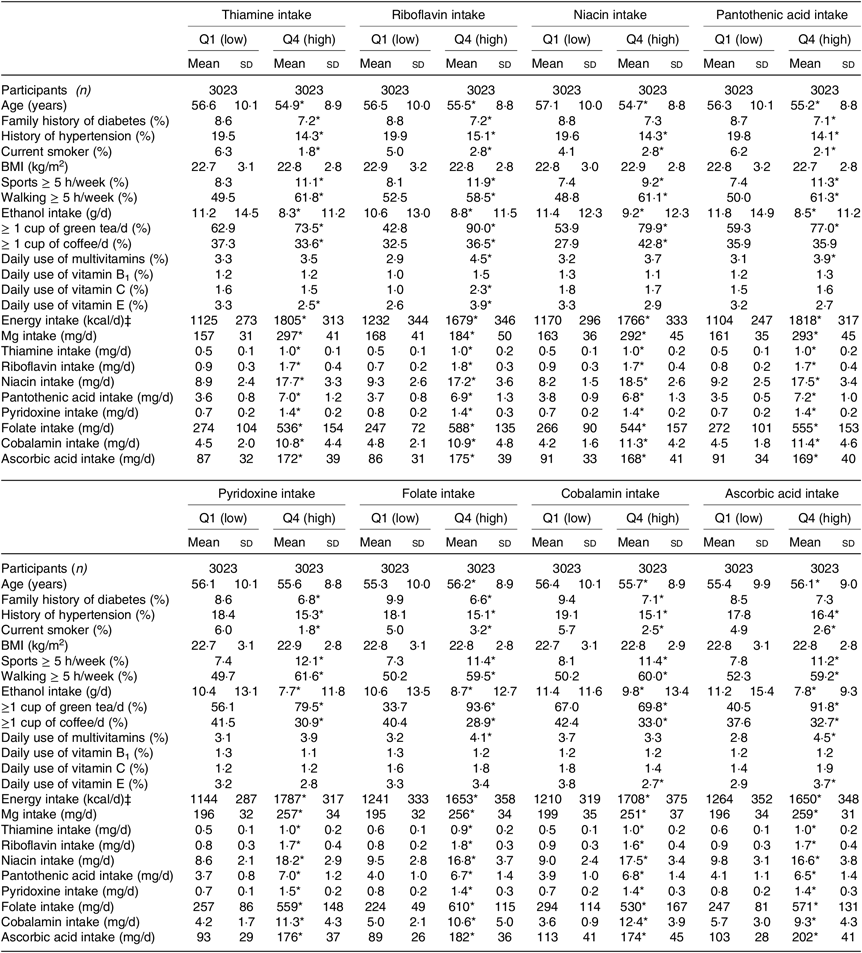
* P for trend < 0·05.
† Linear regression was used for continuous variables, and logistic regression was used for categorical variables.
‡ To convert kcal to kJ, multiply by 4·184.
In the multivariable-adjusted model, increasing dietary intakes of vitamins C, B2 and folate among women, but not men, were associated with the significant reduced risk of T2DM with 39, 44 and 30 % reduced risk in the highest v. lowest quartiles of intakes, respectively. No significant associations with dietary intakes of vitamins B1, B5, B6 or B12 were observed. The significant inverse trend for risk of T2DM across increasing quartiles of vitamin B3 intake among both men and women lost its significance in the multivariate analysis (Table 3).
Table 3. Risk of 5-year incidence of type 2 diabetes according to quartiles of dietary intakes of water-soluble vitamins (Odds ratios and 95 % confidence intervals)
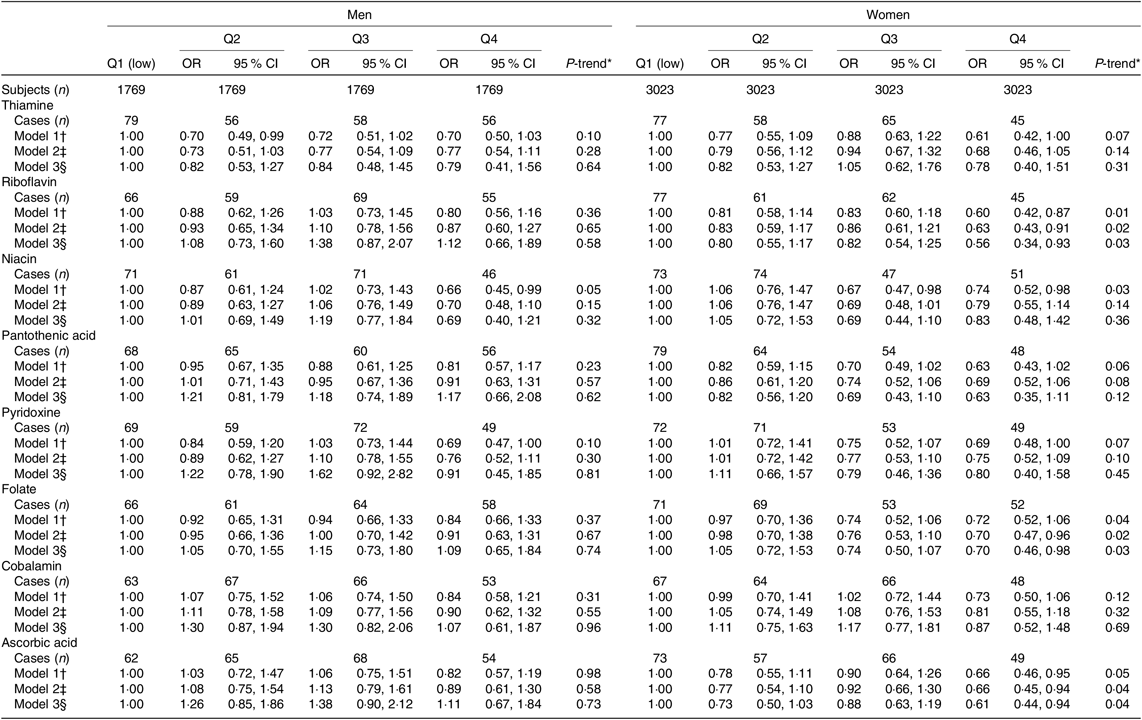
* Median values of water-soluble vitamins’ intakes in each quartile were used to test for a linear trend across quartiles.
† Model 1 age-adjusted OR (95 % CI) by logistic regression model.
‡ Model 2 adjusted further for past history of hypertension, family history of diabetes, BMI, smoking status, alcohol intakes, hours of exercise, hours of walking, supplement use of vitamins E, C, B1 and multivitamins, intakes of coffee and green tea and quartiles of total energy and Mg intakes.
§ Model 3 adjusted further for quartiles of fat-soluble vitamins (A, K, E, D) intakes.
There were no significant interactions with age, smoking status, BMI or having a family history of diabetes: P > 0·10 (data not shown in tables).
Discussion
In this large prospective study, we studied the associations between the dietary intakes of the whole set of water-soluble vitamins and risk of T2DM in Japanese men and women. The findings indicated higher dietary intakes of vitamins C, B2 and folate for risk reduction of T2DM in women. The reduced risk of T2DM with higher dietary intakes of vitamin B3 did not reach statistical significance in either men or women. The dietary intakes of the rest of the B-group vitamins (B1, B5, B6 and B12) were not associated with T2DM risk in either sex.
The pathogenesis of T2DM involves oxidative reactions that foster insulin resistance and impede insulin secretion(Reference Valdés-Ramos, Guadarrama-López and Martínez-Carrillo4,Reference Martini, Catania and Ferreira5) ; thus, the antioxidative properties of vitamin C may counteract this oxidative stress by acting as a reducing agent in free radical–mediated oxidation processes(Reference Zhou, Na and Shan6,Reference Christie-David, Girgis and Gunton8) . Serum vitamin C levels in individuals with diabetes were 30 % lower compared with those in individuals without diabetes as indicated from a review of twenty-three observational studies(Reference Will and Byers22). However, no difference was found in other studies, especially after controlling for dietary vitamin C intakes(Reference Montonen, Knekt and Järvinen23).
Similar to our findings, high dietary intakes of vitamin C were inversely associated with the risk of T2DM in two population-based Chinese cohort studies; the multivariable-adjusted hazard ratio in the highest v. lowest quartiles were 0·46 (95 % CI 0·30, 0·71; P-trend = 0·002) in 3483 men and women of the Harbin People’s Health Study and 0·76 (95 % CI 0·64, 0·89; P-trend = 0·003) in 7595 men and women of the Harbin Cohort Study on Diet, Nutrition and Chronic Non-communicable Diseases(Reference Martini, Catania and Ferreira5). On the contrary, no association between dietary vitamin C intakes and the risk of T2DM was seen in 4304 subjects of the Finnish Mobile Clinic Health Examination Survey(Reference Montonen, Knekt and Järvinen23). The reasons for the inconsistent findings are not clear; however, differences in sample size, participants’ characteristics and the tools and criteria used to diagnose diabetes may be considered. For example, in our study, of 19 168 participants aged 40–79 years, the exposure variables were estimated via a FFQ, outcome data were self-reported and our model was adjusted for several dietary variables that were associated with the risk of diabetes, including alcohol, coffee, green tea, Mg and fat-soluble vitamins intakes. Whereas the Finnish study used a dietary history interview for dietary data and the Finnish nationwide social insurance system registry of patients receiving antidiabetic treatment to ascertain the diabetes among 4304 men and women aged 40–60 years(Reference Montonen, Knekt and Järvinen23). The Chinese study involved 11 078 participants aged 20–74 years in whom diet was investigated by a FFQ and diabetes was diagnosed by an oral glucose tolerance test(Reference Zhou, Na and Shan6). Both the Finnish and the Chinese studies estimated the risk of diabetes with no adjustment for any dietary variables except total energy intake. Moreover, when looking at inconsistencies between studies, the level of intake of the micronutrients in different populations could be important. If all have plenty of vitamin C, for example, there may not be an association; conversely if the majority is well below requirements, perhaps the association will be evident. The average intake of vitamin C (80–160 mg/d) in the Nordic countries is higher than the recommended intake (75 mg/d)(Reference Fagt24); while 65·1 % of Chinese were shown to have insufficient vitamin C intake in a recent study(Reference Jia, Wang and Zhang25).
Smoking is a risk factor for vitamin C deficiency(Reference Zhou, Na and Shan6–Reference Christie-David, Girgis and Gunton8); thus, effect modifications by smoking status on the associations between vitamin C and risk of diabetes could be expected. However, similar to our findings, no interaction of smoking status with dietary or supplemental vitamin C intakes towards the risk of diabetes was reported in the previous studies(Reference Zhou, Na and Shan6–Reference Christie-David, Girgis and Gunton8).
The reduced risk of T2DM with vitamins C, B2 and folate intakes was evident for women, but not for men, in our study. This could be ascribed to chance, but the higher median intakes of vitamin C in women – 140 mg/d compared with that in men, 114 mg/d – should be considered. Zhou et al. suggested 140 mg/d increment in vitamin C intake for a 5 % risk reduction of T2DM(Reference Zhou, Na and Shan6). Moreover, the impact of dietary water-soluble vitamins might not be enough to counteract the high-risk profile in men, such as the high proportions of smokers and the higher amount of alcohol intake.
The most common source of vitamin B2 in Japanese diet is dairy products(Reference Watanabe, Suemura and Taniguchi26). Dairy products have been inversely associated with the risk of T2DM in Japanese women, but not men; OR in the highest v. lowest quartiles of intake were 0·71 (95 % CI 0·51, 0·98) and 1·18 (95 % CI 0·90, 1·56), respectively(Reference Kirii, Mizoue and Iso27). This may partially explain the inverse association of vitamin B2 intake and risk of T2DM observed in women of the present study. Although the biological mechanisms by which vitamin B2 can reduce T2DM risk are not yet established, vitamin B2 possesses antioxidant properties and has an efficient-reducing capacity of ferric Fe-based haeme proteins(Reference Ashoori and Saedisomeolia28). Therefore, vitamin B2 can help reduce Fe overload which has been involved in the pathogenesis of T2DM(Reference Eshak, Iso and Maruyama2). Moreover, high plasma homocysteine levels were reported causally related to the development of T2DM(Reference Huang, Ren and Huang29), and the vitamin B2-derived flavin adenine dinucleotide is needed to activate the methylenetetrahydrofolate reductase enzyme which metabolises folate to 5-methyltetrahydrofolate, the methyl donor needed in homocysteine remethylation(Reference Power30).
So far, there is only one randomised, placebo-controlled trial that assessed the effect homocysteine-lowering treatment (a combination of folic acid and vitamins B6 and B12) on the risk of T2DM among women, and reported no apparent effect of the combination treatment(Reference Song, Cook and Albert10). We also did not find any significant associations between dietary intakes of vitamins B6 and B12 with the risk of T2DM; however, folate intake was inversely associated with the risk. Title et al. found that a supplement containing folate and other antioxidants did not significantly improve the endothelial function, whereas a supplement containing folate alone did(Reference Title, Cummings and Giddens31). Endothelial dysfunction, independent of other known risk factors, was shown to predict T2DM in a prospective, nested case–control study of 32,826 women within the Nurses’ Health Study(Reference Meigs, Hu and Rifai32). It seems that dietary v. supplementary intakes and folate alone v. combinations with other vitamins might modify the association between B-group vitamins and risk of T2DM due to some unfavourable interactions between folate and other vitamins.
High homocysteine levels can be metabolised through two ways, trans-sulfuration and remethylation. Pyridoxine is needed for the first, whereas the second depends on vitamins B2, folate and B12 (Reference Huang, Ren and Huang29–Reference Title, Cummings and Giddens31,33) . The available evidence showed inconsistent effects of supplementary vitamin B6, and a stronger homocysteine-lowering activity for folate, rather than vitamin B12 (Reference Song, Cook and Albert10,33) . Accordingly, the reduced risk of T2DM with higher folate, rather than vitamins B6 or B12 intakes in women of the present study, is plausible.
An inverse trend of T2DM risk was observed across the increasing quartiles of dietary vitamin B3 intake in both sexes of our study; however, this trend lost its significance in the multivariable model, especially after adjusting for alcohol and magnesium intakes. Despite its beneficial effects on cardiovascular health through lipid modification(Reference Goldie, Taylor and Nguyen15,Reference Sazonov, Maccubbin and Sisk16,Reference Goldberg and Jacobson34) , vitamin B3 therapy in clinical trials was associated with a rise in blood glucose levels in patients with(Reference Goldberg and Jacobson34) and without diabetes(Reference Sazonov, Maccubbin and Sisk16). The only available meta-analysis of randomised controlled trials showed a 34 % increased risk of T2DM with vitamin B3 therapy; relative risk was 1·34 (95 % CI 1·21, 1·49)(Reference González-Ortiz, Martínez-Abundis and Robles-Cervantes14). We do not have a clear explanation for these inconsistent results for dietary and supplemental vitamin B3 intakes. However, the intake of some nutrients may have a U-shaped association with health outcomes. Since the supplement is an additional intake of nutrients, total intake levels may exceed appropriate intake levels even though water-soluble vitamins are believed to not cause vitamin excess. Unfortunately, none of the vitamin B3 supplement clinical trials has reported the baseline dietary intake of vitamin B3, nor did any of them adjust for dietary vitamin B3 intake.
The self-report of diabetes status and dietary intakes is one of the limitations in our analysis. While the self-reported diabetes status showed very high sensitivity and specificity with laboratory and treatment data(Reference Eshak, Iso and Maruyama2,Reference Eshak, Iso and Muraki3) , the FFQ-estimated intake of water-soluble vitamins was underestimated by about 30 % according to the validation study; however, it demonstrated good repeatability and ability to classify subjects into extremes of vitamin intakes(Reference Eshak, Iso and Muraki3,Reference Date, Fukui and Yamamoto20) . Another limitation is the use of data of one-third of the total study subjects due to the lack of information about diabetes status at the 5-year follow-up. We compared the participants’ characteristics such as age, BMI, smoking and drinking habits and dietary intakes of water-soluble vitamins among subjects who reported and those who did not report their diabetes status at the 5-year follow-up, and there were no significant differences. Despite the control over a large bulk of confounding factors, residual confounding should be addressed as a limitation. For example, phytochemicals are considered to be a protective factor of diabetes, but we had no data on such dietary factors. Last, the absence of data on the use of water-soluble vitamin supplement, except for vitamins C and B1, is an additional limitation. However, in the late 1980s and early 1990s, vitamin supplementation was not prevalent in Japan: only 3·5 %, 2·6 % and 1·3 % of our participants reported the daily use of multivitamin, vitamin C and vitamin B1 supplementation, respectively. We controlled for these variables in the multivariate model and ran a sensitivity analysis by excluding those supplement users; however, the associations did not substantially change (data not shown).
Conclusions
In conclusion, this large community-based prospective cohort study suggested higher dietary intakes of vitamins C, B2 and folate for the reduced risk of T2DM among Japanese women. Further evidence from other observational studies and clinical trials are necessary to confirm that water-soluble vitamins are beneficial for the prevention of T2DM. However, because food sources of water-soluble vitamins are abundant, the availability, accessibility, affordability and preferences of dietary patterns rich in vitamins C, B2 and folate may be emphasised as important public health and dietary guideline measures for the prevention of T2DM.
Acknowledgements
The authors thank all staff members involved in the present study for their valuable help in conducting the baseline survey and follow-up.
The present study was supported by Grants-in-Aid for Scientific Research from the Ministry of Education, Science, Sports and Culture of Japan (61010076, 62010074, 63010074, 1010068, 2151065, 3151064, 4151063, 5151069, 6279102, 11181101, 17015022, 18014011, 20014026, and 20390156); and Comprehensive Research on Cardiovascular and Lifestyle Related Diseases (H26-Junkankitou [Seisaku]-Ippan-001).
The authors’ contributions were as follows: A. T. and H. I. designed the research, E. S. E. conducted the analyses and prepared the manuscript; H. I., I. M. and A. T. made critical revisions of the manuscript; E. S. E. and H. I. had primary responsibility for final content. All authors read and approved the final manuscript.
There are no conflicts of interest.
Supplementary material
For supplementary material/s referred to in this article, please visit https://doi.org/10.1017/S000711451900062X






