Trial registration
ClinicalTrials.gov ID; URL: http://www.clinicaltrials.gov/:NCT00932529
Significant outcomes
∙ Global cognition and several cognitive subdomains continued to improve beyond the initial phase of acute psychosis treatment and into the later phase of follow-up.
∙ Reduction of the C-reactive protein (CRP) level in the initial acute phase was associated with delayed improvement of global cognition. Inverse associations between CRP levels and cognitive subdomains were found for learning, verbal abilities, and attention.
Limitations
∙ There was considerable attrition in the study during follow-up.
∙ CRP was the only inflammatory marker measured in the study.
Introduction
Cognitive dysfunctions are core features of schizophrenia that detrimentally affect functional outcome (Reference Kahn and Keefe1–Reference Howes and Murray3). The neurobiological disturbances involved in cognitive dysfunctions are, however, largely unknown. Abnormal myelination, white matter changes, and immunological processes, including low-grade inflammatory responses, have been suggested as possible mechanisms (Reference Kroken, Loberg, Dronen, Gruner, Hugdahl, Kompus, Skrede and Johnsen4). Many studies have indicated that the cognitive impairments seem to stabilise after the first episode of psychosis (Reference Heaton, Gladsjo, Palmer, Kuck, Marcotte and Jeste5,Reference Rodriguez-Sanchez, Ayesa-Arriola, Perez-Iglesias, Perianez, Martinez-Garcia, Gomez-Ruiz, Tabares-Seisdedos and Crespo-Facorro6), although there have also been some findings suggesting cognitive improvements (Reference Ito, Nemoto, Tsujino, Ohmuro, Matsumoto, Matsuoka, Tanaka, Nishiyama, Brunel, Suzuki, Kinoshita, Ozawa, Fujita, Shimodera, Kishimoto, Matsumoto, Hasegawa and Mizuno7–Reference Martinez-Cengotitabengoa, Mico, Arango, Castro-Fornieles, Graell, Paya, Leza, Zorrilla, Parellada, Lopez, Baeza, Moreno, Rapado-Castro and Gonzalez-Pinto10). The inconsistent literature might at least in part be explained by differences in the patient samples, the duration of follow-up, and the cognitive test batteries. Taken together, recent findings indicate that cognitive dysfunction is not always irreversible and can be dynamic, especially in the acute phases (Reference Johnsen, Fathian, Kroken, Steen, Jorgensen, Gjestad and Loberg11,Reference Anda, Bronnick, Johnsen, Kroken, Jorgensen and Loberg12). Importantly, current treatment options in psychosis, including psychosocial interventions and antipsychotic medications, have few if any beneficial effects on cognitive performance (Reference Kroken, Loberg, Dronen, Gruner, Hugdahl, Kompus, Skrede and Johnsen4,Reference Lee and Lee13–Reference Nielsen, Levander, Kjaersdam Telleus, Jensen, Ostergaard Christensen and Leucht17). Thus, this central area of dysfunction in psychosis is in need of a better understanding and novel treatment approaches.
Inflammation has been the focus of recent studies addressing the pathophysiology of psychotic disorders. Immune abnormalities in the blood, cerebrospinal fluid, and central nervous system, including immune cell numbers, inflammatory markers, and antibody titers, have been demonstrated in schizophrenia (Reference Dickerson, Stallings, Origoni, Vaughan, Khushalani and Yolken18–Reference Miller and Goldsmith23). There is increasing evidence for underlying inflammatory mechanisms relevant to cognitive functioning (Reference Kroken, Loberg, Dronen, Gruner, Hugdahl, Kompus, Skrede and Johnsen4,Reference Ribeiro-Santos, Lucio Teixeira and Salgado24–Reference Kahn and Sommer26). Indeed, neuroinflammation with white matter pathology has been demonstrated in the early stages of psychosis (Reference Najjar and Pearlman27), and it has been suggested that these changes can lead to both structural and functional dysconnectivity and cognitive dysfunction (Reference Khandaker, Cousins, Deakin, Lennox, Yolken and Jones21). Low-grade elevation of the CRP, a well characterised and standardised marker of inflammation (Reference Deodhar28), has been found in some studies in schizophrenia (Reference Horsdal, Kohler-Forsberg, Benros and Gasse29–Reference Miller, Culpepper and Rapaport35). An inverse association between the serum levels of CRP and cognitive function has been reported (Reference Dickerson, Stallings, Origoni, Vaughan, Khushalani and Yolken18,Reference Dickerson, Stallings, Origoni, Boronow and Yolken36,Reference Dickerson, Stallings, Origoni, Vaughan, Khushalani and Yolken37). However, these studies are predominantly cross-sectional and include patients in the chronic phase. This might restrict the generalisability of the results, and longitudinal studies with repeated cognitive assessments are scarce.
We have recently shown an inverse association between the serum level of CRP and cognitive performance in the acute phase of psychosis (Reference Johnsen, Fathian, Kroken, Steen, Jorgensen, Gjestad and Loberg11). What remains unresolved, however, is whether the levels and change of CRP in the initial phase of acute psychosis influence cognition in later phases. The main aim of this study was therefore to investigate how the CRP level in the first acute phase of psychosis correlates with cognitive function during a 6 months follow-up.
Material and methods
The study is part of a pragmatic, randomised trial comparing four different second-generation antipsychotics in the treatment of psychosis (Fig. 1). Importantly this study reports data for all treatment groups collectively, and does not compare treatment groups. We have previously published cross-sectional comparisons of treatment groups, and no differences were found (Reference Johnsen, Fathian, Kroken, Steen, Jorgensen, Gjestad and Loberg11). The main objective of the present study was to investigate overall associations between longitudinal changes in CRP and cognition in the whole group. The clinical sample and methods have been described in detail elsewhere (Reference Johnsen, Kroken, Wentzel-Larsen and Jorgensen38).
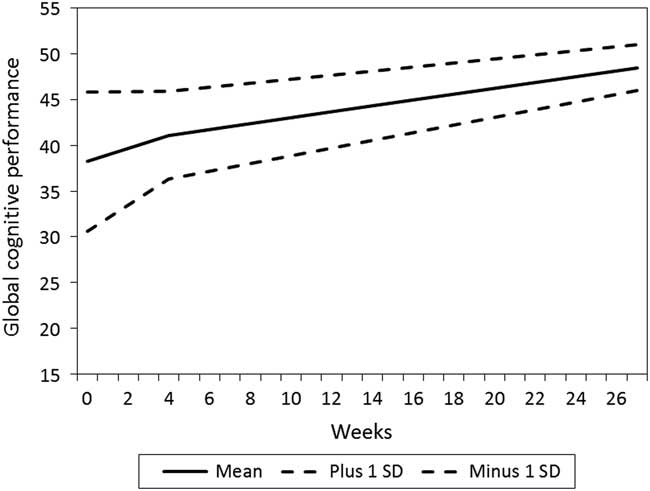
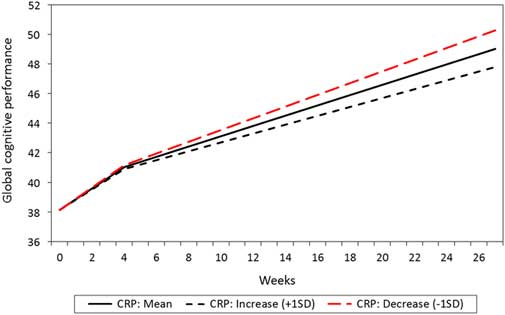
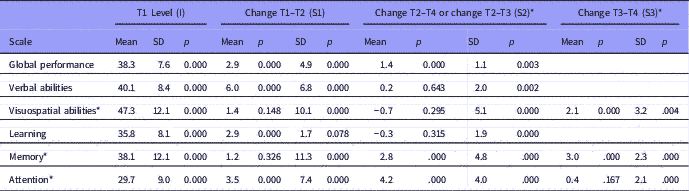
Figure 1 Estimated mean level and change in global cognitive score over time, with standard deviations to display individual variation (N=181).
Clinical sample
The sample consists of 208 consecutive, acute phase patients recruited at admittance for psychosis, who underwent CRP measurements and cognitive assessment. The project was designed and approved by the Ethics committee with two phases: The first was the quality assurance phase from admission to discharge or 6 weeks at the latest, and was approved by the Ethics Committee without the requirement of informed consent as this phase included only elements of best clinical practice. At admission all psychotic patients admitted to the hospital for symptoms of acute psychosis were consecutively included, if they were to use the hospital’s standard antipsychotic medication regimen, and could cooperate with clinical assessments of condition. This phase should assure that psychosis patients were offered best-quality guideline-concordant treatment for psychosis. The second phase (research phase) was based on informed consent provided at discharge or after 6 weeks at the latest. This included invitation to visits and tests at 3, 6, 12, and 24 months after admission. In this part of the project there were procedures beyond usual clinical standard, such as collections of data for use in psychiatric basic research within genetics and brain functioning.
The recruitment period was from March 2004 until February 2009 at Haukeland University Hospital, Bergen, Norway, with a catchment population of about 400 000. The study was approved by the Regional Committee for Medical Research Ethics, and by the Norwegian Social Science Data Services. The study was publicly funded and did not receive any financial or other support from the pharmaceutical industry.
All adult patients were eligible for participation if they were acutely admitted to the emergency ward for symptoms of active psychosis, as determined by a score of ≥4 on one or more on the items Delusions, Hallucinatory behaviour, Grandiosity, Suspiciousness/persecution, or Unusual thought content in the Positive and Negative Syndrome Scale (PANSS) (Reference Kay, Fiszbein and Opler39) and were candidates for oral antipsychotic drug therapy. All eligible patients met the International Statistical Classification of Diseases and Related Health Problems (ICD)-10 diagnostic criteria (http://apps.who.int/classifications/icd10/browse/2010/en) for schizophrenia, schizoaffective disorder, acute and transient psychotic disorder, delusional disorder, drug-induced psychosis, major depressive disorder with psychotic features, bipolar disorder except manic psychosis. Manic psychosis was excluded based on a priori expectation that these patients would not be able to cooperate with the assessments in the acute phase.
Patients were excluded from the study if they were unable to use oral antipsychotics, were suffering from manic psychosis or, for other behavioural or mental reasons related to the state of illness, were unable to cooperate with assessments, did not understand spoken Norwegian, were candidates for electroconvulsive therapy, or were medicated with clozapine on admittance. Patients with drug-induced psychoses were included only when the condition did not resolve within a few days and when antipsychotic drug therapy was indicated.
The patients were assessed at baseline (T1: N=208 with data on CRP level and/or cognition), at the first follow-up (on average after 4 weeks; T2: N=103), at the second follow-up after 3 months (T3: N=43), and at the third follow-up after 6 months (T4: N=35). This period corresponds to the treatment of the acute phase during which the largest reduction of psychotic symptoms is seen, and treatment response is expected in the majority of patients (Reference Robinson, Woerner, Alvir, Geisler, Koreen, Sheitman, Chakos, Mayerhoff, Bilder, Goldman and Lieberman40).
Clinical and biochemical assessments
Eligible patients underwent the PANSS structured clinical interview at baseline, before inclusion. All tests were performed by personnel independent of the clinical departments, who were trained and with inter-rater reliability training. Intra-class correlation coefficients were calculated based on inter-rater assessments and showed high inter-rater reliability (0.92). Furthermore, the Calgary Depression Scale for Schizophrenia (Reference Addington, Addington and Schissel41), and the Clinical Drug and Alcohol Use Scales (Reference Drake, Rosenberg and Mueser42) were used, and the patients were rated according to the Clinical Global Impression-Severity of Illness Scale (Reference Guy43), and the Global Assessment of Functioning-Split Version, Functions Scale (Reference Pedersen and Karterud44). Illicit drug use data was based only on information from the participant.
For analysis of CRP, a blood sample was collected from the patients in the fasting state between 08:00 and 10:00 h in the morning at baseline (T1) and after 4 weeks follow-up (T2). The laboratory changed the routine CRP analysis method in January 2005, and hence only data obtained after this revision is used in the present work. The CRP level was measured by the Tina-quant C-reactive Protein (Latex) method from Roche Modular P® (Mannheim, Germany), which measures CRP levels >1 mg/l.
Cognitive assessment
Cognitive assessments were conducted at baseline (T1) and follow-up (T2, T3, and T4). A brief neuropsychological screening instrument with alternative forms; the Repeatable Battery for the Assessment of Neuropsychological Status (RBANS), was used to minimise potential practice effects (Reference Beglinger, Gaydos, Tangphao-Daniels, Duff, Kareken, Crawford, Fastenau and Siemers45–Reference Wilk, Gold, Bartko, Dickerson, Fenton, Knable, Randolph and Buchanan47), since longitudinal studies on cognitive functioning usually do not sufficiently address the issue of practice effects (Reference Goldberg, Keefe, Goldman, Robinson and Harvey48,Reference Goldberg, Goldman, Burdick, Malhotra, Lencz, Patel, Woerner, Schooler, Kane and Robinson49). The RBANS has previously shown to have good reliability and validity in psychosis (Reference Wilk, Gold, Bartko, Dickerson, Fenton, Knable, Randolph and Buchanan47). To increase clinical validity, a more comprehensive cognitive battery, but with the same cognitive subdomains, was administered at T3 (Table 1). In a preliminary analysis, a satisfactory relationship between the RBANS/short battery and equivalent domains from the long battery/more comprehensive neuropsychological battery 3 months later emerged, even despite of symptom-level differences at the two time points (Reference Loberg, Langeland and Jorgensen50). The five cognitive domains were the same as for the RBANS: Language; Visuospatial/constructional; Immediate memory; Delayed memory; and Attention. We have used the already set domains from the RBANS, which does not include executive functioning. We do argue, however, that both Trails B and Stroop do depend as much on attentional resources, as executive functioning making it correct to group them together with the Attention domain. If we had an executive domain we would have grouped in this domain also. The policy of grouping test with several domains when they do capture several cognitive processes is what we consider the most transparent way of taking the complexity into account. The grouping of WAIS III digit span with both Attention and Learning was done for the same reason. In addition, in the before mentioned preliminary analysis, this method of grouping the tests was the one that obtained a satisfactory relationship with the RBANS (Reference Loberg, Langeland and Jorgensen50). Raw scores for the neuropsychological variables were converted to t-scores using the best available norms from corresponding manuals (when available) or published papers. The final summary score based on the mean t-scores across the five cognitive domains defined the overall cognitive function t-score. Neuropsychological assessment by cognitive domain and time are shown in Table 1.
Table 1 Neuropsychological assessment by cognitive subdomain and time
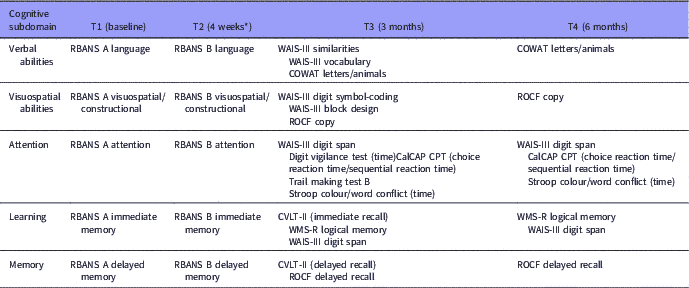
CalCAP CPT, California Computerized Assessment Package-Continuous Performance Test; COWAT, Controlled Oral Word Association Test; CVLT, California Verbal Learning Test; RBANS, Repeatable Battery for the Assessment of Neuropsychological Status; ROCF, Rey Osterrieth Complex Figure Test; WAIS-III, Wechsler Adult Intelligence Scale; WMS-R, Wechsler Memory Scale-Revised.
* Mean follow-up time at T2 was 4.1 weeks after baseline.
Statistical analyses
Descriptive statistics (mean, standard deviation, frequency) and independent sample-t-test were analysed with SPSS version 24.0. The program Mplus 8 (Reference Muthén and Muthén51) was used to analyse the level and change in variables with latent growth curve and time contrast models (Reference Wang and Wang52). The standard linear change assumption was tested based on the goodness of fit measures, residual results, and plots of the observed and estimated change model were used to decide the change pattern. If this assumption was not met, piecewise growth was explored. If the model indicated one piece based on two measurements only, this part is a difference score model (Reference Newsom53). Some residuals had to be set as constant to fit such a model. The models were evaluated based on their goodness of fit measures, as follows: threshold values in comparative fit index and Tucker–Lewis index beyond 0.95; root mean error of approximation (RMSEA)<0.05 as close fit, RMSEA <0.08 as fair fit, and RMSEA <0.10 as a mediocre fit (Reference Wang and Wang52). Model fit measures were presented for unconditional level and change models, but not for prediction models, as these models solely are predictions with statistical significant and non-significant relations and not structural models. The estimator was set to maximum likelihood with robust standard errors (MLR) to account for non-normality in data (Reference Kline54). The full information maximisation likelihood (FIML) method uses all available data under the assumption that missing data are random (‘Missing at Random’) (Reference Muthén and Muthén51,Reference Kline54). Thus, the level and change results are estimated and not observed values based on all observations. After analysing the variables separately, level and change in CRP were related to levels and changes in neurocognitive scales. A sensitivity analysis was performed for CRP levels <10 mg/l.
Finally, we controlled for confounders, known to have impact on both CRP level and cognition, as metabolic syndrome, smoking, being antipsychotic medication-naive, illicit drug use, and educational level (Reference Johnsen, Fathian, Kroken, Steen, Jorgensen, Gjestad and Loberg11).
Results
The study included 208 patients with data in one or more of the outcome variables. At baseline, there were CRP measurement for 158 patients and cognitive assessments for 169 patients and a total of 123 patients had both measurements. In total, 181 patients had observations on cognitive performance and 169 on CRP at any time point. The demographic and clinical characteristics are shown in Table 2. The patients that were tested only at baseline were not statistically different from those with follow-up data for the clinical or demographic characteristics at baseline, with the exception of a higher PANSS negative subscale score in patients with baseline test only compared to those with two or more visits [independent samples t-test: p=0.034; mean difference 2.2 points; 95% confidence interval (CI): 0.2–4.2], and fewer years of education (independent samples t-test: p=0.027; mean difference, 0.8 years; 95% CI: 0.10–0.6).
Table 2 Baseline demographics and clinical characteristics (N=208)
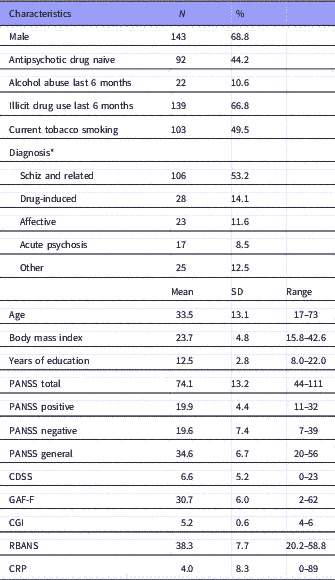
N, number of patients; Antipsychotic drug naive, no life-time exposure to antipsychotic drugs before index admission; Abuse, abuse or dependence according to the Clinical Drug and Alcohol Use Scales (CDUS/CAUS), patients with no illicit drug use could be included in the category alcohol use last 6 months; Schz and related, schizophrenia and related disorders: Schizophrenia, schizoaffective disorder, acute polymorphic psychotic disorder with symptoms of schizophrenia, acute schizophrenia-like psychotic disorder, delusional disorder; Acute psychosis, acute psychosis other than those categorised under Schz and related; Affective, bipolar and unipolar depression; Other, miscellaneous psychotic disorders.
CDSS, the Calgary Depression Scale for Schizophrenia; CGI, the Clinical Global Impression, severity of illness scale; CRP, C-reactive protein (mg/l); GAF-F, the Global Assessment of Functioning, split version, Functions scale; PANSS, the Positive and Negative Syndrome Scale; RBANS, the Repeatable Battery for the Assessment of Neuropsychological Status.
All diagnoses are according to ICD-10;
* Patients with missing diagnoses are not included in the list. According to the naturalistic design, patients were included on the basis of the presence of active psychosis as determined by the PANSS, and not based on diagnosis.
Levels and changes in cognitive performance
The mean and individual levels and changes in cognitive subdomains are presented in Table 3. The mean level of global cognitive performance increased over time in a non-linear manner, being most pronounced in the T1–T2 interval (Fig. 1). The figure also shows more individual change variation in the T1–T2 interval than in the later intervals. Statistically significant improvements over time were also found for verbal abilities, learning and attention in the T1–T2 interval, and for memory and attention in the T2–T4 interval, respectively. Individual differences in change (standard deviation) were also present. Some patients improved their performance, whereas others showed decline. The goodness of fit measures showed close fit between model and data for global cognitive performance, verbal abilities and learning scales, while the other three scales were based on saturated models and thereby giving no fit measures (please see Supplementary Table 1).
Table 3 Levels and changes in global cognition and cognitive subdomains over time
The time unit for change is months. T1, baseline; T2, 4 weeks; T3, 3 months; T4, 6 months.
S1: change from T1–T2; S2: change from T2–T4 if supported by data; and S3: change T3–T4. Change in the T3–T4 interval was estimated if a linear change from T2–T4 was not supported. In these models, S2 consists of the change between T2–T3.
Group mean and individual differences (standard deviation, SD) for both level and change are reported.
Statistical p-values are presented for individual differences at baseline level (T1 Level), and mean change and individual differences in change (standard deviation).
* Three change factors had to be estimated: S1: T1–T2; S2: T2–T3; and S3: T3–T4.
The relationship between levels and changes in CRP and cognition
The mean change in CRP from T1 to T2 was not statistically significant (0.52 mg/l/ month, p=0.652, baseline mean level: 3.99 mg/l, SD =8.32). However, the individual variation in change was statistically significant (SD =12.13, p=0.031). In the total sample, a reduction in the CRP level from T1 to T2 was associated with an increase in global cognitive performance in the T2–T4 interval, whereas no such association was found between CRP and cognition during the T1–T2 interval (Table 4). Patients with most reduction in CRP also were the patients with most improvement in global cognitive performance, illustrated with a steeper increase in their performance, compared to those with a smaller reduction or an increase in CRP (Fig. 2).
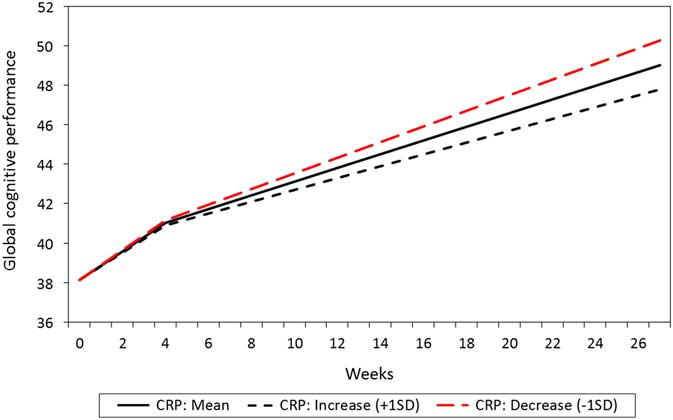
Figure 2 Relationship between estimated level and change in C-reactive protein (CRP) and global cognitive performance total score (N=208).
Table 4 Baseline level and changes over time in cognitive performance predicted by baseline and change in C-reactive protein (CRP) level
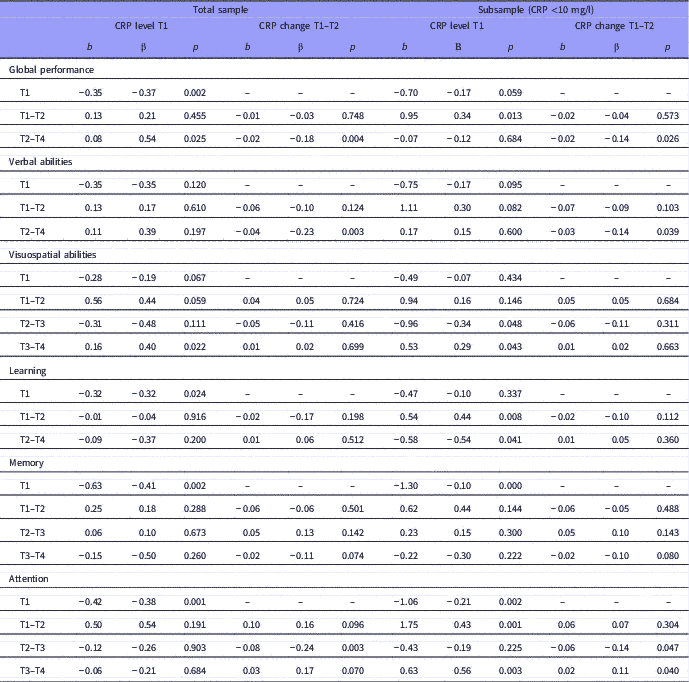
The table presents results for all patients (i.e. total sample) and patients with CRP <10 mg/l (i.e. subsample). The time unit for change is months.
Relations are given by unstandardised and standardised regression weights (b and β).
For the cognitive subdomains, we observed a statistically significant association between reduction of the CRP level and increase in verbal abilities for the T2–T4 interval and in attention for the T2–T3 interval (Table 4).
For patients with CRP <10 mg/l (N=191), the results remained unchanged regarding the association between changes in global cognitive performance (T2–T4), verbal abilities (T2–T4), and attention (T2–T3), and CRP level changes. However, we also found additional statistically significant associations in this sub-sample compared to the total sample (Table 4).
A separate model for the sub-sample of patients diagnosed within the schizophrenia spectrum showed that change in CRP level did not predict T2–T4 change in the global cognitive performance as was found in the total sample (b=−0.01, p=0.208). Two new statistically significant associations were found between change in CRP level and attention in the T1–T2 interval (b=0.12, p=0.045) and in the T3–T4 interval (b=0.03, p=0.026).
Finally, the models were adjusted for the covariates metabolic syndrome, smoking, being medication naïve, illicit drug use, and the educational level. The association between baseline CRP level and global cognitive performance for the whole follow-up remained essentially unchanged. Baseline CRP level and visuospatial performance in the T3–T4 interval was no longer statistically significant (p=0.120), whereas a statistically significant association was found between CRP baseline level and this subdomain in the T1–T2 interval (b=0.66, p=0.018). CRP level change and verbal abilities in the T2–T4 interval was no longer statistically significant associated (p=0.067), while such an association was found in the T1–T2 interval (b=−0.09, p=0.039). Additional statistically significant associations were found between CRP baseline level and attention in the T3–T4 interval (b=0.31, p<0.001), and CRP level change and attention in the T1–T2 interval (b=0.12, p=0.045), and in the T3–T4 (b=0.03, p=0.026), respectively.
A separate model for the sub-sample of patients diagnosed within the schizophrenia spectrum showed some differences. Change in CRP did not predict T2–T4 change in the global score as it did in the total sample (b=−0.01, p=0.208), whereas change in CRP was found to be associated with change in attention for the T1–T2 (b=0.12, p=0.045) and T3–T4 (b=0.03, p=0.026) intervals, respectively.
In the schizophrenia group, the inclusion of covariates showed that higher CRP baseline level was related to lower baseline level in global cognitive performance (b=−0.26, p=0.036). CRP baseline level was found to be associated with verbal abilities in the T1–T2 interval (b=0.64, p=0.016). In addition, CRP level change was found to be associated with verbal abilities in (b=−0.09, p=0.024) and learning in the T1–T2 interval (b=−0.04, p=0.009). The model with the attention outcome variable did not converge, even after increasing the number of iterations.
Discussion
The main finding of the present study was that the global cognitive performance continued to improve from the initial phase (baseline to 4 weeks) of acute psychosis to the later phase (4 weeks to 6 months), and was predicted by the reduction of the CRP level as observed during the initial phase (baseline to 4 weeks) of the treatment. Similar associations were found for several of the cognitive subdomains. These findings might indicate a prolonged effect of inflammatory processes on cognition after an acute psychosis, stretching beyond the initial phase.
The stability of cognitive dysfunctions in patients with psychosis has been debated for decades. Most studies have suggested stability or decline in cognitive functioning (Reference Bilder, Lipschutz-Broch, Reiter, Geisler, Mayerhoff and Lieberman55–Reference Tyson, Laws, Roberts and Mortimer58). A study of first-episode schizophrenia spectrum disorders and controls with follow-up intervals of 1-year and 3-years showed that, although patients performed worse than controls at any given time, the cognitive performance of the patients improved in a similar way as the controls in all domains, except for verbal and visual memory, which showed greater improvement in controls (Reference Rodriguez-Sanchez, Ayesa-Arriola, Perez-Iglesias, Perianez, Martinez-Garcia, Gomez-Ruiz, Tabares-Seisdedos and Crespo-Facorro6). Another longitudinal study demonstrated improvement of general cognitive function, working memory, and verbal learning after 12 weeks, but these changes were mediated by improvements in both positive and negative symptoms (Reference Trampush, Lencz, DeRosse, John, Gallego, Petrides, Hassoun, Zhang, Addington, Kellner, Tohen, Burdick, Goldberg, Kane, Robinson and Malhotra59).
There is substantial evidence that inflammatory processes are involved in the cognitive performance in psychosis (Reference Miller and Goldsmith23,Reference Mondelli, Pariante, Navari, Aas, D’Albenzio, Di Forti, Handley, Hepgul, Marques, Taylor, Papadopoulos, Aitchison, Murray and Dazzan60,Reference Buckley, Pillai, Evans, Stirewalt and Mahadik61). Oxidative stress and inflammation have been suggested to be associated with specific aspects of cognitive functioning in first-episode psychosis patients (Reference Martinez-Cengotitabengoa, Mac-Dowell, Leza, Mico, Fernandez, Echevarria, Sanjuan, Elorza and Gonzalez-Pinto62). Of particular interest, several studies have shown a link between CRP and cognitive function (Reference Bulzacka, Boyer, Schurhoff, Godin, Berna, Brunel, Andrianarisoa, Aouizerate, Capdevielle, Chereau-Boudet, Chesnoy-Servanin, Danion, Dubertret, Dubreucq, Faget, Gabayet, Le Gloahec, Llorca, Mallet, Misdrahi, Rey, Richieri, Passerieux, Roux, Yazbek, Leboyer and Fond25,Reference Horsdal, Kohler-Forsberg, Benros and Gasse29,Reference Dickerson, Stallings, Origoni, Vaughan, Khushalani and Yolken37,Reference Micoulaud-Franchi, Faugere, Boyer, Fond, Richieri, Faget, Cermolacce, Philip, Vion-Dury and Lancon63,Reference Misiak, Stanczykiewicz, Kotowicz, Rybakowski, Samochowiec and Frydecka64). CRP levels have been associated with cognitive impairment in patients with schizophrenia (Reference Bulzacka, Boyer, Schurhoff, Godin, Berna, Brunel, Andrianarisoa, Aouizerate, Capdevielle, Chereau-Boudet, Chesnoy-Servanin, Danion, Dubertret, Dubreucq, Faget, Gabayet, Le Gloahec, Llorca, Mallet, Misdrahi, Rey, Richieri, Passerieux, Roux, Yazbek, Leboyer and Fond25,Reference Dickerson, Stallings, Origoni, Boronow and Yolken36). In one study, abnormal CRP levels were correlated with poorer cognitive functioning of general intellectual ability, abstract reasoning, memory, working memory, semantic memory, learning abilities, attention, mental flexibility, and processing speed (Reference Bulzacka, Boyer, Schurhoff, Godin, Berna, Brunel, Andrianarisoa, Aouizerate, Capdevielle, Chereau-Boudet, Chesnoy-Servanin, Danion, Dubertret, Dubreucq, Faget, Gabayet, Le Gloahec, Llorca, Mallet, Misdrahi, Rey, Richieri, Passerieux, Roux, Yazbek, Leboyer and Fond25). In another, longitudinal study, CRP levels did not predict changes in cognitive performance (Reference Dickerson, Schroeder, Stallings, Origoni, Katsafanas, Schwienfurth, Savage, Khushalani and Yolken65), but this study included chronic phase patients with an average illness duration of 22.5 years, who are likely to have a lesser potential for inflammation related cognitive improvements.
Taken together, the present study supports a link between inflammation and cognitive performance in schizophrenia. Our study shows that early low-grade inflammation can predict cognitive improvement in later phases. Some studies have suggested that low-grade inflammation as indicated by elevated CRP levels is related to cerebral microstructural disintegration, involving frontal lobe executive functions (Reference Wersching, Duning, Lohmann, Mohammadi, Stehling, Fobker, Conty, Minnerup, Ringelstein, Berger, Deppe and Knecht66). It has been suggested that increased levels of CRP can increase blood–brain barrier permeability, which might lead to inflammatory-related cognitive impairment (Reference Hsuchou, Kastin, Mishra and Pan67). As neuroinflammation has been demonstrated to be associated with white matter pathology (Reference Najjar and Pearlman27), any psychosis-related inflammation could, in principle, have a negative impact on brain tissue components of relevance to cognition, and the reversibility of such effects would likely include more slow regenerative processes.
There are some limitations to this study. First, attrition was substantial, but generally not related to any of the baseline characteristics except a higher PANSS negative subscale score and lower educational level in those patients tested only at baseline. As negative symptoms have been found to correlate with cognitive dysfunctions (Reference Anda, Bronnick, Johnsen, Kroken, Jorgensen and Loberg12), those with the most pronounced cognitive difficulties may have dropped out during follow-up.
It is, however, difficult to predict how any selective dropout might have biased our results. Furthermore we used statistical methods that reduce the effect of missing data (Reference Bollen68), and give improved statistical power and generalisability to the results, as all information in the data set is taken into consideration, rather than using the listwise deletion method that gives a net sample based on intact data in all variables under the assumption of missingness to be completely at random. However, the FIML method does not rule out the possibility of missingness to be non-random. The study included several models for different outcome variables in the total sample and in the schizophrenia sub-sample. In addition, several covariate variables were included in sensitivity analyses. This strategy has implication for statistical power, which has consequences particularly for small effects. Studies with larger samples are needed to increase the validity of our findings.
Being exploratory, we did not adjust for multiple testing. The high number of tests could theoretically result in statistical type I error. However, we find it very unlikely that such a high number of statistically significant findings could be the result of chance.
According to the naturalistic design, patients were included on the basis of the presence of active psychosis as determined by the PANSS, and not based on diagnosis. Hence all patients were psychotic and in need of antipsychotic medication. The lack of specific diagnosis for nine patients probably reflects that some patients may have dropped out or been discharged before the treating clinician were able to make a proper diagnostic evaluation. This is clearly a limitation, but the direction of any influence on our results is difficult to predict.
Moreover, CRP was the only inflammatory marker that was measured, at baseline and the first follow-up (T2). Further measurements during the follow-up period would have allowed for analyses of associations between potential later changes in the CRP level and cognitive performance. We are also aware of the limitation that other factors potentially confounding the relationship between CRP and cognition might exist that we have not accounted for, although many relevant factors were included in the analyses. Finally, the cognitive test battery at the T3 follow-up was more comprehensive but assessed the same cognitive domains as the test battery at T1, T2, and T4. We have no reason to suspect that this difference could have influenced the results.
We have shown that initial changes in the serum level of CRP in the acute phase of psychosis may predict cognitive function in later phases of the disease. These findings create an opportunity for future RCT research efforts to develop more individualised treatments with add-on anti-inflammatory agents (Reference Bumb, Enning and Leweke69). Some efforts have already been made in this field of research, but the results are mixed. A double-blind, randomised, placebo-controlled, add-on study with Celecoxib, a cyclo-oxygenase-2 inhibitor showed significant effect on the total PANSS score and the cognition factor of PANSS scale in patients with schizophrenia (Reference Muller, Riedel, Schwarz and Engel70). N-acetyl cysteine significantly improved working memory compared to placebo in patients with psychosis, however, these preliminary data require replication (Reference Rapado-Castro, Dodd, Bush, Malhi, Skvarc, On, Berk and Dean71). A double-blind, randomised study with minocycline treatment was associated with improvement in negative symptoms and executive functioning in early phase schizophrenia (Reference Levkovitz, Mendlovich, Riwkes, Braw, Levkovitch-Verbin, Gal, Fennig, Treves and Kron72), whereas another study with add-on minocycline treatment showed improvement in negative symptoms, but not in cognition (Reference Chaudhry, Hallak, Husain, Minhas, Stirling, Richardson, Dursun, Dunn and Deakin73). Aspirin given as add-on treatment in another study reduced the total and positive PANSS score, but did not affect cognitive function (Reference Laan, Grobbee, Selten, Heijnen, Kahn and Burger74). Finally, omega-3 fatty acid (ethyl eicosapentaenoic acid) add-on treatment did not affect cognition, positive or negative symptoms (Reference Fenton, Dickerson, Boronow, Hibbeln and Knable75). Possible explanations for the equivocal findings might at least in part be related to small, unselected samples both with and without signs of an increased inflammatory status, with predominantly chronic phase psychosis, and with anti-inflammatory agents under investigation typically of low anti-inflammatory potency (Reference Sommer, van Westrhenen, Begemann, de Witte, Leucht and Kahn76). Therefore, a more targeted approach might be to investigate more potent anti-inflammatory agents, for example, corticosteroids, in selected samples with signs of low-grade inflammation as determined by, for example, elevated CRP levels.
Acknowledgements
The authors thank research nurses Ingvild Helle and Marianne Langeland at the Research Department, Division of Psychiatry, Haukeland University Hospital for their contributions. We also wish to thank the Division of Psychiatry, Haukeland University Hospital for financial support, and the Clinical Departments for enthusiasm and cooperation. Authors’ Contributions: All authors made substantive intellectual contributions to the study. F.F. designed the study, contributed to the statistical analyses and interpretations of the data, and wrote the first draft of the manuscript. E.-M.L. collected data and contributed to interpretations of the data and drafting the manuscript. R.G. contributed to the statistical analyses and interpretations of the data and contributed to the drafting of the manuscript. R.A.K. collected data and contributed to interpretations of the data and drafting of the manuscript. V.M.S. was the co-designer of the project and contributed to the interpretations of the data and drafting of the manuscript. H.A.J. designed the project, assisted in data collection and contributed to interpretations of the data and drafting of the manuscript. E.J. was the co-designer of the project, collected data, contributed to the statistical analyses, and co-drafted the manuscript. All authors have read and given final approval of the latest version of the manuscript.
Financial support.
Funding for the project was provided grants to Erik Johnsen from the Research Council of Norway (#213727) and Helse Vest RHF (#911679) and partly supported by the Stiftelsen Kristian Gerhard Jebsen in Norway and the Research Council of Norway through its Centres of Excellence funding scheme (#223273). The funding sources had no role in the study design, collection, analyses, and interpretation of the data, writing of the report, or the decision to submit the paper for publication.
Ethical standards.
The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national and institutional committees on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008.
Supplementary materials
To view supplementary material for this article, please visit https://doi.org/10.1017/neu.2018.25