Brain lateralization refers to the asymmetric location of functional elements within the bilateral brain of animals (Corballis, Reference Corballis2017) and humans (Wey, Phillips, McKay et al., Reference Wey, Phillips, McKay, Laird, Kochunov, Davis and Fox2014). Thus far, five lateralized functions have been recognized in humans. These are handedness (Kencht, Drager, Deppe et al., Reference Knect, Drager, Deppe, Bobe, Lohmann, Floel and Henningsen2000), language ability (Knecht, Deppe, Drager et al., Reference Knect, Deppe, Drager, Bobe, Lohmann, Ringelstein and Henningsen2000; Stroobannt, Buijs, & Vingerhoets, Reference Stroobant, Bujis and Vingerhoets2009), spatial skills (Badzakova-Trajkov, Haberling, Roberts, & Corballis, Reference Badzakova-Trajkov, Habeling, Roberts and Corballis2010; Kang, Herron, Ettlinger, & Woods, Reference Kang, Herron, Ettlinger and Woods2015), facial recognition (Morita, Saito, Ban et al., Reference Morita, Saito, Ban, Shimada, Okamoto, Kosaka and Naito2017), and emotion recognition (Innes, Burt, Burch, & Hausmann, Reference Innes, Burt, Birch and Hausmann2015).
Often, several of these specialized processing elements have been found to be parceled to the right hemisphere, while others can independently appear in the left hemisphere (Corballis & Haberling, Reference Corballis and Haberling2017). Individuals vary greatly in the brain laterality of these functions, which appear to be genetically set independently at a very young age (Yamazaki, Easwar, Polonenko et al., Reference Yamazaki, Easwar, Poloneko, Jiwani, Wong, Papsin and Gordon2018). For example, although people more commonly have their language skills located in the left hemisphere and spatial abilities in the right, this often can be reversed. Occasionally, some people are found with both language and spatial functions in the same hemisphere (Haberling, Badzakova-Trajkov, & Corballis, Reference Haberling, Badzakova-Trajkov and Corballis2011). Handedness is now known to be sorted independently of the language function (Mazoyerr, Zago, Jobard et al., Reference Mazoyer, Zago, Jobard, Crivello, Joliot, Perchey and Tzourio-Mazoyer2014).
Based upon the surprisingly different responses obtained from each of the isolated hemispheres of split-brain subjects (Bogen, Reference Bogen2000; Gazzaniga, Reference Gazzaniga2000; Gazzaniga, Bogen, & Sperry, Reference Gazzaniga, Bogen and Sperry1962; Geschwind, Iacoboni, Mega et al., Reference Geschwind, Iacoboni, Mega, Zaidel, Cloughesy and Zaidel1995), it was early proposed by investigators that the right and left cerebral hemispheres are characterized by inbuilt, qualitatively different and mutually antagonistic modes of data processing, separated from interference by the major longitudinal fissure of the brain (Levy, Reference Levy1969; Sperry, Reference Sperry1982). In this model, the left hemisphere specialized in top-down, deductive, cognitive dissection of local detail (Fink, Halligan, Marshall et al., Reference Fink, Halligan, Marshall, Frith, Frackowiak and Dolan1996), while the right hemisphere produced a bottom-up, inductive, perceptual synthesis of global structure (Floegel & Kell, Reference Floegel and Kell2017; Gazzaniga, Reference Gazzaniga2000; Sperry, Reference Sperry1982). This context has been reinforced by known laterality differences between them. That is, there are striking differences in input to each hemisphere, differences in internal neuronal-columnar architecture, and differences in hemispheric output (Hutsler & Galuske, Reference Hutsler and Galuske2003; Jager & Postma, Reference Jager and Postma2003; Kosslyn, Chabris, Marsolek, & Koenig, Reference Kosslyn, Chabris, Marsolek and Koenig1992; Kosslyn, Koenig, Barrett et al., Reference Kosslyn, Koenig, Barrett, Cave, Tang and Gabrieli1989; Schuz, & Preissl, Reference Schuz and Preissl1996; Stephan, Fink, & Marshall, Reference Stephan, Fink and Marshall2006).
Supporting the above global view is a large body of detailed evidence that the left cerebral hemisphere in most right-handed individuals manifests facilities for language (Broca, Reference Broca1863), has an orientation for local detail (Robertson & Lamb, Reference Robertson and Lamb1991), has object abstraction-identification abilities (Kosslyn, Reference Kosslyn1987), and appears to possess a hypothesis-generating, event “interpreter” (Gazzaniga, Reference Gazzaniga1989, Reference Gazzaniga2000; Wolford, Miller, & Gazzaniga, Reference Wolford, Miller and Gazzaniga2000). In contrast, the right hemisphere has been demonstrated to excel in global analysis (Proverbio, Zani, Gazzaniga, & Mangun, Reference Proverbio, Zani, Gazzaniga and Mangun1994; Robertson & Lamb, Reference Robertson and Lamb1991), object localization (Kosslyn et al., Reference Kosslyn, Koenig, Barrett, Cave, Tang and Gabrieli1989), facial recognition (Milner, Reference Milner1968), and spatial construction (Sperry, Reference Sperry1968).
Recently, a sixth asymmetric functional element bearing on personality has been discovered (Morton & Rafto, Reference Morton and Rafto2010, Reference Morton and Rafto2017). It is the larger side of the split bilateral anterior cingulate cortex (ACC). This appears to be the final output element of the executive system, of which in the bilateral vertebrate brain, by logic there can only be one (“The buck stops here”). Which side is the larger varies among the general population in a seemingly idiosyncratic manner, yet with a genetic basis because true-breeding lineages exist.
Earlier, executive laterality had been unwittingly approached by the popular topic of right brain, left brain personality differences, called hemisphericity. This had been stimulated by laterality findings of split-brain subjects (Bogen, Reference Bogen2000; see also, Gazzaniga, Reference Gazzaniga2000; Gazzaniga et al., Reference Gazzaniga, Bogen and Sperry1962; Sperry, Reference Sperry1982). However, due to poor definitions and weak measurement instruments, after hundreds of publications, the intuitively correct preliminary idea of hemisphericity was statistically debunked (Beaumont, Young, & McManus, Reference Beaumont, Young and McManus1984). This placed a pall on brain laterality research for decades.
Recently, right brain or left brain personality differences have been more accurately determined. This was done by replacing the original flawed definition of brain laterality. That definition consisted of a 10 step gradient between right and left extremes. As a result, subjects mainly gave intermediate values for their answers. This resulted in poor statistics and the ultimate rejection of the idea of hemisphericity. Here, the gradient idea has been replaced with a binary measure where a person is inherently born either right or left brain oriented. This binary executive laterality construct has been named hemisity (Morton & Rafto, Reference Morton and Rafto2010, Reference Morton and Rafto2017).
The binary definition has facilitated the development of a statistically robust set of nine binary biophysical assays and behavioral questionnaires. The four biophysical tests were the Dichotic Deafness Test (Morton, Reference Morton2001, Reference Morton2002), Phased Mirror Tracing Test (Morton, Reference Morton2003a), the Two Hand Line Bisection Test (Morton, Reference Morton2003b), and the MRI Hemisity Assessment Method (Morton & Rafto, Reference Morton and Rafto2010), none of which depend upon personality. The four behavioral questionnaires were the Polarity Questionnaire (Morton, Reference Morton2002), the Asymmetry Questionnaire (Morton, Reference Morton2003c), the Binary Questionnaire (Morton, Reference Morton2012a), and the Hemisity Questionnaire (Morton, Reference Morton2012a), plus the earlier less accurate Zenhausern’s Preference Questionnaire (Morton, Reference Morton2002; Zenhausern, Reference Zenhausern1978).
Crucially, these hemisity methods have been validated by the MRI-based discovery that the larger side of the bilateral ACC is on the same side as one’s hemisity, making MRI the primary standard for hemisity determination (r = 0.96) (Morton & Rafto, Reference Morton and Rafto2010). The asymmetric sides of the ACC are separated by the midline fissure of the brain. The larger side in Areas 24 and 24’ appears to contain the element producing the final output of the executive system. The relative sizes of the two sides of the ACC vary in a seemingly idiosyncratic manner (Huster, Westerhausen, Kreude, Schweiger, & Whittling, Reference Huster, Westerhausen, Kreuder, Schweiger and Whittling2007). There are at least 30 measurable differences in individual characteristics and behaviors between those persons with a larger executive final output element on the right compared to those with it on the left (Morton, Reference Morton2013).
1. Materials and Methods
1.1 Nomenclature of hemisity
R = right brain-oriented hemisity, L = left brain-oriented hemisity, M = male, F = female, P = person, RM-LF = patripolar couple, RF-LM = matripolar couple. RM-RF and LM-LF are hybrid couples. Handedness is irrelevant.
1.2 Subjects
The hemisity of a total of 2929 subjects was determined by methods described here.
These groups were as follows: 1089 were volunteers from the University of Hawaii community, ranging in age from 7 to 75 yrs, 44.0 yrs mean age, +/- 14.5 yrs S.D. In a subset (n = 399), 47.3% (189) were female, 11% (45) claimed left-handedness, and 77% (309) were Caucasian, the rest being predominantly Asian. Based upon an earlier MRI study (Morton & Rafto, Reference Morton and Rafto2010) with 399 subjects, there were 45.1% (180) right brain-oriented persons (RPs) and 54.9% (219) left brain-oriented persons (LPs). The above RPs were composed of 48.3% (87) right brain-oriented females (RFs) and 51.7% (93) right brain-oriented males (RMs). The LPs consisted of 47.9% (105) left brain-oriented females (LFs) and 52.1% (114) left brain-oriented males (LMs)].
For the hemisity pairing study, the 412 members of long-term couples (>5 yrs), the majority were recruited from members of the University of Hawaii at Manoa community. Others were obtained from families participating in children’s soccer leagues in Honolulu and from those waiting for flight connections at the Honolulu International Airport. The 191 children of 96 couples also came from these groups.
The hemisity of 1428 individuals was determined in later studies (Morton, Svard, & Jensen, Reference Morton, Svard and Jensen2014). These consisted of 1049 junior and senior high school students from Kapolei High School on Oahu in Hawaii and Provo School District in Provo, Utah, in addition to 379 professional musicians.
1.3 Hemisity measurement methods
The following nine independent hemisity methods of Table 1 are described in greater detail elsewhere. They are as follows:
Table 1. Overall correlations and reliability of preference questionnaire scores and biophysical measurements regarding predetermined subject hemisity subtypes

* = % yield refers to the percentage of questionnaire statements that were significantly associated with subjects neuroanatomical hemisity.
1.3.1. The four biophysical assays for hemisity
1.3.1.1 The Dichotic Deafness Test (Morton, Reference Morton2001, Reference Morton2002; Morton & Rafto, Reference Morton and Rafto2006)
The “Tonal and Speech Materials for Auditory Perceptual Assessment,” Disc 1.0 (1992), purchased from the Long Beach Research Foundation, was used to measure minor ear deafness of 115 pseudo-randomly selected subjects during simultaneous, and 90 ms-separated, presentations of dichotic consonant–vowel syllables. Attention bias (Iaccino & Houran, Reference Iaccino and Horan1989) was reduced by instructing subjects to write the syllables heard in each ear.
1.3.1.2 Phased Mirror Tracing (Morton, Reference Morton2003a)
Mirror tracings of pentagonal stars were produced by both hands of 131 subjects with the aid of a Lafayette Instruments, Mirror-drawing apparatus, Model 31010. Competitive mean elapsed time outcomes between hands were phase adjusted by use of the Affective Laterality Test (Morton, Reference Morton2003a).
1.3.1.3 Best Hand Task (Morton Reference Morton2003b, Reference Morton2003d)
Forms containing 20 horizontal lines for each hand to bisect were completed by 142 subjects, measured, phased, and scored according to Morton (Reference Morton2003b).
1.3.1.4 MRI Hemisity Determination (Morton, 2010)
The largest side of the ACC in Areas 24 and 24’ was identified as the right or left brain hemisity orientation of the subject.
1.3.2 The five questionnaire measurements of hemisity
Sensitive topics were sought by posing hundreds of contrasting items to subjects and excluding those not causing sorting. Target comparisons causing sorting were then packaged into preference questionnaires. The questionnaire statements had little or no relationship to psychological or neurological theory.
1.3.2.1 Zenhausern’s Preference Questionnaire (Morton, Reference Morton2002; Zenhausern, Reference Zenhausern1978)
A preexisting laterality instrument was used here. It was the best of the earlier generation hemisiphericity assays, albeit quite weak, as demonstrated here. Subjects ranked 21 statements on a ten-point gradient from “strongly agree to strongly disagree.”
1.3.2.2 The Polarity Questionnaire (Morton, Reference Morton2002)
This is a binary questionnaire of 11 neutral statements, which were assessed by each subject, using true or false answers. For example: “Given the opportunity, I am more of an early morning than a late night person,” or “I would rather maintain and use good old solutions than find new better ones,” or “I am comfortable and productive in the presence of disorder and disorganization.”
1.3.2.3 The Asymmetry Questionnaire (Morton, Reference Morton2003c)
Subjects selected between 15 binary statement pairs as to their preference.
1.3.2.4 Binary Preference Questionnaire (Morton, Reference Morton2012a)
It is a questionnaire consisting of 40 either-or preference choices, neither choice is wrong, just a personal preference or lack of such.
1.3.2.5 Hemisity Questionnaire (Morton, Reference Morton2012a)
This is another questionnaire of 21 either-or preference choices regarding personal orientation.
1.4 MRI corpus callosum cross-sectional area determinations
1.4.1 MRI assessment of corpus callosum thickness
MRI assessments employed a General Electric Signa 1.5 Tesla MRI instrument. A midsagittal plane setup calibration protocol was run for 3 minutes using a T1 weighted spin echo sequence (TR = 400 msec, TE = 1/Fr) to image 5 mm slices from the midline plane and two adjoining sagittal planes 6 mm on either side. Whole-head photographic images were prepared from these three planes, and additionally, a 2.3x enlargement of the most medial plane, centering on the corpus callosum (CC). These four exposures were printed on a single 35 cm x 43 cm film sheet for each subject.
Sagittal corpus callosal cross-sectional areas were determined by tracing the corpus callosal outline of the 2.3x midline enlargement upon computer printer paper (Weyehauser 1180, 20 lb stock) of predetermined weight per unit area. The 8 ½ x 11 inch pages varied in weight by +/- 0.6%. Corpus callosal cutouts weighed on a microbalance varied in weight by +/- 35%. These data were converted to absolute CC cross-sectional areas by use of predetermined magnification and paper weight constants. Subject corpus callosal areas ranged from 4.5 to 10.1 cm2.
1.4.2 MRI assessment of ACC laterality (hemisity primary standard)
MRI assessments (Morton & Rafto, Reference Morton and Rafto2010) were obtained employing a General Electric Signa 1.5 Tesla MRI instrument. A midsagittal plane setup calibration protocol was run for 3 minutes to image 5 mm thick slices from the midline plane and two adjoining sagittal planes 6 mm on either side. Whole-head photographic images were prepared from these three planes. These three exposures were printed on a single film sheet for each subject. This procedure enabled both cortical walls on either side of the midline fissure to be visualized and measured, thus allowing sub-element lateralities of the ACC to be evaluated directly from the film. At two ACC sites on each side of the brain, one in Area 24 and the other at Area 24’ (Vogt et al., Reference Vogt, Nimcheniski, Vogt and Hof1995), estimations of the relative thickness of the ventral gyri (vgACC) there were made. This abbreviation and these four ACC locations within Areas 24 and 24’ are not to be confused with the more frontal ventral region of the perigenual ACC. The vgACC locations where these relative thickness estimations were made are illustrated by the arrows in Figure 4. Two lines were extended outward perpendicularly from the inner edge of the CC, ending in one case at a more frontal point in Area 24 and in the other at a more dorsal point in Area 24’. Both points were in the plane of the cingulate sulcus and arbitrarily selected, based upon the sites in the region giving the largest vgACC thickness for each brain side involved. The average of these two lateral relative thickness estimates from the vgACC of each side was then used to determine upon which side of each subject’s brain the vgACC was thicker.
1.5 Statistical analysis
The Statistica 6.0 package was used to assess the strength of these data and their associations with the various hemisity methods, as illustrated in Table 1.
1.6 Ethics and safety
The Committee of Human Studies of the University of Hawaii Institutional Review Board approved all appropriate elements of this unfunded research which was conducted in compliance with the Code of Ethics of the World Medical Association and posed no significant risks to participants.
2. Results
The above nine methods were used earlier to determine the hemisity of 143 subjects, as shown in Table 1 (mean number of methods/subject was 7.3). As may be seen, each method was able to determine the hemisity of a subject to some degree. The MRI method was superior (r = .93) and became the primary standard against which the others were validated. The average of the seven non-MRI hemisity tests, excluding Zenhausern’s Preference Questionnaire, was r = .46; that of Zenhausern’s Preference Questionnaire (Reference Zenhausern1978) was r = .24.
Clearly, all eight of the questionnaires were superior to the earlier hemisphericity standard, Zenhauser’s Preference Questionnaire (Reference Zenhausern1978). Although the MRI method is most accurate, it was costly, time consuming, and intensely scheduled. The three other biophysical methods were also time consuming. However, it was found that combining three of the preference questionnaires for a given subject gave hemisity concordance values (r = 0.89, 91% accurate) comparable to the MRI method alone. This breakthrough allowed the rapid and accurate hemisity determination of large numbers of subjects.
Thirty significant behavioral contrasts of hemisity are shown in Table 2. These hemispheric asymmetries subtly, but measurably, influence the person’s personality and behavioral orientation, depending on the side of his or her executive output.
Table 2. Thirty binary behavioral correlates of hemisity
2.1 Ten properties of hemisity
2.1.1 Stability of hemisity
Regarding the stability of one’s hemisity over time, it has been found that brain laterality is present at very young ages and develops further in adolescence (Yamazaki et al., Reference Yamazaki, Easwar, Poloneko, Jiwani, Wong, Papsin and Gordon2018). This lateralization completes with final maturation. It is inconceivable for laterality of the ACC to reverse itself, requiring the rewiring of the entire brain. Thus, one’s hemisity can be considered permanent and unchangeable.
2.1.2 Relationship of hemisity to handedness
No relationship was found between hemisity and handedness in 113 subjects (Morton & Rafto, Reference Morton and Rafto2006), or 1049 subjects (Morton, Svard, & Jensen, Reference Morton, Svard and Jensen2014). Left brain-oriented, right-handed individuals commonly exist, as do right brain-oriented, left-handed individuals.
2.1.3 Hemisity in the general population
Among 703 students at Kapolei High School near Honolulu, Hawaii, we found a nearly even split between RPs (328) and LPs (355) (Table 3). The same relationship was found among 346 students attending high schools in the Provo School District in Provo, Utah: an even distribution of RPs (175) and LPs (171). Finally, among the summated population of these two public high school juniors and seniors (n = 1049), we found an even distribution of RPs (523) and LPs (526) (Morton, Svard, & Jensen, Reference Morton, Svard and Jensen2014). Since these high school students were unsorted, it may be concluded that in the general population, there are approximately equal numbers of RPs and LPs.
Table 3. The apparent hemisity of 1049 high school students

* indicates significance, p < .05, ** indicates significance, p < .01.
2.1.4 Hemisity and corpus callosum size
Individuals differ in the number of CC nerve fibers interconnecting
their cerebral hemispheres by about threefold. Using quantitative MRI, we found the midline CC area of 113 subjects was significantly correlated, not with handedness or sex, but with hemisity (Figures 1 and 2) (Morton, 2006). That is, right brain-oriented individuals of either sex had significantly larger CCs than left brain-oriented persons.
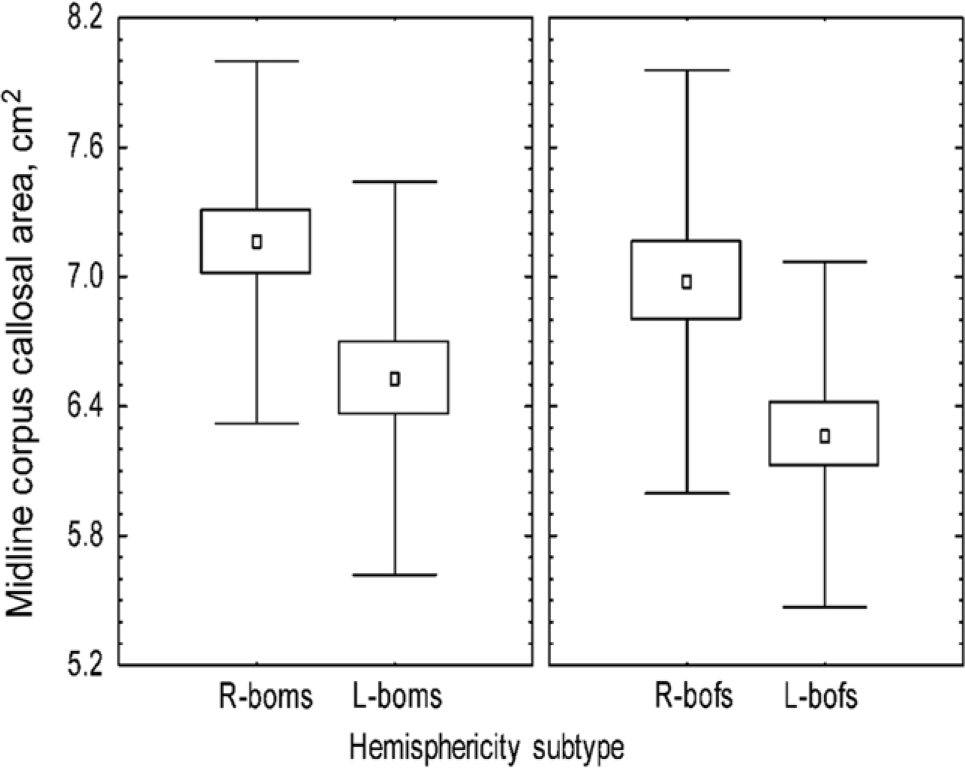
Figure 1. Effect of sex and hemisphericity upon corpus callosal area.
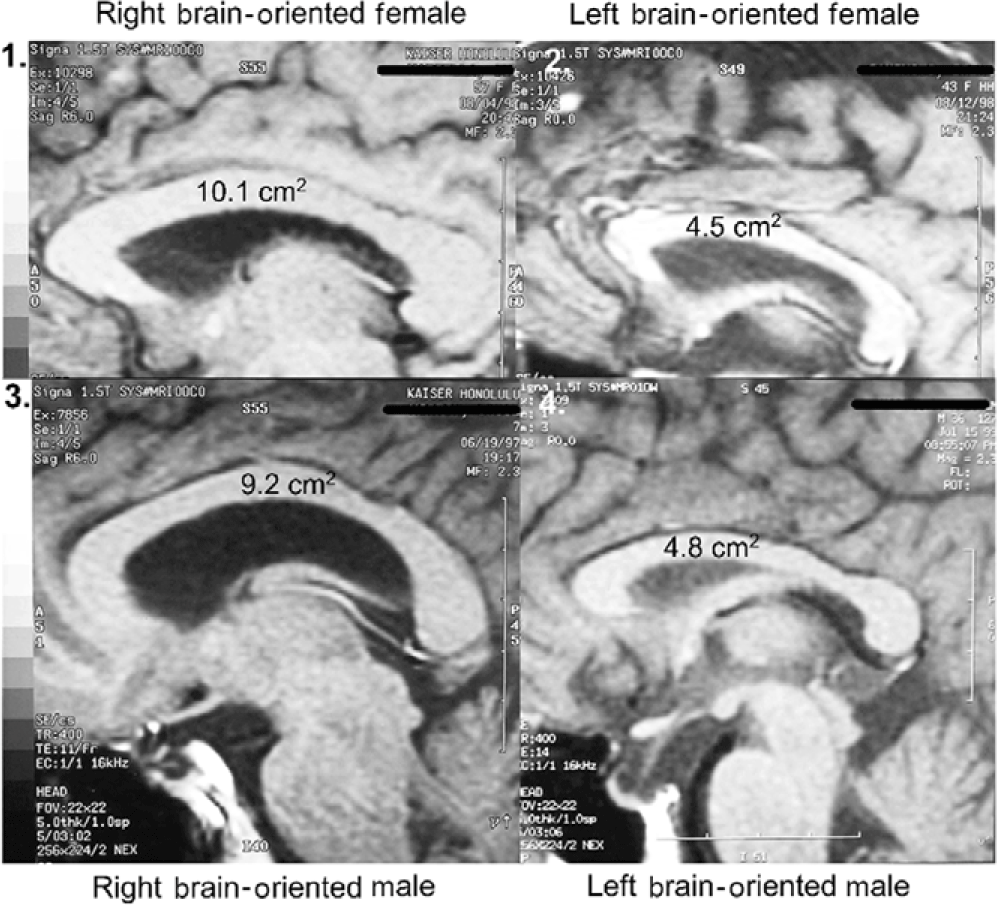
Figure 2. Hemisphericity vs. sex: size range of corpus callosal area.
2.1.5 Evidence that hemisity is inherited
Twin studies clearly show that corpus callosal size is inherited (Tramo, Loftus, Stukel et al., Reference Tramo, Loftus, Stukel, Green, Weaver and Gazzaniga1998). Since hemisity is tied to callosal size, and corpus callosal size does not change over time, it would appear that hemisity is inherited. Hemisity of offspring results also support this.
2.1.6 Hemisity distribution by sex
Although the right and left hemisity distributions were equal; interestingly, the distribution of hemisity subtypes between the sexes was quite different (n = 1049). Instead, in Figure 3, it may be seen that among the Hawaiian students, we found a reciprocal complementary relationship between RMs (37%, n = 132) and LFs (38%, n = 133) and correspondingly between LMs (62%, n = 216) and RFs (63%, n = 222). This same reciprocal complementary relationship was also found among genders in the Utah population between RMs (44%, n = 74) and LF (43%, n = 77) and correspondingly between LM (56%, n = 94) and RF (57%, n = 101). These paired numbers appear to be too close to be coincidental. Rather, it appears that they somehow reflect the hemisities of future couples, as follows.

Figure 3. Reciprocal complementary relationship between hemisity and gender in both Hawaiian and Utahan populations of high school students, n = 1049.

Figure 4. Asymmetries of the human anterior cingulate cortex.
2.1.7 Hemisity of reproductive pairs
Using a group of 412 paired individuals from the general population, the above seen distribution coupling of RMs to LFs and RFs to LMs was mirrored by the finding that these pairs actually formed the predominant marital partnerships of the RF-LMs and RM-LFs (Table 4). These pairing relationships also matched the larger number of related RF, LM, and RM-LF values of single high school students above (Figure 3). The abundance of the four naturally occurring heterosexual pairs was 40% RF-LM, 27% RM-LF, 20% LM-LF, 13% RM-RF. Of these, 67.5% consisted of opposite hemisity pairs (RM-LF, RF-LM), and 32.5% were composed of like–like (RM-RF, LM-LF) hemisity pairs. These observations support the hypothesis that in general twice as many opposite hemisity pairs bond to each other than like–like pairs and that “opposites attract” is the more common evolutionary pair-forming pattern.
Table 4. Hemisities of 206 couples in the general population
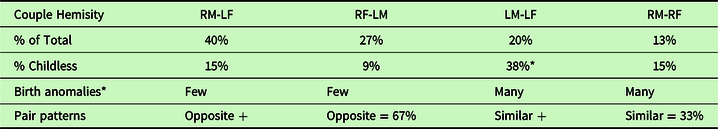
n = 206 of couples > 5 yrs. 50-50 MF. 53%LPs, 47% RPs.
* = birth anomalies: dyslexia, bisexuality, homosexuality.
2.1.8 RP hemisity dominance
Of the couples with opposite hemisities, the RP globally oriented partners were more dominant, and the LP detail-oriented partners were more supportive as shown in Table 5. This might be predicted from the observation that RPs are bold and outgoing, as compared to LPs who are more cautious and conservative. RPs also may be more cross-connected because their executive output often comes from the hemisphere opposite their language hemisphere. This may contribute to the observation that the medial CC area is considerably larger in RPs (Morton & Rafto, Reference Morton and Rafto2010).
Table 5. Six partner dominance-oriented items from the preference questionnaires

2.1.9 Discovery of the neuroanatomical basis of hemisity
As mentioned above, ACC asymmetry is present in humans (Morton & Rafto, Reference Morton and Rafto2010), one side being larger than the other in a seemingly idiosyncratic manner (Figure 4). The larger side was congruent with hemisity subclass. If it was larger on the right, the person was found to be a right brain-oriented dominant male or female. If the ACC was larger on the left, the subject was determined to be a left brain-oriented supportive male or female.
2.1.10 Hemisity of offspring from parents of known hemisity
Table 6 portrays the hemisity of 191 offspring of 91 of the four possible heterosexual pairs. As may be seen, for the RM-LF and RF-LM pairs of opposite hemisity, there was a trend toward offspring of the same sex as the parent having the same hemisity, that is, “like father, like son and like mother like daughter.” In contrast, the offspring of the RM-RF same–same pairs appeared to be sorted randomly. For those of the LM-LF pairs, percentage of RP was greatly reduced, in keeping with the high level of reproductive failure and childlessness of this pair due to partial sterility (Table 4). Offspring from both like–like parent pairs showed certain reproductive anomalies including dyslexia, bisexuality, and homosexuality. Pedophilia could not be assessed. The differences between the polarities at the human familial level are shown in Table 7, where the opposite behavioral roles are contrasted between the RM-LF and RF-LM reproductive pairs.
Table 6. Hemisity of 191 offspring from 95 parental pairs

Table 7. Human personality trait comparisons between the two familial polarities

3. Discussion
This is the report of a sixth lateralized brain function called hemisity. It is found to depend upon the idiosyncratic laterality of the larger, dominant side of the ACC. When the larger side of the ACC is on the right, the 30 behavioral traits of right hemisity (Table 2) were predominantly found. Correspondingly, when the larger side of the ACC is on the left, the person showed mostly left hemisity traits. That one’s right or left hemisity can be determined by questionnaires based upon three biophysical, non-behavioral measures (dichotic deafness, mirror tracing, best hand test (not counting MRI)), which give strong support to the existence of hemisity, especially because their results are similar to those of the five behavioral questionnaires, each confirmed by MRI (Table 1).
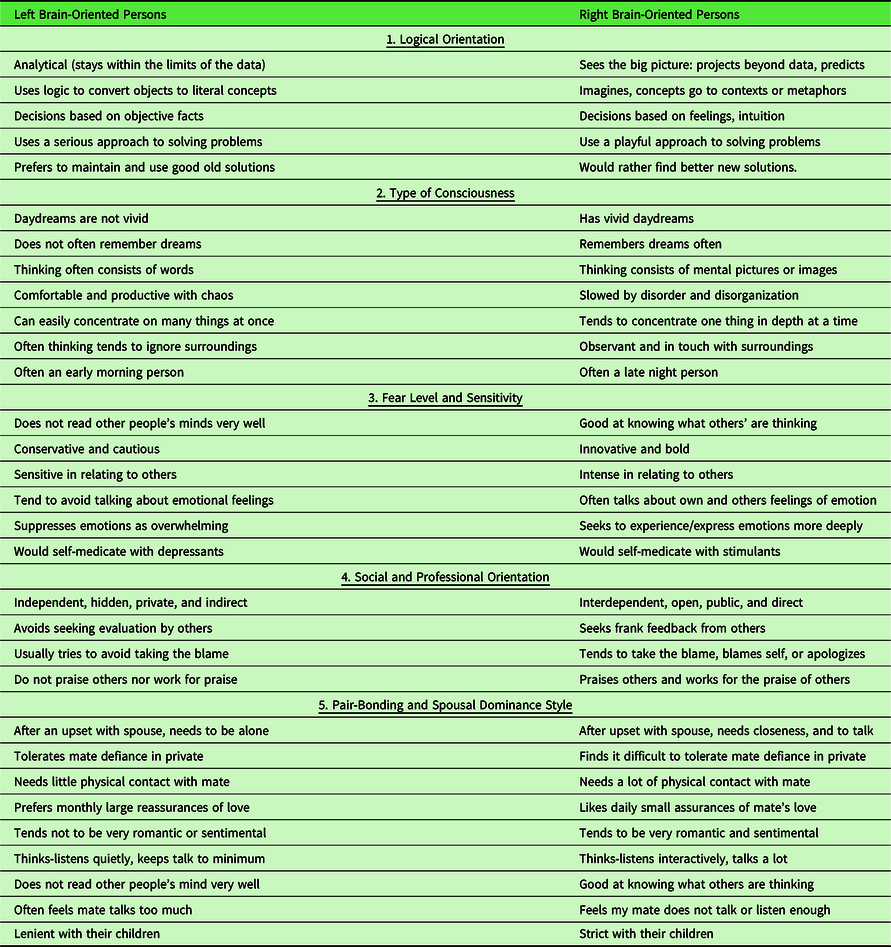
The 30 binary behavioral correlates of hemisity (Table 2) are organized under the following five categories: logical orientation, type of consciousness, fear level and sensitivity, social and professional orientation, and pair-bonding and spousal dominance style. Each of the 30 binary choices has a probability of 80% for agreement in hemisity. Thus, the differences in hemisity are fairly obvious, L hemisity traits, for example: independent hidden, private, and indirect vs. R hemisity traits: interdependent, open, public, and direct. Or L hemisity traits: often an early morning person vs. R hemisity traits: often a late night person. It thus becomes very clear whether a person’s hemisity is right or left.
3.1 Ten properties of hemisity
Hemisity supplies an important information packed missing factor in the categorization of individuals, of couples, and of groups. Ten properties of Hemisity are identified as follows:
1. It is irreversibly set by birth.
2. It is unrelated to handedness.
3. It is inherited.
4. In the general population, the numbers of RPs and LPs are approximately equal.
5. In RPs, the CC was up to three times larger than in LPs.
6. The numbers of both RMs and LFs have inexplicably been found to be similar, as were the more numerous LMs and RFs. That is, Both the RMs and LFs were about 40% of the LMs and RFs.
7. There were twice as many “opposites attract” RF-LM and RM-LF reproductive pairs than “same–same” RM-RF and LM-LF couples. This may have evolutionary significance.
8. In the “opposites-attract” mating pairs, RPs were the dominant partner. In the “same–same” pairs, dominance was random.
9. The side of the brain with the larger half of the split ACC determines whether a person will be an RP or LP.
10. Opposites-attract reproductive RF-LM and RM-LF pairs were true breeding, usually producing similar offspring. However, childlessness was high in L-L couples, whose remaining LP offspring were often homosexual. Offspring of R-R couples had many RP dyslexics and LP bisexuals.
3.2 Hemisity and corpus callosum size
It was found that those RPs with their final ACC executive output on the right side of the brain have significantly larger corpus callosi (Morton, 2006). This may be a consequence of their executive output module being on the opposite side of the brain from the commonly left hemisphere language module. Those LPs with their executive output on the same side as their language module would not be expected to need as much trans-hemisphere connectivity as RPs would. Thus, LPs would thus be expected to have smaller corpus callosi. Beyond this may be other factors that are related to the top-down, bottom-up specialization of the left and right cerebral hemispheres (Levy, Reference Levy1969; Sperry, Reference Sperry1982).
3.3 Reproductive partner choice
When marital partners are chosen, the two complimentary pairs (RF-LM and RM-LF) result more than twice as often as pairs with similar hemisity partners (LM-LF, RM-RF). The opposite hemisity pairs would appear to be evolutionarily preferred over similar hemisity pairs. This is because, in terms of survival, opposite hemisity partners may logically contribute many more survival skills as a reproductive package, such as morning and evening orientations, than two partners of the same limited skills. Besides this, L-L and R-R pairs are not true breeding and produce reproductive anomalies, such as dyslexia and homosexuality.
3.4 Distribution of hemisity subtypes and sex subtypes
The distribution of hemisity subtypes between the sexes was not the same as the equal distribution of right and left brainers in the population. That is, among the 1049 high school students there were more RFs and LMs than RMs and LFs. Furthermore, the numbers of the each of the two hemisity sets of high school individuals were almost identical. This suggests that there may somehow be a relationship between the unpaired high school student data and the later self-selected RM-LF and RF-LM reproductive pairs.
3.5 Hemisity subtype pair dominance
Among the “opposites attract” reproductive pairs, dominance between the partners almost always goes to the RP, whether male or female. This is not to say that LPs outside the home do not have a strong dominance hierarchies. They notoriously do, especially LMs. However, in the home, between partners, the RP almost always has the final word. Although one may be biased in judging the hemisity of others by one’s own hemisity orientation, the existence within the home of either male dominant or female dominant families is readily visible to the educated eye. However, in homes of like–like hemisity marital partners, this can be confusing because they follow a more random pattern.
3.6 Correlation of ACC size laterality and hemisity subtype
The fortuitous finding of laterality size differences between the divided ACCs was the key unifying factor when it was discovered that the larger ACC side was very highly correlated with hemisity subtype. That is, RPs almost invariably had larger ACCs on the right and LPs on the left. This gave hemisity a neuroanatomical basis.
3.7 Breakthrough: the true breeding nature of opposite hemisity Pairs
A new chapter began when it was found that two complementary hemisity pairs (RM-LF and RF-LM) were true breeding, producing offspring identical in hemisity to their parents (like mother, like daughter, like father, like son). In contrast, the hemisity of offspring from parents of the RM-RF partners was of random hemisity with anomalies, such as dyslexia, bisexuality, and homosexuality common. For the offspring of L-L couples, there was a large amount of childlessness. It may be that most of the RPs of the LM-LF pairs were aborted leaving only homosexual and bisexual LP offspring.
The ability to determine the right- or left brain hemisity of individuals has opened doors to many significant previous inaccessible social and historical issues. These include not only the personality and dominance of individuals but also the identification of the hemisity of reproductive pairs, that of their offspring, that of their geographic distributions, with consequent historical insights. The integration of all the facts presented here begins to suggest the existence of two human species (Morton, Reference Morton2012b). This topic will be developed in a future publication.
Acknowledgments
The author gratefully acknowledges the University of Hawaii environs and the thousands of subjects, colleagues, and friends therein who enthusiastically devoted their time and energy to this unfunded study.
Conflict of Interest
None present.













