INTRODUCTION
Noroviruses (NoVs) are now recognized as the leading aetiological agent of non-bacterial acute gastroenteritis (AGE) outbreaks, which are responsible for at least 50% of all AGE outbreaks worldwide, and a major cause of foodborne illness [Reference Hall1]. NoVs are extremely contagious and can infect people of all ages [Reference Hall1]. NoVs cause a self-limiting infection with AGE symptoms of typically 2–3 days duration. In the USA, NoVs cause 19–21 million illnesses and lead to 56 000–70 000 hospitalizations and 570–800 deaths annually [Reference Hall2].
NoVs are transmitted via the faecal–oral route, and the infections usually spread through person-to-person contact, foodborne or waterborne [Reference Hall1]. Of these routes of transmission, the foodborne transmission of NoV infection typically occurs via an infected food handler and contaminated food items such as raw oysters, fruits, and vegetables [Reference Le Guyader, Atmar and Le Pendu3]. Raw oysters are one of the well-known vehicles for the transmission of NoVs, which results in foodborne AGE outbreaks [Reference Costantini4]. NoVs are known to be capable of persisting in the tissues of oysters for long periods [Reference Le Guyader, Atmar and Le Pendu3]. Furthermore, oysters accumulate a large amount of NoVs when harvested from sewage-contaminated water [Reference Le Guyader5]. Therefore, NoV outbreaks associated with raw oyster consumption have been well documented worldwide [Reference Le Guyader, Atmar and Le Pendu3–Reference Le Guyader5].
In South Korea, raw oysters are one of the favourite food items and are usually consumed during the winter season. Furthermore, the fermentation of raw oysters with 5–10% salt at room temperature for approximately 2 weeks, for the preparation of ‘eoriguljeot’, is a traditionally popular technique in South Korea practised so that raw oysters can be enjoyed over a longer period [Reference Seo6]. During 2007–2012, various seafood items including raw oysters were reported as a major vehicle and NoV was the most common pathogen identified in foodborne disease outbreaks in South Korea [Reference Moon7]. However, no epidemiological and virological studies of oyster-associated NoV outbreaks have been reported from South Korea.
In May 2013, an AGE outbreak was reported at a high school in Anseong, Gyeonggi Province, South Korea. We therefore conducted epidemiological investigations to characterize the extent of the outbreak, to identify the route of transmission, and to determine the causative agent with virological tests. Our findings indicate that the AGE outbreak was closely associated with NoVs from fermented oysters.
METHODS
Epidemiological investigation
On 24 May 2013, a physician reported a suspected AGE outbreak to a health centre after eight students from one particular high school visited the local clinic with typical AGE symptoms of diarrhoea, vomiting, abdominal pain and nausea. In accordance with the guidelines for epidemiological investigations for waterborne and foodborne diseases, published by the Korea Center for Disease Control & Prevention (KCDC), all AGE outbreaks should be promptly and thoroughly investigated by technically trained public health officials to identify the causative agents and stop the spread of the disease [Reference Cho8, Reference Park9]. Public health authorities visited the high school to investigate the outbreak and the eight affected students indeed showed AGE symptoms from 21 to 22 May 2013. The students were then asked to complete a standardized questionnaire to identify which food items from the school restaurant they consumed, along with clinical information about the AGE symptoms. The results of the questionnaire were used for a subsequent case-control study to detect the vehicle for this outbreak. Cases were defined as students who presented at least three episodes of diarrhoea; or at least one episode of vomiting, nausea, abdominal pain or fever within 72 h of eating breakfast at the school restaurant on 21 May 2013. Controls were defined as students who did not meet the definition of a case, but who ate breakfast at the school restaurant on 21 May. The number of control subjects was approximately three times that of the number of case subjects. Odds ratios (OR) with 95% confidence intervals (CI) and P values to estimate the associations between the AGE symptoms and potential exposures were calculated using Excel 2010 (Microsoft Corp., USA).
Collection of clinical, food, and environmental specimens
On 24 May 2013, a total of 16 stool specimens were collected by public health officials from the eight AGE students and eight staff members who were working at the restaurant in the high school. The collected stool specimens were stored at 4 °C and were transferred to Gyeonggi Province Institute of Health and Environment (GIHE) in order to identify the aetiological agent responsible for the outbreak. In addition, samples of preserved food items served at breakfast on 21 May, together with environmental samples from kitchen knives, chopping boards, and dishcloths, and drinking-water and tap-water samples were collected and transferred to GIHE to identify the causative pathogens.
Microbiological investigation
The stool, food, and environmental samples collected were tested for ten species of bacteria (Escherichia coli, Salmonella spp., Shigella spp., Vibrio spp., Staphylococcus aureus, Clostridium perfringens, Campylobacter jejuni, Listeria monocytogenes, Yersinia enterocolitica, Bacillus cereus). The samples were cultured for bacterial pathogens at GIHE. Enteric bacterial pathogens were examined as described previously [Reference Park9]. The stool samples and uncooked food samples (fermented oysters and cabbage kimchi) were tested for five species of viruses (norovirus, group A rotavirus, astrovirus, adenovirus, sapovirus) [Reference Park9]. The drinking-water and tap-water samples from the school were tested for general bacteria, total coliforms, and E. coli [Reference Cho8].
Norovirus detection and sequence analysis
For virus detection, the faecal specimens were diluted to 10% in phosphate-buffered saline (PBS) and clarified by centrifugation at 800 g for 15 min, and the supernatants were then collected. The fermented oyster samples were tested for the presence of norovirus by using proteinase K digestion [Reference Rajko-Nenow10]. Briefly, the fermented oyster samples were rinsed twice with water to remove the seasoning. The digestive diverticula were removed and dissected using sterile scissors and forceps. Approximately 1·5 g digestive tissues from the oysters was obtained and wholly homogenized with the same amount of proteinase K (Sigma-Aldrich, USA) and incubated at 37 °C with shaking at 320 rpm for 1 h. The samples were then incubated at 60 °C for 15 min. Next, the samples were clarified in two rounds of centrifugation at 4000 g for 5 min, and the final supernatants were obtained for the measurements. Viral RNA was extracted from the 140 µl supernatant of faecal and oyster samples using the QIAamp viral RNA mini kit (Qiagen, Germany) according to the manufacturer's instructions. To detect the NoVs, semi-nested reverse transcriptas–polymerase chain reaction (RT–PCR) was performed using specific primer sets (NV-GIF1M/NV-GIR1M/NV-GIF2 for NoV GI; NV-GIIF1M/NV-GIIR1M/NV-GIIF3M for NoV GII), targeting the capsid gene (region C) as described previously [Reference Cho8]. For human samples, the final PCR products were then purified and bi-directionally sequenced using nested PCR primers. For oyster samples, the amplified fragments were purified and then cloned into pGEM-T Easy vector (Promega, USA) according to the manufacturer's recommendations. Plasmids were purified and then sequenced. The MEGA software program v. 6.0 was used for the phylogenetic analysis with reference strains [Reference Jeong11].
RESULTS
Epidemiological analysis
On 24 May 2013, a local clinic physician notified Anseong Public Health Centre that eight students from the same high school in Anseong, Gyeonggi Province, South Korea had developed AGE symptoms 2 days previously. Initial epidemiological investigations were undertaken to identify the common feature in the affected population. Two common factors were observed; first, all the patients used the dormitory of the school, and second, they all ate breakfast at the school restaurant. Students using the dormitory of the school usually eat breakfast at the school restaurant, whereas other students eat breakfast at home, which was one of the critical differences between the two sets of students. Furthermore, none of the students who did not use the dormitory showed AGE symptoms. Therefore, in order to identify the cause of this outbreak, epidemiological and microbial investigations focused on the 130 students using the dormitory and who ate breakfast at the school restaurant were conducted.
Of the 130 students, eight (6·2%) exhibited AGE symptoms (Table 1). The epidemic curve for this outbreak showed that the index case was found at 23:30 hours on 21 May, the other seven patients were identified by 22 May and no secondary cases were found. The main gastrointestinal symptoms reported were vomiting (n = 6), nausea (n = 6), diarrhoea (n = 5), fever (n = 5) and chills (n = 4) with a range of onset times from 16 to 40 h after eating breakfast at the school restaurant. The average incubation time was 26·9 h and the median duration of the illness was 2 days (Table 1).
Table 1. Epidemiological features of the oyster-borne NoV-associated outbreak in Gyeonggi Province, South Korea, 24 May 2013

* Compared to linked clinical specimens.
† Other viruses included group A rotavirus, enteric adenovirus, astrovirus, and sapovirus.
A case-control study was performed using the asymptomatic students who used the dormitory of the school as control subjects. Although the OR of the fermented oyster samples was not calculated owing to zero count of not-eaten group in case subjects (Table 2), the results of our case-control study revealed that the AGE outbreak could be significantly linked to fermented oysters among the five food items served to the students (Table 2).
Table 2. Association between the acute gastroenteritis symptoms and the breakfast menu served on 21 May 2013 in the school restaurant
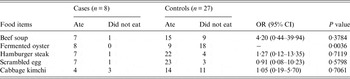
OR, Odds ratio; CI, confidence interval.
Microbiological analysis
NoVs were detected in the faecal samples of all eight AGE students, whereas none of the faecal samples from food handlers showed the presence of NoVs. None of the other enteric bacteria or viruses tested was found to be present in the same eight specimens. The drinking- and tap-water samples were also negative for general bacteria, total coliforms, and E. coli. Multiple genotypes of NoV were detected in the faecal specimens of the cases; NoV GII.4, GII.11 and GII.14 (Table 1, Fig. 1). Furthermore, the GII.4 and GII.14 NoV strains were also identified in fermented oysters served from the breakfast menu on the day. The nucleotide sequence similarity between the NoV strains from the clinical samples and those from the fermented oysters was 99·5–100 % (Fig. 1).
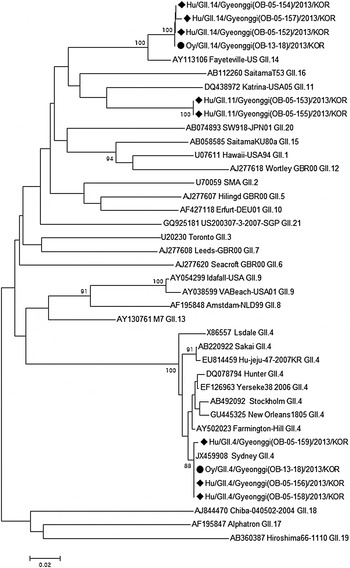
Fig. 1. A phylogenetic tree was constructed using 218 base-pair nucleotide sequences from the capsid gene of the NoV strains isolated from the clinical specimens (♦) and fermented oyster samples (•) associated with the acute gastroenteritis outbreak. ClustalW and the neighbour-joining method with bootstrap analysis (n = 1000) were used for DNA sequence alignments and dendrogram construction, respectively. Oy, Fermented oyster; Hu, human patient; OB, outbreak.
DISCUSSION
In this study, both the epidemiological and the virological evidence supported fermented oysters to be associated with the NoV outbreak that occurred at a high school in May 2013 in South Korea. The affected students suffered from AGE symptoms, and analysis of the faecal specimens obtained from the symptomatic students confirmed NoVs as the causative agent. The results of case-control study suggested a strong correlation between AGE symptoms and consumption of fermented oysters. NoVs were detected in fermented oysters and the sequence similarity between the NoVs from the clinical and fermented oyster samples was found to be more than 99·5%.
The association between AGE outbreaks and the consumption of shellfish is well established [Reference Le Guyader, Atmar and Le Pendu3]. From 2006 to 2013, 102 NoV outbreaks were reported in Gyeonggi Province, South Korea [Reference Cho12]. Fifteen of these outbreaks were associated with bivalve shellfish such as oysters and mussels, but no NoVs were detected in the shellfish owing to the lack of leftover samples, although groundwater and kimchi were identified as vehicles in the NoV outbreaks [Reference Cho8]. However, fermented oysters were fortunately obtained as preserved food by the school in this outbreak, and multiple NoV genotypes were identified from these fermented oysters, which was consistent with other previous reports of oyster-borne NoV outbreaks [Reference Le Guyader, Atmar and Le Pendu3, Reference Le Guyader5, Reference Rajko-Nenow10].
Fermented oysters are one of the representative fermented foods and have never been reported as a vehicle of transmission in NoV outbreaks in South Korea previously [Reference Seo6]. A recent study reported that murine and feline NoVs were significantly inactivated in fermented oysters during the 2 weeks of fermentation [Reference Seo6]. However, in the present study, fermented oyster samples could not be obtained directly from the food company that supplied them to the school. Furthermore, this company was not the manufacturer, only a mid-distributor in the complex distribution system of fermented oysters. Therefore, additional information about the fermented oysters could not be obtained because the company did not know the production area or the fermentation period of these oysters. Moreover, since none of the faecal samples from the food handlers at the high school showed the presence of NoVs, contamination of the food by asymptomatic NoV carriers at the high school is unlikely.
Only eight of the 130 exposed students were affected. This low attack rate can be explained by individual preference for fermented oysters; fermented oysters develop a distinctive flavour after curing, therefore they may not be preferred by many of the students [Reference Seo6]. In addition, a dose-dependent relationship between fermented oyster consumption and AGE symptoms is considered as a strong indicator of the involvement of the oysters in AGE outbreaks [Reference Loury13]. However, this aspect was not studied in this epidemiological investigation, which was one limitation of this study. Despite the limitations mentioned above, based on our results, we assume that raw oysters contaminated by NoVs were used to prepare the fermented oysters, and that these fermented oysters harbouring active NoVs were served to the students, which resulted in this AGE outbreak.
In conclusion, our results indicate that the fermented oysters were very likely to be the vehicles for NoVs responsible for this AGE outbreak. Our findings can contribute to a greater understanding of the risks associated with the consumption of NoV-contaminated oysters. Therefore, to prevent further outbreaks, proper management of raw oysters prior to the preparation of fermented oysters is necessary, and the food industry should be aware of the risks of viral gastroenteritis posed by the consumption of fermented oysters contaminated with NoVs.
ACKNOWLEDGMENTS
This study was supported by the Korea Environmental Industry & Technology Institute (2013000550009) and a grant of the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea (grant no.: HI15C1781).
DECLARATION OF INTEREST
None.






