Postprandial glycaemia has been implicated in the development of chronic metabolic diseases such as obesity, type 2 diabetes mellitus and CVD( Reference Blaak, Antoine and Benton 1 ). Both the amount and type of carbohydrates (CHO) consumed contribute to glycaemia and insulinaemia( Reference Wahlqvist, Wilmshurst and Richardson 2 ). In 1981, the concept of glycaemic index (GI) was introduced by Jenkins et al. ( Reference Jenkins, Wolever and Taylor 3 ) to rank different foods according to their glycaemic response. Moreover, low GI diets have been shown to improve glycaemic control( Reference Brand-Miller, Hayne and Petocz 4 ) and insulin sensitivity( Reference Frost, Leeds and Trew 5 , Reference Rizkalla, Taghrid and Laromiguiere 6 ) in diabetic patients. They are also independently associated with reduced risk of chronic diseases( Reference Barclay, Petocz and McMillan-Price 7 ).
Starch is one of the most important glycaemic CHO in cereal products. Among the various classes of processed starchy foods, wheat-based cereal products exhibit a very wide range in GI( Reference Foster-Powell, Holt and Brand-Miller 8 , Reference Englyst, Vinoy and Englyst 9 ). Intrinsic properties of starch (gelatinisation, amylopectin:amylose ratio, etc.) as well as the nutritional composition of food products influence the rate of starch digestion( Reference Liljeberg, Granfeldt and Bjorck 10 – Reference Lehmann and Robin 12 ). The rate and extent of starch digestion has been measured in vitro using a method developed by Englyst et al. ( Reference Englyst, Kingman and Cummings 13 – Reference Englyst, Englyst and Hudson 15 ), which classifies starch into three major fractions: rapidly digestible starch (RDS), slowly digestible starch (SDS) and resistant starch.
Statistical analyses using regression models have been performed to explain the influence of starch digestibility in cereal products or other plant food products on GI( Reference Englyst, Vinoy and Englyst 9 , Reference Englyst, Veenstra and Hudson 14 , Reference Garsetti, Vinoy and Lang 16 , Reference Kai, Shujun and Copeland 17 ). The number of tested products and product categories in these studies was low( Reference Englyst, Vinoy and Englyst 9 , Reference Garsetti, Vinoy and Lang 16 ) and results are therefore difficult to extrapolate. The contribution of the physico-chemical characteristics of the food products was not extensively investigated. In addition to GI, insulinaemic index (II) is also investigated because of its role in glucose homeostasis and its regulating effects on lipid metabolism( Reference Miller, Pang and Broomhead 18 – Reference Saltiel and Kahn 21 ).
The aim of the present work was thus to test the influence of CHO digestibility and the nutritional characteristics of a large sample of different categories of cereal-based foods on GI, II and on various parameters of glycaemic and insulinaemic response curves; to evaluate the contribution of the interactions among the different parameters in cereal-based foods; to discuss the physiological relevance of glycaemic and insulinaemic descriptors.
Materials and methods
Subjects and in vivo studies
Since 1998, 190 cereal products have been tested according to the international standard methodology for GI( 22 , 23 ) using 50 g glucose dissolved in 250 ml water as the reference food (GI = 100) as proposed by Brouns et al. ( Reference Brouns, Bjorck and Frayn 24 ). The procedures followed during the clinical studies were in accordance with the Declaration of Helsinki and the ethical standards of the responsible institutional or regional committees on human experimentation, and all the subjects gave informed written consent.
A total of 591 volunteers (in forty-nine trials) consumed the cereal products in test portions containing 50 g of glycaemic CHO with 250 ml water within 10–15 min. Capillary blood samples were collected at the consumption time (T0), and after 15, 30, 45, 60, 90 and 120 min to build the glycaemic and insulinaemic response curves for each test product. As indicated in the International Standards (ISO 26 642:2010)( 23 ), the subjects selected for the GI test were healthy human subjects of both sexes who complied with two additional inclusion criteria: fasting glycaemia < 6·1 mm and 2-h glycaemia following the ingestion of a 50-g glucose solution < 8·9 mm. Mean age and BMI of the study subjects were, respectively, 24·7 (sd 4·7) years and 22·3 (sd 1·9) kg/m2. Fasting glycaemia and 2-h glycaemia (mm) were, respectively, 5·11 (sd 0·40) and 4·62 (sd 0·72).
The GI of a given food reflects how much of its digestible CHO raise blood glucose levels. It is defined as the iAUCg after consumption of a portion of test food providing 50 g of the available CHO, expressed as a percentage of the average iAUCg to the same amount of CHO from a reference food (glucose) ingested by the same subject on a separate occasion. The iAUCg is the incremental area under the blood glucose response curve (calculated for 2 h following the ingestion of the tested product: 0–120 min), ignoring the area beneath the fasting concentration. In the present work, the iAUCg was calculated according to the trapezoidal method as recommended in the International Standard( 23 ). For an individual subject, the GI of a tested food is given by: iAUCg test food/average iAUCg reference food × 100. Other parameters defining the shape of the response curves over 2 h were also investigated: iAUCg, C max g (maximum concentration of blood glucose) and Δpeak g (Δpeak of glycaemic response; difference between C max and baseline concentration). Moreover, II was calculated similarly to GI in measuring the extent to which a food raises plasma insulin concentration( Reference Wolever, Jenkins and Jenkins 25 ). The same parameters (incremental area under the blood insulin response curve (iAUCi), maximum concentration of blood insulin (C max i), Δpeak of insulin response (Δpeak i)) as those of the glycaemic response curve were used to describe insulinaemic response.
Product database with characteristics of the cereal products by category
An internal database of 190 products representing a wide range of processed cereal products was used for the statistical analyses. These products were classified into four categories according to the manufacturing process: extruded cereals, dried bakery products and crackers, soft bakery products and biscuits. Nutritional composition and starch digestibility parameters in the cereal products used in this analysis are summarised in Table 1. The nutritional parameters were analytically measured (fibres: AOAC 985-29; total starch: enzymatic method as described in the French standard V18-121; fat and moisture: methods described in the French Decree of 08/09/1977; proteins: Kjeldahl method). In vitro starch digestibility was assessed using the method developed by Englyst et al. ( Reference Englyst, Kingman and Hudson 26 ). It involved several steps that simulate the in vivo enzymatic digestion of CHO in the stomach and the small intestine and the release of glucose at several experimental times. This method makes it possible to measure the amounts of different starch and sugar fractions according to their digestibility( Reference Englyst, Veenstra and Hudson 14 , Reference Englyst, Englyst and Hudson 15 ) (Fig. 1).
Table 1 Mean of nutritional composition and starch digestibility parameters (g/portion size providing about 50 g available carbohydrates) of the 190 cereal products classified by category (Mean values and standard deviations; median values and minimum and maximum values)

CHO, carbohydrate; RAG, rapidly available glucose; RDS, rapidly digestible starch; SDS, slowly digestible starch; RS, resistant starch.

Fig. 1 Sugar and starch components and their successive digestible fractions as determined by the Englyst method( Reference Englyst, Kingman and Hudson 26 ).
The starch fractions are defined as the RDS (the starch fraction digested in vitro within 20 min of hydrolysis), the SDS (the starch fraction digested in vitro in between 20 and 120 min of hydrolysis) and the resistant starch. The resistant starch fraction is calculated as the released glucose from further hydrolysis of the starch remaining in the main incubation tube at the end of 120 min. The notion of rapidly available glucose has been proposed as being the amount of glucose likely to be available for rapid absorption in the human small intestine( Reference Englyst, Englyst and Hudson 15 ). Regarding cereal products, slowly available glucose can be assimilated into SDS. We will therefore use the term SDS in the present paper.
Statistical analyses
For investigating the impact of cereal product characteristics on GI, II and glucose and insulin response parameters (iAUC, C max, Δpeak), a step-by-step statistical analysis was applied using JMP Software version 10.0 (SAS Institute Inc.). Partial least squares (PLS) regression analysis was first performed to select the variables of product composition (among nutritional composition and starch digestibility parameters) that may impact on GI, II and each of the six metabolic response parameters. In this PLS regression analysis, eight individual variables were tested on the four glycaemic response parameters (GI, iAUCg, C max g and Δpeak g) as a first step: fat, dietary fibres, total available CHO, proteins, fructose, free sugar glucose, RDS and SDS. Only parameters with a variable importance in projection (VIP) value greater than 0·8, indicative of their significant impact, were considered in the following steps. Another PLS regression analysis was then performed on the four glycaemic response parameters including the product variables selected in the first step and all the possible interactions between them, including the quadratic terms of the variables. These terms are representative of a non-linear effect between product variables and glycaemic response. As for the first step, only interactions with a VIP value greater than 0·8 were considered. Finally, product parameters and their interactions with a contribution to the model greater than 5 % were kept in the final model. In the case of interactions selected for the models, all the parameters involved in the interactions were also kept. Once selected, these product variables were introduced into a new PLS analysis integrating the four glycaemic response parameters. The prediction formulas of this model for each glycaemic response parameter were extracted and a linear regression analysis was done between the actual value of each glycaemic response parameter and its predicted value to obtain a R 2 value for each parameter with this model. A prediction profiler was also built on Excel Software version 10.0 (Microsoft Corporation) using the prediction formula obtained from the model developed for glycaemic responses. It was used to understand and illustrate the impact of the product variables and their interactions on metabolic response parameters.
The same PLS methodology was followed for the insulinemic response parameters. However, it is known that plasma insulin tests are not standardised and that the use of different analytical kits can significantly impact the analytical results( Reference Miller, Thienpont and Van Uytfanghe 27 , Reference Manley, Luzio and Stratton 28 ). We also investigated whether the type of analytical kit used in the laboratories could impact the results on II, iAUCi, C max i and Δpeak i. This was done by using an ANOVA on the iAUCi obtained after the consumption of the reference product (glucose).
Results
Characteristics of the cereal products
All values are expressed as means with standard deviations and as median, minimum and maximum. Nutritional characteristics in cereal products are shown per portion of product providing 50 g of available CHO (Table 1). The biscuit category had the lowest average value of GI (49 (sd 10)) and II (62 (sd 15)), while the extruded cereal category had the highest GI value (67 (sd 13)). The highest II was observed in the soft bakery products (79 (sd 21)). Dried bakery products and crackers had the highest rapidly available glucose content and biscuits the lowest (42·6 (sd 2·5) v. 29·6 (sd 3·3) g, per 50 g available CHO). The biscuits had the highest SDS content (10·8 (sd 3·2) g, per 50 g available CHO).
Selection of the nutritional composition and starch digestibility parameters impacting the glycaemic response
All the analyses were performed on the full range of product characteristics present in the database. The variables of nutritional composition (fat, proteins, total available CHO, dietary fibres) and starch digestibility (fructose, free sugar glucose, RDS, SDS) were integrated into the PLS analysis testing the four parameters of glycaemic response: GI, iAUCg, C max g and Δpeak g. All the analyses were performed on the whole range of product characteristics in the database. Testing these four parameters at the same time showed that their changes were very similar and thus strengthened our analysis. From this first step, SDS, fat, dietary fibres and RDS displayed a VIP value greater than 0·8 and were kept in the second step of the PLS analysis. In this second step, we also introduced all the interactions among these four variables. These four variables (fat, fibres, RDS and SDS) and seven interactions (fibres2, fat2, SDS2, fat × SDS, fat × fibres, SDS × RDS and fat × RDS) displayed a VIP >0·8. A final step consisted of selecting variables and interactions contributing for more than 5 % in the model. Therefore, the final model included four variables (SDS, fat, fibres and RDS) and four interactions (fibres2, SDS × RDS, fat × fibres and fat2). Here, the main contributors to the model built over the full range of the biscuits characteristics were SDS, fibres2 and fat with contributions of 17·4, 15·9 and 13·7 %, respectively (Table 2).
Table 2 Factors significantly influencing glycaemic response (glycaemic index, incremental area under the blood glucose response curve, Δpeak of glycaemic response and maximum concentration of blood glucose)

SDS, slowly digestible starch; RDS, rapidly digestible starch.
* Kept as this variable is involved in interactions.
The same PLS approach was followed to select the product variables impacting the insulinaemic response parameters. The analysis indicated that SDS, fat, fibres and proteins are the characteristics of the cereal products that influence II and the insulin response parameters (iAUCi, C max i and Δpeak i) the most. When integrating the interactions between these components in the PLS analysis, the best model was the one including four variables (fat, fibres, proteins and SDS) and six interactions (fibres2, fibres × fat, fat × SDS, fat2, fibres × proteins and SDS2). When selecting only variables contributing for more than 5 % in the model, three variables (SDS, fibres and fat) and five interactions (fat × fibres, fibres2, fat × SDS, SDS2 and fat2) were included in the final model (Table 3).
Table 3 Factors significantly influencing insulin response (insulinaemic index, incremental area under the blood insulin response curve, Δpeak of insulin response and maximum concentration of blood insulin)

SDS, slowly digestible starch.
* Kept as this variable is involved in interactions.
Relationships between glycaemic response parameters and product characteristics
Almost half (48·9 %) of the glycaemic response was explained by the model including conjointly the four selected variables and the four interactions tested. In this model, the components SDS and fat impacted the glycaemic response the most. Fibres2, SDS × RDS and fat × fibres interactions also had an important influence on the glycaemic response. The regression analysis performed to determine the individual contributions of the selected variables to the glycaemic parameters over the full range of biscuits indicated that the variables explained 52·9, 41·4, 41·2 and 60·1 % of the variance in GI, iAUCg, C max g and Δpeak g, respectively (all P< 0·0001). The GI and Δpeak g are the glycaemic response parameters that are best explained by the model.
Contour plots for modelised GI clusterised on three levels of SDS content (Fig. 2(a)) indicated that for identical contents of fat and fibres in products, GI is higher with low SDS products (range 80–50). With low and medium SDS contents in the cereal products, increased fat and fibres lowers the GI value (from 80 to 40 and from 70 to 20, respectively), indicating that fat and fibres make important contributions to GI. With high SDS content (>13 g/portion), the contributions of fibres and fat to GI are limited (the GI values do not fluctuate much, ranging from 40 to 60). A similar profile of contour plot for Δpeak g was obtained, indicating the same influence of the product characteristics on Δpeak g.

Fig. 2 Impact of fat and fibres on (a) glycaemic index (GI) and (b) Δpeak of glycaemic response (Δpeak
g) values clusterised as low, medium and high levels of slowly digestible starch (g/portion) in the cereal products. (a) Predicted GI: (![]() ), 20·0–30·0; (
), 20·0–30·0; (![]() ), 30·0–40·0; (
), 30·0–40·0; (![]() ), 40·0–50·0; (
), 40·0–50·0; (![]() ), 50·0–60·0; (
), 50·0–60·0; (![]() ), 60·0–70·0; (
), 60·0–70·0; (![]() ), 70·0–80·0. (b) Predicted Δpeak g: (
), 70·0–80·0. (b) Predicted Δpeak g: (![]() ), 0·50–1·00; (
), 0·50–1·00; (![]() ), 1·00–1·50; (
), 1·00–1·50; (![]() ), 1·50–2·00; (
), 1·50–2·00; (![]() ), 2·00–2·50; (
), 2·00–2·50; (![]() ), 2·50–3·00; (
), 2·50–3·00; (![]() ), 3·00–3·50.
), 3·00–3·50.
To further understand the effect of the fibres × fat interaction on GI, another approach was used. It consisted in profiling the effect of fibre content on GI at the medium SDS level (8·5 g/portion) and for three levels of fat: 0·5, 9·3 and 18·1 g/portion (corresponding to minimum, medium and maximum fat contents observed in the product database) (Fig. 3). The graph shows that GI decreases when the fibre content of the product portion increases. This effect is reduced as the fat content increases until being lost with the highest fat content.
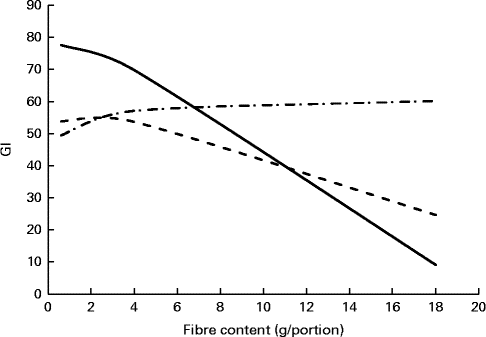
Fig. 3 Example of prediction profiler showing the fibre content effect on glycaemic index (GI) at different fat levels at a medium level of slowly digestible starch. Ordinates correspond to the predicted GI values. Fat levels correspond to minimum (0·5 g/portion), medium (9·3 g/portion) and maximum (18·1 g/portion) fat contents of products present in the database. ![]() , 0·5 g of fat/portion;
, 0·5 g of fat/portion; ![]() , 9·3 g of fat/portion;
, 9·3 g of fat/portion; ![]() , 18·1 g of fat/portion.
, 18·1 g of fat/portion.
Relationships between insulinaemic response parameters and product characteristics
The model developed for insulinaemic response parameters including the three product variables (fat, fibres and SDS) and the five interactions (fibres2, fat2, SDS2, fat × fibres and fat × SDS) explains 43·3 % of the four insulinaemic descriptors considered together. When testing the kit effect, the ANOVA indicated that the type of kit used for insulin quantification significantly impacted the results (P< 0·0001). Therefore, we did not further analyse the insulin parameters as the observed kit effect biases the results.
Discussion
This is the first study that evaluated the effect of starch fraction and macronutrient contents on the metabolic responses of an important dataset (190 cereal products). The present results, based on various categories of cereal products, indicate that 53 % of GI variability is explained by a model involving both parameters of starch digestibility (SDS and RDS) and nutritional composition (fat and fibres). We found that SDS was the main parameter influencing glycaemic response, accounting for 17·4 % in its variance, while RDS explained only 5·5 % of the variance. In previous studies, rapidly available glucose was highly correlated to GI and glycaemic response( Reference Englyst, Veenstra and Hudson 14 , Reference Englyst, Englyst and Hudson 15 ). Moreover, it has been reported both on twenty-three cereal products of different categories (cereals, bakery products, crackers and biscuits)( Reference Englyst, Vinoy and Englyst 9 ) and on twenty-four plain sweet biscuits( Reference Garsetti, Vinoy and Lang 16 ) that SDS was the major contributing factor to GI variation. The results of the present study, obtained on a larger dataset of 190 cereal products, confirm these previous observations.
Moreover, in this statistical analysis, we showed a 13·7 % contribution of fat in the glycaemic response. Fat content has already been shown to influence the glycaemic response in several studies( Reference Englyst, Vinoy and Englyst 9 , Reference Garsetti, Vinoy and Lang 16 , Reference Normand, Khalfallah and Louche-Pelissier 29 , Reference Flint, Moller and Raben 30 ). In thirteen European meals, Flint et al. ( Reference Flint, Moller and Raben 30 ) showed that GI was more strongly correlated with either fat, protein or total energy content than with CHO content alone. As noted by the authors, available CHO are fixed to 50 g and fat and proteins vary; therefore, it is not surprising that the best prediction model included both fat and protein contents of the test meals, which are directly related to increased energy. The CHO content in the present study was also fixed at 50 g of available CHO, but protein and fat contents in the cereal products were below the ranges of the meals used by Flint et al. ( Reference Flint, Moller and Raben 30 ): 2–22 g for protein and 0·5–18 g for fat in the present study, v. respectively 5–28 and 3–42 g in the test meals in the study of Flint et al. ( Reference Flint, Moller and Raben 30 ). This may explain why no impact of proteins on the glycaemic response was found in the present study. Another possible explanation could be that the meals were composed of various foods leading to more intricate interactions than with cereal products tested alone. The quality of lipids and proteins contained in the products was not investigated in the present study. However, many studies have evaluated these parameters on postprandial glycaemic and insulinaemic responses in both healthy subjects and patients with type 2 diabetes. The main effect of lipid quality seems to be on exacerbated insulin response( Reference Lardinois, Starich and Mazzaferri 31 , Reference Rasmussen, Lauszus and Christiansen 32 ). However, similar effect was not confirmed by more recent work( Reference Thomsen, Storm and Holst 33 – Reference Shah, Adams-Huet and Brinkley 35 ). Regarding the quality of proteins, several studies have investigated the impact of proteins from different sources on glycaemia and insulinaemia( Reference Esteves de Oliveira, Pinheiro Volp and Alfenas 36 ). Insulinotropic effect of proteins was related specifically to animal proteins such as whey proteins, due to their high content of branched-chain amino acids. However, literature review did not allow reaching a consensus on the impact of proteins on glycaemic and insulinaemic responses.
We also showed that fibre contributes to glycaemic response and is especially relevant within the interactions included in the model. Other previous studies that tested the impact of dietary fibres have shown that they do influence the GI( Reference Bjorck and Elmstahl 37 , Reference Tosh 38 ). The importance of fat and fibre contents, alone or in combination, in glycaemic response may be explained by physiological mechanisms. Interactions of starch with fibre and other food components can prevent effective diffusion and adsorption of the α-amylase into the substrate( Reference Lehmann and Robin 12 , Reference Colonna, Leloup and Buleon 39 ). However, management of blood glucose levels may be achieved through means other than the ones influencing the susceptibility of starch to digestion. The ingredients present within the food matrix may influence the glucose metabolism through regulation of gastric emptying, gut hormone profile and glucose absorption( Reference Lehmann and Robin 12 ). Soluble fibres can reduce the rate of gastric emptying by increasing the digestate in the upper part of the gastrointestinal tract. However, the viscosity properties of fibres seem to be more important in regulating the glycaemic response than the fibre quantity( Reference Juntunen, Niskanen and Liukkonen 40 ). SDS has previously been shown to reduce the glycaemic response by decreasing the glucose appearance rate in blood circulation( Reference Nazare, Rougemont and Normand 41 , Reference Vinoy, Normand and Meynier 42 ). Fat may also reduce postprandial glycaemia by slowing down gastric emptying( Reference Cecil, Francis and Read 43 ). Normand et al. ( Reference Normand, Khalfallah and Louche-Pelissier 29 ) found that while medium fat addition (15 g) to starch decreases the initial glycaemic peak, the addition of a larger amount of fat (45 g) to starch appears to be unfavourable to glycaemic response by leading to a feature of insulin resistance.
The originality of the present study was to bring out the contribution of interactions between components in the glycaemic response. The interactions of fibres2, SDS × RDS, fat × fibres and fat2 accounted for 15·9, 10·5, 9·5 and 6·8 %, respectively, to the glycaemic response. The importance of these interactions has been highlighted by the present results, showing that not only the SDS level but also the fat and fibre contents impact glycaemic response. We also showed with our food database that fat and fibre contents have an impact on GI for low and medium SDS levels, but this effect is not observed when the SDS level is high. In addition, fat interacts with fibres to minimise the effect of fibre on glycaemic response. The present results show that the relative macronutrient contents modulate the glycaemic response differently.
The limitation of the present study is that it focused on processed cereal products only, which have defined ranges of macronutrient contents. Our database is not composed of equal amounts of information for each category, with a higher sample of products being rotary moulded biscuits. However, this database can be considered as representative of marketed sweet cereal products. In addition, the recipes of these tested cereal foods make it possible to estimate the role of each macronutrient within the context of a complex food matrix.
In a database including 1126 food products from all categories, Brand-Miller et al. ( Reference Brand-Miller, Stockmann and Atkinson 44 ) showed that GI was correlated to some attributes of the glycaemic response, including the absolute peak response. The present results are in accordance with this and suggest that not only GI but also the Δpeak of the glycaemic curve can be explained by the product characteristics for cereal products. Indeed, the GI does not take into account the shape of the glycaemic response curve and its measurement is obtained, thanks to the 2-h postprandial response. To take the shape of the curve into account, Rosen et al. ( Reference Rosen, Silva and Andersson 45 ) propose measuring the glycaemic profile (min/mm) of the products to evaluate the course of post-meal glycaemia. The glycaemic profile is defined as the time (min) during which the blood glucose is above fasting concentration (3-h postprandial glucose evaluation) divided by the blood glucose Δpeak value (mm) for each subject and test meal( Reference Rosen, Silva and Andersson 45 ). The present results indicate that the Δpeak, which takes into account the magnitude of the glycaemic response, may bring added value to GI to describe the glycaemic response. It would be of public health interest to take into account, in addition to the GI, the magnitude of the glycaemic response by investigating the Δpeak as a high Δpeak leads to greater insulin stimulation, which may induce deleterious effects in the diabetes genesis( Reference Ceriello, Colagiuri and Gerich 46 ).
This is also the reason why we investigated the insulin response in addition to the glycaemic response. However, as noted by others, we found that the type of kit used for the insulin analyses significantly impacted the results, and we emphasise the need to standardise the kits for insulin detection analysis to confirm that the lower glycaemic responses obtained are not followed by an excessively high insulin response( Reference Miller, Thienpont and Van Uytfanghe 27 , Reference Manley, Luzio and Stratton 28 ).
Finally, the high contribution of some cereal product characteristics (SDS, fat and fibres) and their inter-dependance in the GI and glycaemic response parameters, such as Δpeak g, contends for focusing on both the optimisation of the formula and the manufacturing process of cereal products to decrease postprandial glycaemic response. Reducing glycaemic response has been recognised as relevant to the prevention of metabolic diseases( Reference Blaak, Antoine and Benton 1 , Reference Barclay, Petocz and McMillan-Price 7 , Reference Livesey, Taylor and Hulshof 47 ).
Acknowledgements
We are grateful to Klaus Englyst (Englyst Carbohydrates-Research & Services Limited, Southampton, UK) for his collaboration in determining the CHO digestibility profile of the cereal products; Jennie Brand-Miller (Sydney University, Australia) for her advices related to GI tests.
The work described in this article was sponsored by Mondelez France R&D SAS (6 Rue René Razel – Bâtiment K, 91400 Saclay, France).
A. M., A. G. and S. V. are employees of the Nutrition Department, Mondelez International R&D; O. B. is owner of Statistique Industrielle KHI2 Consulting (KSIC), Esches, France; F. A. is an employee of the Human Nutrition Unit of the University of Sydney.
The authors' responsibilities are as follows: A. M., A. G. and S. V. designed the research (project conception, development of overall research plan, and study oversight); F. A. was in charge of the GI tests; A. M., A. G. and S. V. discussed the results and planned the manuscript; O. B. and A. M. performed the statistical analyses. A. M., A. G., F. A., O. B. and S. V. edited and approved the final manuscript.
A. M., A. G. and S. V. are employees of Mondelez France R&D SAS. O. B. received payment from Mondelez France R&D SAS for performing the statistical analyses. O. B. and F. A. declare that they have no known conflicts of interest.









