Maternal obesity is a major risk factor for adverse short- and long-term health outcomes for both mother and child, and is on the rise globally(Reference Abarca-Gómez, Abdeen and Hamid1,Reference Godfrey, Reynolds and Prescott2) (Fig. 1). In England, more than half of all women live with overweight (31 %) or obesity (30 %), with only half of women of childbearing age with BMI within the normal range(3). The prevalence of maternal obesity in early pregnancy in England has doubled from 8 to 16 % between 1989 and 2007, while starting pregnancy within the normal weight range declined by 12 % from 66 to 54 %(Reference Heslehurst, Rankin and Wilkinson4). Women from deprived backgrounds and those who are multiparous are at particular risk of starting their pregnancy with obesity(Reference Heslehurst, Rankin and Wilkinson4,Reference Ziauddeen, Roderick and Macklon5) . A recent systematic review of seventy-nine studies found that children born to mothers with obesity before pregnancy are more likely to develop childhood obesity (OR 3⋅64, 95 % CI 2⋅68, 4⋅95)(Reference Heslehurst, Vieira and Akhter6). Maternal obesity is consistently found to be a key predictor for the risk of childhood obesity(Reference Ziauddeen, Roderick and Macklon7).
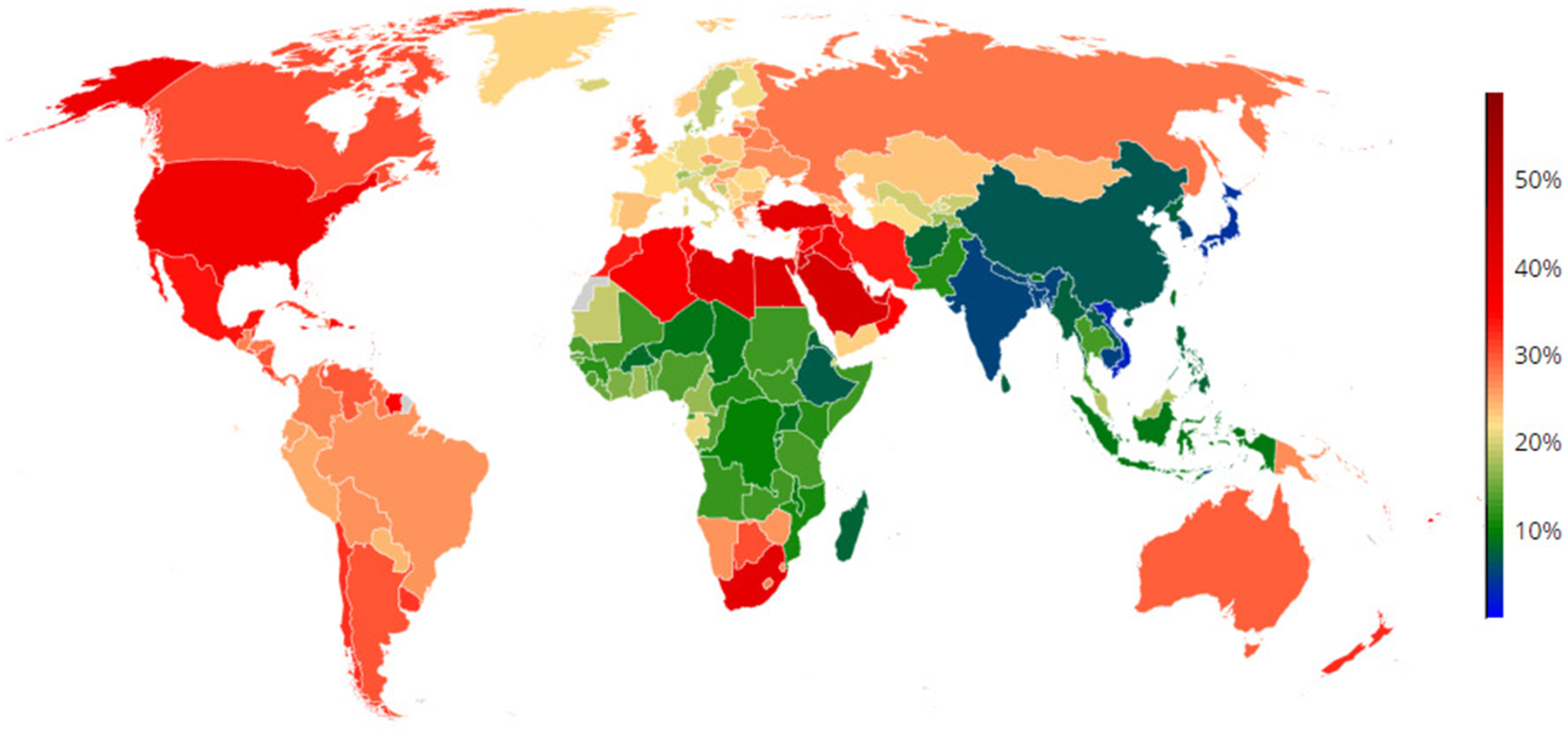
Fig. 1. (Colour online) Global prevalence of obesity in women (≥30 kg/m2) in 2016(Reference Abarca-Gómez, Abdeen and Hamid1).
Childhood obesity is a global public health problem on the rise(Reference Abarca-Gómez, Abdeen and Hamid1) (Fig. 2). Worldwide between 1980 and 2013, the proportion of children or adolescents with overweight and obesity has substantially increased, with just under a quarter of all children in high-income countries and about 13 % in low- and middle-income countries overweight or obese(Reference Ng, Fleming and Robinson8). In 2016, 50 million girls and 74 million boys worldwide were obese(Reference Abarca-Gómez, Abdeen and Hamid1). About one in five children in the final year of primary school, and one in ten of those entering primary school in England live with obesity, with those living in the most deprived areas having double the prevalence of obesity(9). Children with overweight or obesity in early life are over four times more likely to also have overweight or obesity at age 15 years(Reference Yoshida, Kimura and Noda10). Childhood obesity has adverse effects on cardiovascular structure and function, with an increased lifetime risk of CVD(Reference Ayer, Charakida and Deanfield11).

Fig. 2. (Colour online) Trends in the prevalence of childhood obesity (a) boys (b) girls (1975–2016)(Reference Abarca-Gómez, Abdeen and Hamid1).
Source: http://ncdrisc.org/obesity-population-stacked-ado.html.
There is abundant evidence supporting the developmental origins of obesity, with it being influenced by maternal behavioural and environmental experiences during and before pregnancy(Reference Gluckman, Buklijas, Hanson and Rosenfeld12). Findings based on the developmental origins of health and disease paradigm can help shape the early prevention agenda of major public health problems such as obesity. However, how and when to intervene are still open questions. Maternal nutrition during pregnancy influences offspring metabolic health outcomes through lasting effects on offspring organ development, physiology and metabolic function(Reference Godfrey and Barker13). Transient environmental influences may permanently alter gene expression through durable changes in epigenomic features (e.g. DNA methylation, histone modification)(Reference Waterland and Michels14). These can even be induced by preconception exposures(Reference Roseboom, de Rooij and Painter15). The available evidence from both human and animal research supports the importance of the periconceptional period as a critical time shaping the later risk of chronic disease in the offspring(Reference Fleming, Watkins and Velazquez16). Hence, optimising health and wellbeing of women of reproductive age in the preconception period is essential(Reference Barker, Dombrowski and Colbourn17).
The global fertility rate is just under 2⋅5 children per woman(Reference Roser18). Most women in England and Wales have two or more children in their lifetime (63 %). This includes 37 % with two, 16 % with three and 10 % with four or more(19). The interpregnancy interval (IPI) is the interval between the birth of a child to the conception of the next child and thus is the preconception period for the next child. It provides a major opportunity for intervention to improve later health outcomes for the mother and the whole family, as this is a period with relatively extensive contact with professionals within the health and care systems, as well as it being relatively short (< 2 years) for a large proportion of women(Reference Ziauddeen, Roderick and Macklon5). This is a critical time to introduce interventions that support mothers to achieve or maintain a healthy weight in preparation for their next pregnancy.
Little research has been done on maternal weight gain between pregnancies and how it is linked to lifecourse obesity and its predictors in the offspring. Our research using anonymised healthcare data of 19 362 women with at least two consecutive births between 2003 and 2018 from the Studying Lifecourse Obesity Predictors (SLOPE) study in Hampshire, South of England showed that 48 % of women gained ≥1 kg/m2 between their first and second pregnancy with 20 % gaining ≥3 kg/m2. Twenty per cent of women presented to the first antenatal care appointment of their second pregnancy overweight and obese having gained weight from their first pregnancy to the higher BMI category. A similar pattern was seen for higher order pregnancies with 19–22 % of women gaining weight to become overweight or obese by the subsequent pregnancy(Reference Ziauddeen, Roderick and Macklon5).
More mothers who gained ≥3 kg/m2 between pregnancies were obese (48 %) at the start of their second pregnancy compared with 16 % of women who gained 1–3 kg/m2, and 9 % of women who remained weight stable. The average first trimester BMI in those who gained ≥3 kg/m2 was 31 kg/m2, compared to 24 kg/m2 in those who lost weight or remained weight stable between their first two live pregnancies(Reference Ziauddeen, Wilding and Roderick20). Over the 15-year period of the study, the prevalence of overweight and obesity at the start of both first and second pregnancies increased, with a decline in the proportion of women starting their pregnancy within the normal BMI range. Overweight and obesity also increased with higher order pregnancies with 13 % obese at the start of the first pregnancy compared to 32 % obese at the start of the fifth pregnancy(Reference Ziauddeen, Roderick and Macklon5).
Women who gained weight between pregnancies were more likely to be unemployed with lower educational attainment, and to be smokers(Reference Ziauddeen, Roderick and Macklon5,Reference Ziauddeen, Wilding and Roderick20) . The average IPI between the first and second pregnancy was 23 months, with 47–52 % of women having an IPI of <2 years. An IPI of 12–23 months was associated with significantly lower risk (adjusted relative risk 0⋅91, 99 % CI 0⋅87, 0⋅95), and an IPI of ≥36 months with significantly greater risk (adjusted relative risk 1⋅11, 99 % CI 1⋅07, 1⋅15), of starting the second pregnancy with a higher body weight compared to an IPI of 24–35 months(Reference Ziauddeen, Roderick and Macklon5).
We will review the epidemiological evidence linking interpregnancy weight change with pregnancy complications and lifecourse obesity predictors for the offspring in the following sections.
Interpregnancy weight change and size at birth
Large-for-gestational age (LGA) birth is defined as >90th percentile weight for gestational age and small-for-gestational age (SGA) is defined as <10th percentile weight for gestational age(21). The incidence of LGA has increased over time in high-income countries(Reference Kramer, Morin and Yang22,Reference Surkan, Hsieh and Johansson23) . Both LGA birth and SGA birth followed by ‘catch-up’ growth carry an increased risk of later obesity(Reference Yu, Han and Zhu24–Reference Ong26). Maternal pre-pregnancy underweight has been linked to an increased risk of SGA birth, and maternal pre-pregnancy overweight and obesity to LGA birth(Reference Yu, Han and Zhu27).
In a US study with 51 086 women, subsequent born infants of women who returned to their pre-pregnancy weight before the next conception weighed less on average than infants of women who retained or gained weight between pregnancies(Reference Hinkle, Albert and Mendola28). In a UK study, women who lost at least 6 kg between their first and second pregnancy had a smaller average increase in birthweight (48 (sd 581) g) of the second baby compared to women who gained 10 kg or more (209 (sd 600) g; in a 1⋅60 m tall woman, 6 kg equates to approximately 2⋅3 kg/m2 and 10 kg to approximately 3⋅8 kg/m2)(Reference Wilcox, Chang and Johnson29).
Our research using the SLOPE population-cohort data showed that the proportion of LGA births was significantly higher in women with an interpregnancy BMI gain of ≥3 kg/m2 (16 %) compared to women who lost weight (12 %) and those who remained weight stable (12 %) between pregnancies. Women with overweight at the start of their first pregnancy who lost ≥1 kg/m2 had a reduced risk of recurrent LGA (adjusted relative risk 0⋅69, 95 % CI 0⋅48, 0⋅97) in their second pregnancy after having an LGA birth in their first. Women who were within the normal weight range at the start of their first pregnancy and gained 1–3 kg/m2 in the IPI, as well as women in both the normal weight and the overweight range who gained ≥3 kg/m2 between pregnancies had an increased risk of LGA birth in their second pregnancy after a non-LGA birth in the first(Reference Ziauddeen, Wilding and Roderick20).
In a population-based cohort of 146 227 women in the USA, women were found to be at an increased risk of LGA birth in the second pregnancy if pre-pregnancy BMI category increased towards overweight or obese between their first and second pregnancies. This applied to all first pregnancy BMI categories, except underweight women who gained weight and became normal weight by the start of their second pregnancy. Overweight and obese women who dropped the BMI category by their second pregnancy had a lower risk compared to women whose BMI category increased between pregnancies but still remained at an increased risk of LGA birth(Reference Getahun, Ananth and Peltier30). However, weight change is likely to be variable as women at the upper end of a BMI category will move up to the higher BMI category after gaining a small amount of weight whereas women at the lower end of a BMI category need to gain a substantial amount of weight to move up to the same higher BMI category and vice versa to lose weight and move down BMI categories.
In a population-based cohort of 151 080 women in Sweden, 5943 women had a LGA birth in the second pregnancy after excluding 2847 women who had a LGA birth in the first pregnancy. The risk of LGA birth in the second pregnancy showed an increase with a weight gain of 1–2 kg/m2 and a progressive increase in risk with an increase in BMI. The association between weight change and outcome of LGA in the second pregnancy was stronger in women with a healthy first pregnancy BMI (<25 kg/m2)(Reference Villamor and Cnattingius31). In 10 444 obese women in the USA, interpregnancy weight gain of ≥2 kg/m2 was associated with an increased risk of LGA and a weight loss of ≥ 2 kg/m2 was associated with decreased risk compared to the reference group of weight maintained between two BMI units. The analysis was adjusted for LGA birth in previous pregnancy in addition to other confounders.
Analysis of interpregnancy weight change between first and second pregnancies in 12 740 women in Aberdeen, Scotland found an increased risk of SGA and a decreased risk of LGA with between-pregnancy weight loss of >1 kg/m2 and an increased risk of LGA with modest (1–3 kg/m2) and large (≥3 kg/m2) weight gain. The effect remained in both categories on stratification by BMI (< or ≥25)(Reference Wallace, Bhattacharya and Campbell32). Analysis by the same group examined the risk of recurrent SGA and LGA (occurring in both first and second pregnancies) in relation to maternal weight change between pregnancies(Reference Wallace, Bhattacharya and Campbell33). The study included 24 520 women of which 706 women had SGA births and 813 women had LGA births in both pregnancies. Interpregnancy weight loss (≥2 kg/m2) was associated with an increased risk of recurrent SGA, while weight gain (≥2 kg/m2) was protective in women with BMI <25 kg/m2 at first pregnancy. Interpregnancy weight gain (≥2 kg/m2) was associated with an increased risk of recurrent LGA, while weight loss (≥2 kg/m2) was protective. Women with BMI <25 kg/m2 were at an increased risk of recurrent LGA on gaining weight whereas women with BMI ≥25 kg/m2 were at a reduced risk of recurrent LGA on losing weight(Reference Wallace, Bhattacharya and Campbell33). Association between interpregnancy weight loss and increased SGA risk in the second pregnancy was also observed in a population-based case–control study, and a sample of obese women with a weight loss of ≥8 kg/m2(Reference Jain, Gavard and Rice34,Reference Cheng, Bommarito and Noguchi35) .
Three systematic reviews and meta-analyses have examined the association between interpregnancy weight change and size at birth(Reference Oteng-Ntim, Mononen and Sawicki36–Reference Timmermans, van de Kant and Oosterman38). The number of studies included in the meta-analysis varied between them, with Teulings et al. including three, Oteng-Ntim et al. including four and Timmermans et al. including six. Two of the six studies included in the meta-analysis categorised weight change differently (<2, −2 to 2 and >2 kg/m2) to the remaining four studies so these were analysed separately. Two studies were published in 2019(Reference Ziauddeen, Wilding and Roderick20,Reference Benjamin, Ethen and Canfield39) but only the analysis conducted by our group was additionally included in both meta-analyses(Reference Ziauddeen, Wilding and Roderick20). Heterogeneity was identified across the studies with different outcome definitions and differences in categorisation. Confounders adjusted for varied across the studies with only two studies adjusting for gestational diabetes (GDM) in the pregnancy which is a key risk factor for LGA birth. All studies were conducted in high-income countries so generalisability remains limited.
All three meta-analyses showed a reduction in the risk of LGA birth with a weight loss of >1 kg/m2 having an estimated reduction in the risk of LGA in the subsequent pregnancy of 20–30 % (Table 1). An increase in risk with a weight gain of 1–3 kg/m2 was identified in two of the meta-analyses. Weight gain of >3 kg/m2 was associated with the highest risk of LGA birth in the subsequent pregnancy, with an estimated increase of 54–85 %. On stratification by BMI at the beginning of the first pregnancy (< and ≥25 kg/m2), women of BMI <25 kg/m2 were at a higher risk of LGA birth in the second pregnancy if they gained ≥3 kg/m2 compared to women with BMI ≥25 kg/m2. A similar trend was observed in women who gained >1 kg/m2(Reference Oteng-Ntim, Mononen and Sawicki36,Reference Teulings, Masconi and Ozanne37) .
Table 1. Summary of the three meta-analyses of interpregnancy weight change and adverse pregnancy outcomes.

*Reference weight category in all the systematic reviews was −1 to 1 kg/m2.
†Results are not presented for the 1–2 and 2–3 kg/m2 weight change categories as this was not part of the meta-analysis and presented the results of one study.
‡Teulings et al. additionally calculated estimates for weight gain >1 kg/m2 for four outcomes (LGA, GDM, pre-eclampsia and gestational hypertension) which are not presented in this table.
Two meta-analyses examined the association between interpregnancy weight change and the risk of SGA. There was a 31–58 % increased risk of SGA birth on weight loss of >1 kg/m2 but only one meta-analysis found a significant decrease in risk (17 %) with interpregnancy weight gain. Studies included in the meta-analysis were different as one was a newly published study(Reference Benjamin, Littlejohn and Canfield40), and the other study was a publication utilising the same data as a later publication by the same team deemed to be of equal quality by the reviews but larger sample size(Reference Wallace, Bhattacharya and Campbell32,Reference Wallace, Bhattacharya and Campbell33) . The inclusion criteria laid out by the reviewers stated that the study with the larger sample size would be included in cases where studies reported data from overlapping study populations.
To summarise, gaining weight between pregnancies is associated with an increased risk of LGA birth, and losing weight is associated with an increased risk of SGA birth; however, baseline BMI at the start of the first pregnancy is an important effect modifier in this relationship. Interpregnancy weight loss in women with overweight or obesity seems to be linked with the favourable outcome of reducing the risk of LGA birth in the second pregnancy.
Interpregnancy weight change and preterm birth
Preterm birth is a leading cause of death and morbidity worldwide(Reference Althabe, Howson and Kinney41,Reference Liu, Oza and Hogan42) . It is a risk factor for later offspring overweight and obesity(Reference Rito, Buoncristiano and Spinelli43), potentially through the infant being SGA(Reference Gaskins, LaGasse and Liu44) and/or through underdevelopment of the infant gut microbiome(Reference Groer, Luciano and Dishaw45). Preterm birth can be spontaneous or indicated. The causes of preterm birth are numerous and, in places, not well understood(Reference Goldenberg, Culhane and Iams46). Maternal underweight and overweight are known risk factors for spontaneous preterm birth, and maternal obesity is a risk factor for indicated preterm birth(Reference Goldenberg, Culhane and Iams46,Reference Smith, Draper and Manktelow47) .
Whilst it is clear that maternal weight affects the risk of preterm birth, the impact of maternal weight change between pregnancies on preterm birth is less clear, due in part to a paucity of research. The mechanisms that may underlie this association may include poor maternal health(Reference Gravett and Rubens48), maternal undernutrition(Reference Goldenberg49), maternal infection and inflammation(Reference Goldenberg, Culhane and Iams46,Reference Goldenberg49) , poor placental function(Reference Blencowe, Cousens and Oestergaard50) and obesity-related co-morbidities(Reference Villamor and Cnattingius51).
The evidence describing the association between interpregnancy weight change and preterm birth is limited. The vast majority of published studies, if not all, are based in high-income countries, such as USA(Reference Benjamin, Littlejohn and Canfield40,Reference Whiteman, Rao and Duan52–Reference Bender, Hirshberg and Levine58) , the UK(Reference Wallace, Bhattacharya and Campbell32,Reference Wallace, Bhattacharya and Campbell33,Reference Wallace, Bhattacharya and Horgan59,Reference Grove, Ziauddeen and Harris60) , Australia(Reference McBain, Dekker and Clifton61) and Sweden(Reference Villamor and Cnattingius51). The rate of preterm birth across these countries differs, with rates per 100 live births of 12⋅0 in America, 7⋅8 in the UK, 7⋅6 in Australia and 5⋅9 in Sweden, compared to 11⋅1 worldwide(Reference Althabe, Howson and Kinney41). The studies also vary in size with the larger studies(Reference Villamor and Cnattingius51–Reference Riley, Carmichael and Mayo53) more likely to detect statistically significant associations.
Our work, using the SLOPE birth cohort included 14 961 women with first and second live births, and 5108 women with second and third live births. We found that women who were in the normal BMI category at booking for their first pregnancy, and had lost >3 kg/m2 by the start of their next pregnancy, were at increased risk of preterm birth (adjusted OR 3⋅50, 95 % CI 1⋅78, 6⋅88). This association was also evident when examining spontaneous preterm births alone (adjusted OR 3⋅34, 95 % CI 1⋅60, 6⋅98), but not when considering indicated preterm births. There was no increased risk of preterm birth associated with weight loss in women who were in the overweight or obese category at the start of their first pregnancy(Reference Grove, Ziauddeen and Harris60).
Additionally, women who lost >3 kg/m2 between their second and third pregnancies were at an increased risk of preterm birth in the third pregnancy, regardless of starting BMI. This association was not significant when looking at subgroups split by starting BMI at the second pregnancy, although it is possible that the analysis was underpowered to detect differences in these subgroups(Reference Grove, Ziauddeen and Harris60). Only one other study explored interpregnancy weight change and preterm birth across more than one IPI. Wallace et al. (n 5079, Scotland) found no significant associations between weight change and spontaneous preterm birth across the first three pregnancies(Reference Wallace, Bhattacharya and Horgan59).
Villamor and Cnattingius's large Swedish cohort (n 465 836) considered both spontaneous and indicated preterm birth separately as well as considering the grade of preterm birth(Reference Villamor and Cnattingius51). They report that normal weight women who gain (>4 kg/m2) or lose (>2 kg/m2) weight are at an increased risk of moderate spontaneous preterm birth. They also report that weight gain is associated with increased indicated preterm birth. However, the evidence of association between weight gain and indicated preterm birth disappears after removing those with obesity-related co-morbidities from the analysis. In contrast, a Whiteman et al. USA study (n 398 950) found that normal weight women who gained weight (moved from normal to overweight or obese category) were at reduced risk of spontaneous preterm birth. They also report that normal weight women who gain weight are at increased risk of indicated preterm birth, and those who lose weight and become underweight are at risk of both spontaneous and indicated preterm birth(Reference Whiteman, Rao and Duan52). Benjamin et al. also found a statistically significant increase in odds of preterm birth in women who lost >1 kg/m2 and in normal weight women who lost any weight between pregnancies, but no association between weight loss and preterm birth in women who were overweight or obese(Reference Benjamin, Littlejohn and Canfield40).
In their US-based study, Riley et al. (n 75 970) found that gaining weight was protective against spontaneous preterm birth, in underweight women and overweight women, as was remaining obese(Reference Riley, Carmichael and Mayo53), though smoking status does not appear to have been accounted for, with it potentially being a strong confounder(Reference Jaddoe, Troe and Hofman62–Reference Canoy, Wareham and Luben64). Wallace et al. (n 12 740, Scotland) also excluded indicated preterm births and found that weight loss was associated with preterm birth, whilst weight gain was protective(Reference Wallace, Bhattacharya and Campbell32). In contrast, McBain et al. (n 5 371, Australia) included both indicated and spontaneous preterm births and found that, amongst overweight women, gaining weight was associated with preterm birth(Reference McBain, Dekker and Clifton61). Analysing both indicated and spontaneous preterm birth together may have diluted any association, as maternal weight seems to impact spontaneous and indicated preterm birth differently. Hoff et al. focused exclusively on women who were overweight at first pregnancy, and found no association between weight change and preterm birth(Reference Hoff, Cai and Okah55).
Three studies have considered recurrent preterm birth. Merlino et al. found a weight loss of ≥5 kg/m2 was associated with an increased risk of recurrent preterm birth(Reference Merlino, Laffineuse and Collin54). Wallace et al. found no significant associations between weight change and recurrent spontaneous preterm birth(Reference Wallace, Bhattacharya and Campbell33). Girsen et al. considered recurrent preterm birth in women who were underweight, and found that remaining underweight or losing more weight was associated with recurrent preterm birth(Reference Girsen, Mayo and Wallenstein56).
Overall, the available evidence seems to indicate that interpregnancy weight change may be associated with preterm birth. The mechanisms are unclear. It may be that the associations seen are in fact due to unmeasured confounders, such as poor health or stress, which increase the likelihood of both weight loss and preterm birth(Reference Gravett and Rubens48,Reference Kivimäki, Head and Ferrie65) . Weight loss could lead to normal weight women becoming underweight, a risk factor for preterm birth in itself(Reference Goldenberg, Culhane and Iams46,Reference Hendler, Goldenberg and Mercer66) . Other mechanisms associated with weight loss could include micro and macro nutrient deficiencies(Reference Hendler, Goldenberg and Mercer66–Reference Jans, Matthys and Bogaerts68), which may result in poor placental function(Reference Wallace, Bhattacharya and Campbell32), insufficient nutrients for the growing fetus(Reference Neggers and Goldenberg67) or an increased risk of infection(Reference Goldenberg, Culhane and Iams46,Reference Gravett and Rubens48) . Overall, associations between weight gain and indicated preterm birth were attenuated after adjusting for confounders. One possible explanation is that the comorbidities associated with increased BMI, such as GDM, hypertension and pre-eclampsia, are the main driver of this association, rather than the weight change, as supported by evidence when those with obesity-related comorbidities were excluded from the analysis(Reference Villamor and Cnattingius51).
Interpregnancy weight change and childhood obesity
Maternal obesity is an important risk factor for childhood obesity(Reference Heslehurst, Vieira and Akhter6). It is postulated that pre-pregnancy obesity, gestational weight gain and glucose intolerance are all involved in the in utero programming of adipose tissue(Reference Howie, Sloboda and Kamal69,Reference Desai, Beall and Ross70) . A study by Lawlor et al. analysed 3340 parent–offspring trios and found that at age 14 years, each standard deviation increase in maternal BMI was associated with an increase in offspring BMI of 0⋅4 sd. This was higher than the corresponding result for an sd increase in paternal BMI; 0⋅2 sd, supporting the fetal overnutrition hypothesis that maternal adiposity programmes offspring adiposity later in life(Reference Lawlor, Smith and O'Callaghan71). Children born to mothers with a normal pre-pregnancy BMI tend to have lower fat mass and body fat per cent than those born to mothers with a BMI in the overweight or obese range prior to pregnancy (standardised mean differences for body fat per cent (0⋅31 %, 95 % CI 0⋅19, 0⋅42), fat mass (0⋅38 kg, 95 % CI 0⋅26, 0⋅50) and fat-free mass (0⋅18 kg, 95 % CI −0⋅07, 0⋅42))(Reference Castillo-Laura, Santos and Quadros72). A recent systematic review and meta-analysis which pooled data from twenty studies (n 88 872 children aged between 1 and 14 years) confirmed the association between pre-pregnancy overweight and obesity with childhood obesity(Reference Heslehurst, Vieira and Akhter6). The odds of childhood obesity, overweight/obesity and overweight were all increased with maternal obesity (OR 3⋅64, 95 % CI 2⋅68, 4⋅95; OR 2⋅69, 95 % CI 2⋅10, 3⋅46; and OR 1⋅80, 95 % CI 1⋅25, 2⋅59, respectively) and the odds of childhood obesity were also increased with maternal overweight (OR 1⋅89, 95 % CI 1⋅62, 2⋅19)(Reference Heslehurst, Vieira and Akhter6).
A limited number of studies have examined the association between interpregnancy weight change and childhood obesity. A study in Australia found that in a sample of 714 sibling pairs, high interpregnancy weight gain, defined as an increase of ≥ 4 kg/m3, increases the odds of a second-born child being affected by obesity (adjusted OR 2⋅20, 95 % CI 1⋅02, 4⋅75) compared to women who remained weight stable between pregnancies. Aside from interpregnancy weight change, Adane et al. also derived preconception weight trajectories and found a strong dose–response between these trajectories and overweight/obesity in children, with a strong association between ‘chronically overweight’ and ‘chronically obese’ maternal BMI trajectories with the risk of childhood obesity (n 2733)(Reference Adane, Dobson and Tooth73).
Similarly, whilst Aucott et al. focus on interpregnancy changes in smoking behaviour, they also reported an increase in child BMI z-score (β = 0⋅13, 95 % CI 0⋅05, 0⋅20) where the interpregnancy weight change was 10 % or more (n 6580 children and 5862 mothers)(Reference Aucott, Bhattacharya and McNeill74). Conversely, Wilmer et al. examined interpregnancy weight loss due to bariatric surgery undertaken between pregnancies. In a small sample of seventy-one sibling pairs, where one sibling was born before surgery and one after, they found no association between interpregnancy differences in early pregnancy maternal BMI and differences in siblings' BMI at age 4 years. Their study was also unable to show any reduction in the prevalence of overweight or obesity between children born before or after surgery and the group of 10-year-old girls who were born after surgery showed higher rates of obesity. The authors note that more girls than boys were born SGA (20 % compared to 10 %) after surgery which may explain this increased prevalence amongst the girls(Reference Willmer, Berglind and Sørensen75).
Preliminary analysis of the SLOPE study data linked to childhood BMI measurements at 4–5 years of age (n 6358) showed a prevalence of second child overweight/obesity for mothers with ≥ 3 kg/m2 interpregnancy gain of 28 %, compared with 19 % of children of mothers whose weight remained stable between pregnancies (−1 to 1 kg/m2). Interpregnancy gain of ≥ 3 kg/m2 was associated with an increased risk of childhood overweight/obesity; however, the relationship was attenuated on adjusting for birthweight of the second child (adjusted relative risk 1⋅09, 95% CI 0⋅95 to 1⋅25), suggesting that it may be acting as a mediator(Reference Ziauddeen and Alwan76).
In summary, there is limited epidemiological evidence that there is a link between maternal interpregnancy weight gain and increased risk of childhood obesity. However, an analysis that properly accounts for the complex relationships between the main exposure of maternal weight change, the outcome and the various time-varying confounders and mediators is needed to establish causality.
Interpregnancy weight change and pregnancy complications
Pregnancy complications such as GDM, pre-eclampsia and gestational hypertension, as well as caesarean section may mediate the relationship between interpregnancy weight gain and childhood obesity. GDM is associated with offspring obesity, potentially independently of maternal adiposity(Reference Nehring, Chmitorz and Reulen77,Reference Woo Baidal, Locks and Cheng78) . There is also evidence that pre-eclampsia predisposes to an increased risk of excess weight gain in the offspring(Reference Davis, Lazdam and Lewandowski79). Birth by caesarean section has been associated with an increased risk of later childhood obesity compared to vaginal birth(Reference Masukume, McCarthy and Russell80), although the evidence is conflicting(Reference Masukume, Khashan and Morton81). The gut microbiome of an infant is affected by delivery method, and compared to infants born vaginally, those born by caesarean section have reduced gut microbiome diversity(Reference Sandall, Tribe and Avery82). A recent study found evidence of a sequential mediation pathway between bacteria in the infant gut and mode of birth and childhood overweight/obesity. Different genera of Lachnospiraceae were found in the guts of infants born vaginally and by caesarean section and were more abundant in infants whose mothers were overweight(Reference Tun, Bridgman and Chari83). The odds of a child being overweight at age 1 year for those delivered by caesarean section to mothers who were overweight compared to those born vaginally to a woman of normal weight were higher (adjusted OR 5⋅02, 95 % CI 2⋅04, 12⋅38) as were the odds for a child born vaginally to an overweight/obese mother, compared to a vaginal birth to a mother of normal weight (adjusted OR 3⋅33, 95 % CI 1⋅49, 7⋅41)(Reference Tun, Bridgman and Chari83).
Three meta-analyses have been carried out on the association between interpregnancy weight change and the risk of GDM in the second pregnancy(Reference Oteng-Ntim, Mononen and Sawicki36–Reference Timmermans, van de Kant and Oosterman38). Women who gained weight between pregnancies were at increased risk of GDM in the second pregnancy, with women who gained ≥3 kg/m2 having the highest risk(Reference Oteng-Ntim, Mononen and Sawicki36–Reference Timmermans, van de Kant and Oosterman38). Women with BMI <25 kg/m2 at the start of their first pregnancy and experienced an interpregnancy weight gain of ≥3 kg/m2 are at higher risk of developing GDM compared to women with BMI ≥25 kg/m2(Reference Oteng-Ntim, Mononen and Sawicki36,Reference Teulings, Masconi and Ozanne37) . A similar pattern to the association between interpregnancy weight gain and GDM was observed for the risk of pre-eclampsia and gestational hypertension. Two meta-analyses considered pre-eclampsia as an outcome and included the same studies in the meta-analysis(Reference Teulings, Masconi and Ozanne37,Reference Timmermans, van de Kant and Oosterman38) . Gestational hypertension was only considered as an outcome in one meta-analysis(Reference Teulings, Masconi and Ozanne37). Moderate and substantial interpregnancy weight gain was also found to be associated with an increased risk of caesarean section in the second pregnancy. Women of BMI <25 kg/m2 at the beginning of the first pregnancy were at an increased risk of caesarean section if they gained weight by the start of their second pregnancy(Reference Teulings, Masconi and Ozanne37,Reference Timmermans, van de Kant and Oosterman38) .
What next?
The epidemiological evidence reviewed earlier gives some support to a relationship between interpregnancy weight change and adverse outcomes, including birth size and childhood obesity. More research is definitely needed using robust analysis methods and adequate study samples, particularly using the definitive outcome of offspring weight in childhood and adulthood. This is particularly needed given that there is more evidence supporting the importance of maternal pre-pregnancy and early pregnancy metabolic status in programming early placenta function and gene expression before and in the first trimester of pregnancy as opposed to later pregnancy exposures and interventions(Reference Catalano and deMouzon84). Preconception and interconception interventions to optimise maternal weight need to be tested. A recent systematic review of information and communication technology-based interventions to support postpartum women achieve a healthy lifestyle and weight control concluded that studies need larger sample sizes and longer follow-up of outcomes to establish effectiveness(Reference Christiansen, Skjøth and Rothmann85).
Interventions delivered by health professionals postpartum also offer an opportunity to optimise preconception health for the next pregnancy given the relatively intensive contact mothers and their families/partners have with healthcare during that period. Although there have been numerous trials of such interventions, those which demonstrate effectiveness do so mostly on behavioural or intermediate outcomes rather than obesity/overweight outcomes(Reference Hennessy, Heary and Laws86). One thing we must be wary of with such informational or behavioural interventions if delivered universally is their tendency to widen the already existing socioeconomic and ethnic inequalities in obesity and its complications by differential take up. For example, interventions that promote dietary change may be difficult to adhere to in disadvantaged families due to financial constraints making it difficult to afford and maintain a regular healthy diet. Recent UK analysis using the Living Costs and Food Survey and the Family Resources Survey found that 27 % of households would need to spend more than a quarter of their disposable income to meet the Eatwell Guide costs(87), with more than half of these households having at least one child. For households with children in the bottom two income deciles, 42 % of after-housing disposable income would have to be spent to meet the Eatwell Guide costs(Reference Scott, Sutherland and Taylor88).
Conclusion
Evidence shows that weight change between pregnancies shifting maternal BMI to outside the normal range by the start of the next pregnancy is linked to adverse maternal and child health outcomes. Improving preconception health and optimising weight before pregnancy could help to tackle the rise in childhood obesity. The time between consecutive pregnancies is usually a period of change providing an opportunity to focus on the health of the mother as well as the baby, and support her to be better prepared for future pregnancies. Future research into interventions to optimise maternal weight and health during this period is needed, particularly in high-risk and disadvantaged groups.
Financial Support
The original analysis conducted by the authors referenced in this review was funded by grants from the Academy of Medical Sciences/Wellcome Trust and NIHR Southampton Biomedical Research Centre(Reference Ziauddeen, Roderick and Macklon5,Reference Ziauddeen, Wilding and Roderick20,Reference Grove, Ziauddeen and Harris60,Reference Ziauddeen and Alwan76) .
Conflict of Interest
None.
Authorship
The authors had joint responsibility for all aspects of preparation of this paper.





