INTRODUCTION
Globally, norovirus (NoV) are a common cause of viral diarrhoea and vomiting in children <5 years [Reference Ahmed1]. Awareness of NoV has heightened with the improvement of diagnostics to detect the virus and the identification of NoV as an important cause of food-borne illness globally [Reference Patel2, Reference Pires3]. A systematic review of NoV in sporadic gastroenteritis cases conducted in 2014 estimated that NoV was responsible for 18% (95% confidence interval (CI) 15–20) of gastroenteritis among children <5 years [Reference Ahmed1]. A previous review in 2008 estimated that NoV was responsible for 12% (95% CI 10–15) of severe diarrhoea in children <5 years and projected 218 000 deaths in children <5 years in developing countries annually [Reference Patel4].
While NoV infections are generally self-limiting, outcomes can be severe in individuals with comorbidities, extremes of age (<5 and >65 years) or following the emergence of new strains [Reference Koopmans5, Reference Robilotti, Deresinski and Pinsky6]. A study conducted in the USA demonstrated that children <5 years have the highest rates of NoV-associated health care visits and adults ⩾65 years have the greatest risk of death due to NoV [Reference Hall7]. NoV disease in children is generally mild, however, severe outcomes do occur with hospitalization rates between 7·2 and 16 hospitalizations/10 000 population <5 and an estimated 9·9–12% of diarrhoeal deaths annually [Reference Hall7, Reference Lopman8].
Mans et al. recently conducted a review of NoV epidemiology in Africa [Reference Mans9]. While data on NoV prevalence in patients with diarrhoea in sub-Saharan Africa are available, most of these studies have duration of <2 years or <300 specimens available for evaluation. Despite these limitations, the review revealed overall NoV prevalence in individuals with diarrhoea of 13·5% (961/7141; 95% CI 12·7–14·3), with children <1 year mainly affected in African settings [Reference Mans9].
Research on NoV in South Africa (SA) dates back to 1993 [Reference Taylor10] and originally focused on seroprevalence rates in all age groups, which ranged from >50% to >90% [Reference Taylor11, Reference Smit12]. An early virus detection study applied a combination of electron microscopy and recombinant enzyme immunoassays against Norwalk (NV)-like and Mexico (MX)-like NoVs and RT–PCR (reverse transcription–polymerase chain reaction) for the detection of caliciviruses in paediatric stool specimens collected between 1991 and 1995 [Reference Wolfaardt13]. NV-like and MX-like NoVs were detected in 0·2% (3/1296) and 2·2% (29/1296) specimens, respectively [Reference Wolfaardt13].
Between November 2009 and February 2012, the multisite birth cohort study investigating pathogen-specific burdens of community diarrhoea (MAL-ED) included a site in Venda, SA [Reference Rouhani14]. NoV incidence at this site was 9·52 (95% CI 7·06–12·83) NoV GII detected per 100 child-months and 5·09 NoV GI detected per 100 child-months [Reference Rouhani14]. Severity of NoV diarrhoea was comparable with other enteric pathogens detected, except RV, and undernutrition was identified as a risk factor for NoV disease [Reference Rouhani14]. NoV infections peaked in children 6–11 months of age [Reference Rouhani14].
In immunocompromised hosts, NoV infections are reported to be more severe with prolonged symptoms and viral shedding [Reference Robilotti, Deresinski and Pinsky6]. However, the majority of these studies have been conducted in transplant recipients with limited information in HIV-infected individuals [Reference Robilotti, Deresinski and Pinsky6]. Persistent diarrhoea, lasting 7 months, was described in an HIV-infected adult patient with poor compliance to antiretroviral therapy and a chronic NoV infection [Reference Wingfield15]. NoVs were detected more often in HIV-infected compared with HIV-uninfected children [Reference Rodríguez-Guillén16, Reference Cegielski17].
In April 2009, a prospective sentinel surveillance system was established to monitor diarrhoeal disease in hospitalized children <5 years of age in SA. We aimed to describe the epidemiology of NoV between 2009 and 2013.
METHODS
Study participants and sites
Hospitalized children <5 years with acute diarrhoea were enrolled in the sentinel diarrhoea surveillance study. Acute diarrhoea was defined according to the World Health Organization (WHO) definition of ‘three looser than normal stools within a 24 h period’. The sentinel sites, located in three provinces, included: Chris Hani Baragwanath Academic Hospital (CHBAH; 2009–2013), Mapulaneng Hospital (MPH; 2009–2013), Matikwane Hospital (MKH; 2009–2013) and Edendale Hospital (EDH; 2010–2013).
Study enrolment and data collection
Written informed consent was obtained from the parents of patients prior to enrolment. Systematic sampling was used to enrol patients on a daily basis from Monday to Friday between 8 am and 5 pm. Patients who refused to participate in the study were noted, but no additional information was collected. Surveillance officers collected demographic, socio-economic and risk factor data from the parents by interview and additional clinical data from medical records on standardized questionnaires. Data on HIV status of the mother and child were obtained during the interview or review of the medical records. If the status of the child was unknown and the parent gave consent, then a dried blood spot was collected for anonymised HIV testing.
Laboratory screening
A stool specimen was collected for enteric pathogen detection. Nucleic acids were extracted from 10% stool suspensions using the QIAamp® Viral RNA Mini Kit (Qiagen Inc., Valencia, CA) according to the manufacturer's instructions and eluted in 60 µl RNase-free water. Briefly, 10 µl extracted RNA was reverse transcribed with random primers using the Transcriptor First Strand cDNA Synthesis Kit (Roche, Mannheim, Germany) according to the manufacturers’ instructions. Samples were screened for NoV GI and GII in monoplex reactions using 5 µl cDNA, LightCycler® 480 Probe Master Kit (Roche) and primers and probes from published methods [Reference Van Stelten18–Reference Kageyama20]. Screening of other enteric viruses, bacteria and parasites has been described elsewhere [Reference Groome21, Reference Samra22].
A fully screened specimen was defined as a specimen where all viral, bacterial and parasite testing had been performed (n = 1354).
Dried blood spots were screened using the AmpliPrep® (Roche) for automated nucleic acid extraction and COBAS® TaqMan® (Roche) for automated real-time detection of HIV-1.
Data analysis
NoV detection rate was calculated using data from years with 12 months of surveillance (2010–2013). Demographic data, clinical characteristics and environmental features associated with NoV detection were compared in patients with or without NoV, genogroup I (GI) or II (GII) using Stata12 (StataCorp LP, College Station TX). In addition, the characteristics of HIV-infected and HIV-uninfected children were compared in NoV-positive cases. NoV-negative cases were defined as cases where neither NoV GI nor GII strains were detected. Mixed infections were defined as detection of NoV GI or GII strains with any of the following pathogens: RV, human adenovirus (any group), astrovirus, sapovirus (SaV), bocavirus, bacteria (Campylobacter spp., diffusely adherent Escherichia coli, enteroaggregative E. coli, enteroinvasive E. coli, enteropathogenic E. coli, enterotoxigenic E. coli, Salmonella spp. and Shigella spp.) and parasites (Ascaris lumbricoides, Cryptosporidium spp., Entamoeba coli, Giardia lamblia and Isospora belli).
The χ 2 tests were used to determine statistical significance (P⩽0·05) of categorical data, while t tests with unequal variance and Welch's approximation were used for means. A Wilcoxon rank-sum test was used to assess differences between medians. Univariate analysis and stepwise multivariable logistic regression analysis was performed to identify environmental features associated with NoV detection in patients with diarrhoea and a separate analysis restricted to NoV-positive cases compared the characteristics of HIV-infected and HIV-uninfected children. Variables that did not yield statistically significant P-values on univariate analysis were not included in the multivariable models or reported in the tables.
RESULTS
Between 20 April 2009 and 31 December 2013, 3103 children <5 years provided stool samples and were included in the surveillance study. All samples were screened for NoV GI and GII, SaV, human adenovirus, astrovirus and bocavirus. In addition, specimens were screened for RV (99·7%; 3093/3103), bacteria (88·6%; 2748/3103) and parasites (48·5%; 1508/3103).
Overall, 2·6% (79/3019) of children admitted to the sentinel hospitals for the treatment of diarrhoea died over the study period. While 10% (8/79) of these children had NoV detected, only two children had NoV GII strains as the sole pathogen detected. Of the eight children that died, four were HIV infected.
NoV detection
Between 2009 and 2013, NoV was detected in 452/3103 (14·6%) of specimens. The detection rate when only years with 12 months of data were included was 16% (396/2468; 2010–2013). NoV GII strains were detected in 13·4% (330/2468; 95% CI 12·1–14·8) of specimens and NoV GI in 3·4% (84/2468; 95% CI 2·4–3·6) of specimens between 2010 and 2013. An additional 47 GII and 10 GI cases were detected between April and December 2009, resulting in 377 NoV GII and 94 NoV GI cases available for analysis (Supplementary Tables 1 and 2). In fully screened NoV-positive specimens, NoV GII strains were detected as the sole pathogen in 35·4% (61/172) and GI in 23·4% (11/47) of cases. Mixed GII and GI NoV detected in 19 (0·6%; 19/3103) diarrhoea cases.
The median cycle threshold (C t) values in these specimens were compared in single and mixed pathogen NoV cases. The median C t values of single (C t = 27·1; IQR 18·6–31·1) pathogen GII cases were significantly lower than mixed (C t = 30·6; IQR 22·0–33·4) pathogen NoV GII cases (P = 0·005). Similarly, the median C t values of single pathogen GI cases were significantly lower than mixed pathogen NoV GI cases (n = 47; single: C t = 27·2 (IQR 21·0–29·0); mixed: C t = 32·0 (IQR 28·0–33·9); P = 0·008).
NoV epidemiology
NoV detection was higher in children 0–6 months and 7–12 months compared with children older than 24 months (0–6 months adjusted odds ratio (aOR) 2·2; 95% CI 1·4–3·6; P = 0·001; 7–12 months aOR 2·2 95% CI 1·3–3·5; P = 0·002; Table 1). A similar age trend was noted in NoV GII cases (Supplementary Table 1). Unlike NoV GII, the odds of NoV GI detection were higher in the second year of life (19–24 months; aOR 2·3; 95% CI 1·2–4·5; P = 0·011; Supplementary Table 2) compared with the 0–6-month age group (Supplementary Table 2).
Table 1. Univariate and multivariable analysis of demographic data, clinical characteristics and environmental features associated with NoV detection (n = 452)
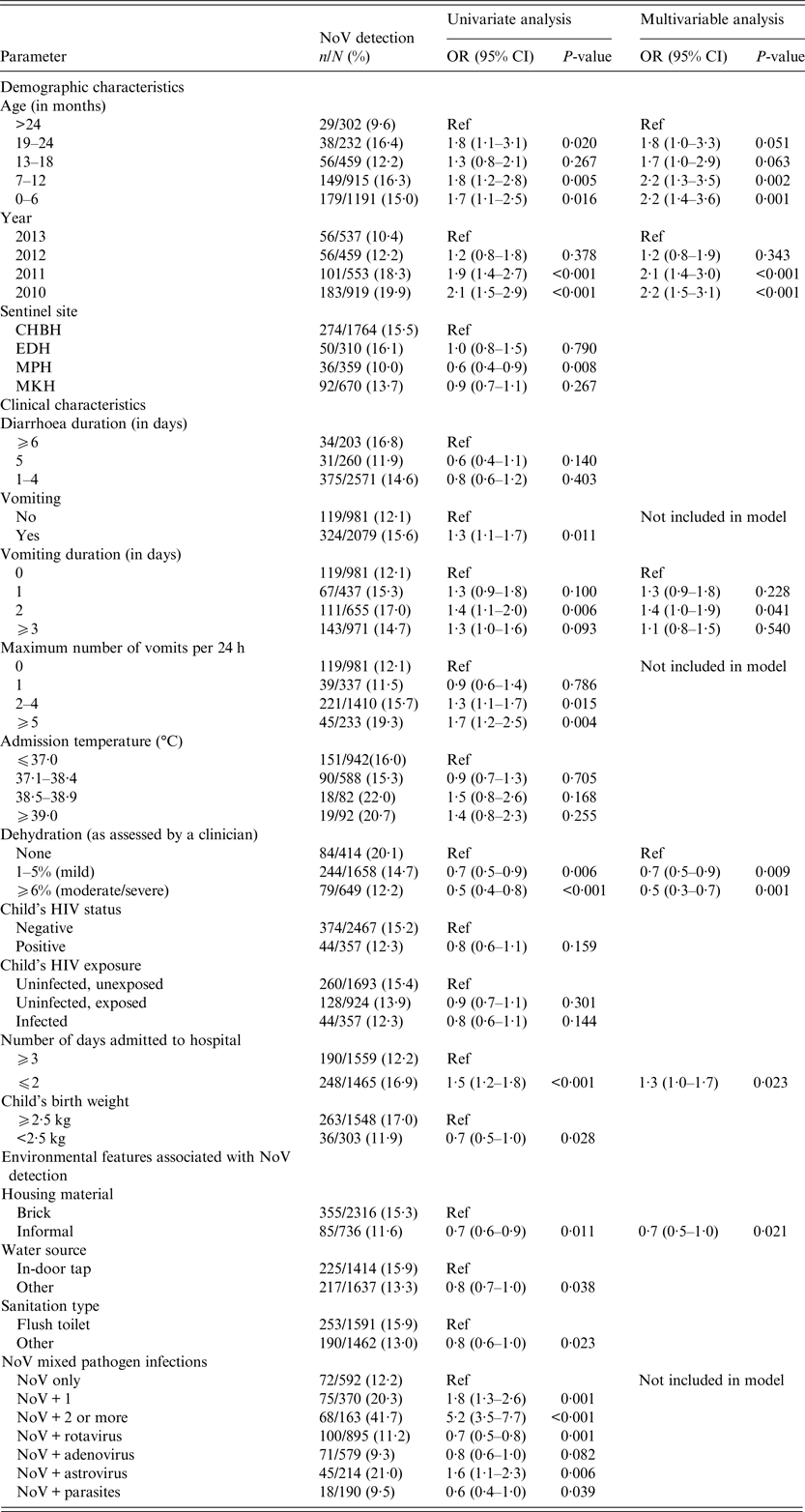
CHBH, Chris Hani Baragwanath Hospital; EDH, Edendale Hospital; MPH, Mapulaneng Hospital; MKH, Matikwane Hospital; HIV, human immunodeficiency virus.
Only variables with P-values <0·2 in the univariate analysis were reported in the table and included in the multivariable model.
NoV strains were frequently detected in 2010 and 2011 compared with 2013 (2010; aOR 2·2 (95% CI 1·5–3·1); P<0·001 and 2011; aOR 2·1 (95%CI 1·4–3·0); P < 0·001; Table 1). This result was echoed in the individual analyses of GII and GI strains although the result was not statistically significant in multivariable analysis of GI (Supplementary Tables 1 and 2). The NoV detection rate by sentinel site was similar ranging from 10·0% in MPH to 16·1% in EDH over the study period (Table 1).
NoV GII were frequently detected from September to December each year (Fig. 1). The percentage-positive NoV GII during this period was 20·5% (163/797) compared with the rest of the year (9·3%; 214/2306; P < 0·001). The monthly detection rate of NoV GI strains in SA was less pronounced than NoV GII although the average detection in late summer (January–April) was 4·3% (43/1009) compared with 2·4% for the rest of the year (May–December; 51/2094; P = 0·01; Fig. 1).
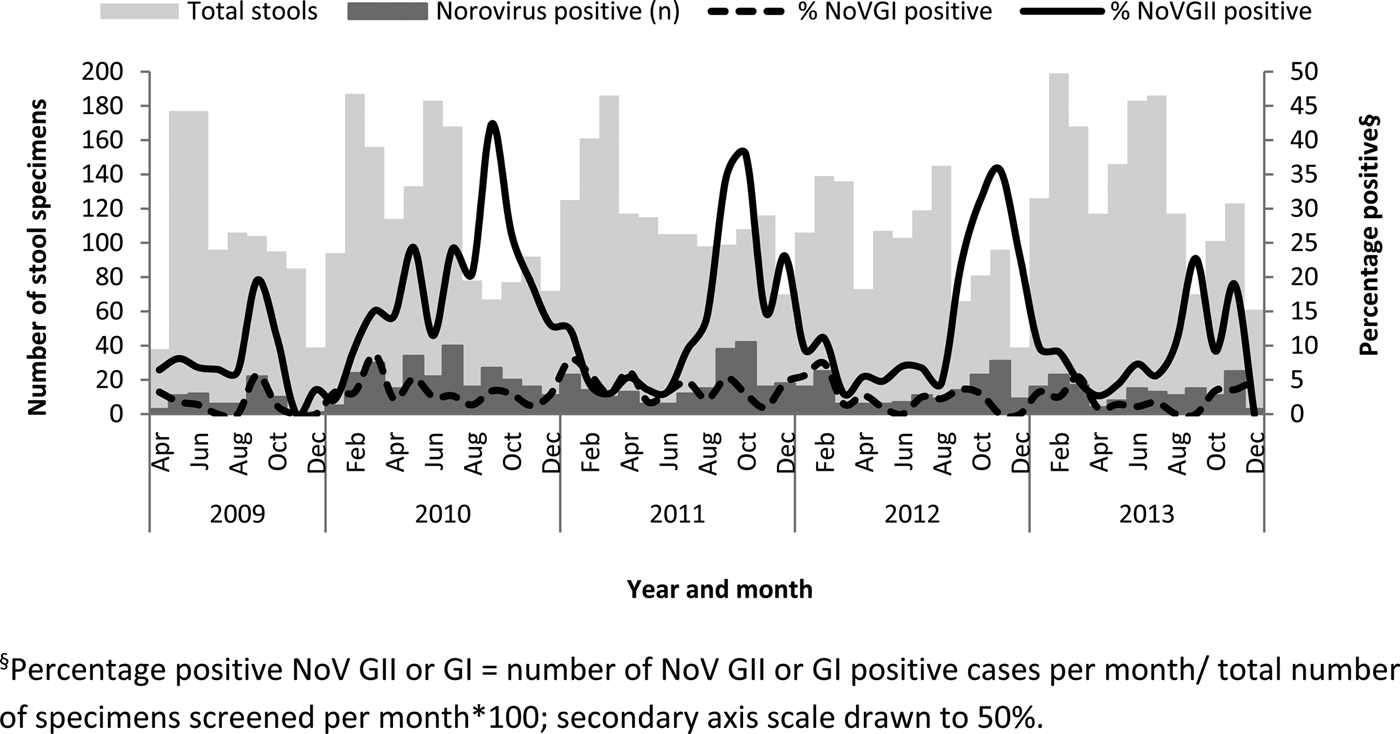
Fig. 1. Total stools tested, number of NoV-positive specimens and the percentage positive for NoV GI and GII by month between 2009 and 2013. §Percentage-positive NoV GII or GI = number of NoV GII- or GI-positive cases per month/total number of specimens screened per month × 100; secondary axis scale drawn to 50%.
Clinical characteristics of NoV infections
Vomiting was more frequently identified in NoV-positive compared with NoV-negative children (OR 1·3; 95% CI 1·1–1·7; P = 0·011; Table 1). NoV GII-positive patients were more likely to report five or more vomiting episodes per day (OR 1·9; 95% CI 1·2–2·8; P = 0·003) and vomiting duration of up to 2 days (aOR 1·8; 95% CI 1·2–2·7; P = 0·005) compared with NoV-negative patients (Supplementary Table 1). However, NoV-positive cases were less likely to be dehydrated when compared with NoV-negative cases (aOR 0·7–0·5; 95% CI 0·3–0·9; P = 0·009; Table 1). Children with NoV detected were admitted to hospital for a median of 2 days (IQR 1–4) compared with 3 days (IQR 1–6) in children without NoV detected (P < 0·001). Low weight at birth (aOR 0·6; 95% CI 0·4–0·9; P = 0·017) compared with normal weight at birth was significantly less common in NoV GII-positive compared with NoV GII-negative patients (Supplementary Table 1).
Environmental features associated with NoV detection
Informal housing compared with brick houses (aOR 0·7; 95% CI 0·5–1·0; P = 0·021; Table 1) was significantly less common in NoV-positive than NoV-negative cases. Water from outdoor taps and boreholes (aOR 0·7; 95% CI 0·5–1·0; P = 0·045) compared with indoor taps and mixed infections with RV (aOR 0·6; 95% CI 0·4–0·8; P = 0·002) and human adenovirus (aOR 0·3; 95% CI 0·2–0·6; P < 0·001) were all significantly less common in NoV GII-positive than NoV GII-negative patients (Supplementary Table 1). No statistically significant environmental or behavioural features were associated with NoV GI detection (Supplementary Table 2).
Compared with NoV-negative cases, mixed pathogen infections were frequently associated with NoV detection (NoV GII + one other pathogen (OR 1·8; 95% CI 1·3–2·6; P = 0·001) and NoV GII + ⩾2 other pathogens (OR 5·2; 95% CI 3·5–7·7; P < 0·001) compared with NoV only; Table 1). These results were also significant when NoVs were stratified according to genogroup (Supplementary Tables 1 and 2).
The odds of detecting NoV GI strains increased 2·0 times when NoV GII strains were present (95% CI 1·2–3·3) and 2·0 times when SaV was present (95% CI 1·1–3·7; Supplementary Table 2) compared with single NoV GI cases. The association between NoV GI and SaV was maintained when adjusted for month of collection (aOR 1·9 (95% CI 1·0–3·5); P = 0·041). Similarly, the NoV GI/NoV GII association also maintained statistical significance when adjusted for month of collection (aOR 1·9 (95% CI 1·1–3·3); P = 0·017).
NoV infection in HIV-infected and -uninfected children
The HIV status was available for 91% (2824/3103) of the children enrolled and for 96% (2974/3103) of mothers. The HIV status was missing in 7% (26/377) of the NoV GII cases and 9% (8/94) of the NoV GI cases. Mixed GII and GI infections were noted in 19 cases with 418 cases available for analysis.
The detection of single (11% vs. 10%) pathogen NoV GII cases was similar in HIV-infected and HIV-uninfected children (P = 1·0). The detection of mixed pathogen NoV GII cases was lower in HIV-infected (9%; 26/278) compared with HIV-uninfected children (14%; 267/1954; P = 0·046). There were no differences in the median NoV GII C t values in HIV-infected (C t = 27·9; IQR 21·4–32·0) compared with HIV-uninfected children (C t = 26·1; IQR 19·7–32·0; P = 0·45).
The detection of single pathogen NoV GI infections was higher in HIV-infected (9%; 6/67) compared with HIV-uninfected (1%; 4/410; P < 0·001) children while the detection of mixed pathogen NoV GI infections was lower in HIV-infected (2%; 5/257) compared with HIV-uninfected children (4%; 71/1758; P = 0·115). Univariate analysis indicated that the odds of detecting single pathogen NoV GI in HIV-infected children were 10·0 times greater (95% CI 2·7–36·4) compared with HIV-uninfected children (P < 0·001). In addition, the median NoV GI Ct values in HIV-infected children were significantly lower (C t = 28·0; IQR 24·4–31·1) compared with HIV-uninfected children (C t = 32·0; IQR 26·9–34·2; P = 0·05) on univariate analysis.
Amongst children with NoV detected, HIV-infected children displayed a higher case fatality rate (9·3% vs. 1·0%; aOR 6·9; 95% CI 1·5–31·9; P = 0·013) and longer hospitalizations (⩾3 days 74·4% vs. 39·9%; aOR 4·3; 95% CI 2·1–8·9; P < 0·001; Table 2) than HIV-uninfected children on multivariable analysis. In addition, HIV-infected children with NoV had 2·4 times greater odds than HIV-uninfected children of experiencing diarrhoea duration of 3 days or more compared with 2 days or less (95% CI 1·2–4·9; P = 0·018; Table 2).
Table 2. Univariate and multivariable analysis of demographic data, clinical characteristics and environmental features associated with NoV detection in HIV-infected and HIV-uninfected children (n = 418)
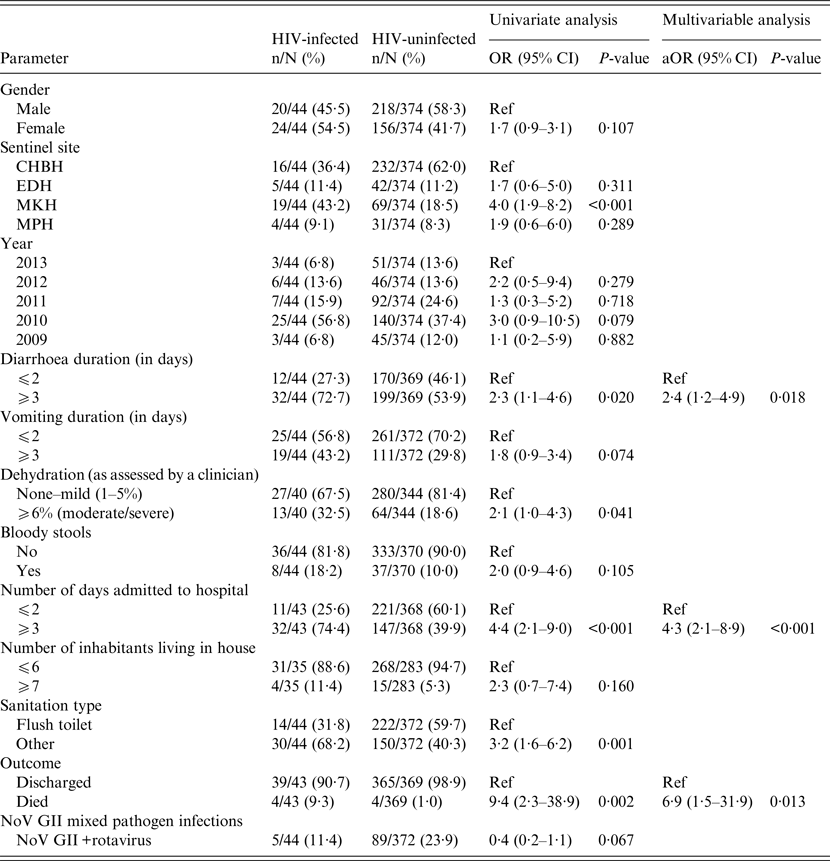
CHBH, Chris Hani Baragwanath Hospital; EDH, Edendale Hospital; MKH, Matikwane Hospital; MPH, Mapulaneng Hospital; HIV, human immunodeficiency virus.
Only variables with P-values <0·2 in the univariate analysis were reported in the table and included in the multivariable model.
DISCUSSION
The study provides epidemiological features of NoV infection in hospitalized children <5 years in a setting with high HIV prevalence. The study demonstrated the importance of NoV in hospitalized children aged <2 years and identified diarrhoea and vomiting with limited dehydration as prominent clinical characteristics in NoV cases. Amongst NoV-infected children, HIV-infection was associated with prolonged hospitalization and increased mortality. It is the first study of its kind in SA that investigates NoV detection in HIV-infected children and NoV diarrhoea post rotavirus vaccine introduction. The study also spans more than 12 months, includes more than one hospital and geographic area and assesses data on clinical characteristics and the environmental features associated with NoV detection in children <5 years.
An earlier study in SA in 2008 detected NoV in 14% of hospitalized children <2 years [Reference Mans23]. Previous studies in Africa have also identified NoV in an average of 13·5% of diarrhoea cases [Reference Mans9]. Similarly, a worldwide systematic review of studies conducted between 1990 and 2008 estimated that NoV was responsible for 12% of severe diarrhoea cases in children <5 years [Reference Pires3]. These results are comparable with the current study findings with NoV detection (GI and GII strains) at 15% in hospitalized South African children.
NoV has often been described as ‘winter vomiting disease’ due to the winter seasonality and occurrence of vomiting symptoms [Reference Robilotti, Deresinski and Pinsky6]. Globally, NoV infections seem to be more common during the winter months [Reference Mounts24, Reference Raboni25]. However, reports from Spain in 2001 and the UK in 2002 show that summer peaks are occasionally present [Reference Lopman26, Reference Boga27]. In SA, NoVs were detected throughout the year with detection increasing during the warmer spring and summer months in SA (between September and November). The results obtained in this study are similar to those obtained by Mans et al. at two hospitals in Gauteng Province in 2008 [Reference Mans23].
During 2010, NoV GII strains were detected at levels above 20% for 5 months (May and July–October), with an uncharacteristic autumn/winter predominance. Whether or not this increase was related to increased tourist activity and the introduction of new GII and GI strains during the Soccer World Cup in SA in June and July of 2010 or the decrease in rotavirus due to the introduction of the rotavirus vaccine in 2009 is unknown. Since the New Orleans 2009 GII.4 variant was first detected in SA in 2008 and was regularly detected from April 2009 to December 2012, the increase is unlikely to be related to changes in the dominant GII.4 variant [Reference Mans28].
NoV GII strains identified in a 2008 study in Pretoria indicated that eight GII (GII.1, GII.4, GII.6, GII.7, GII.10, GII.13, GII.14, GII.16) strains were circulating [Reference Mans23]. While genotyping data from 2010 revealed that GII.2, GII.3, GII.4, GII.12 and GII.17 strains were circulating [Reference Mans29], only limited typing was performed on the 2010 GII strains (39/216; 18%). Therefore, with the current data available, the introduction of new virus genotypes or variants as the cause of the increased detection of NoV GII and GI infections in 2010 could not be established. Continued surveillance and genotyping of NoV strains is recommended to identify the introduction of new variants or types in the South African paediatric population.
In agreement with recent NoV data [Reference Robilotti, Deresinski and Pinsky6, Reference Kirby30], vomiting was a significant symptom associated with NoV detection in South African children hospitalized for diarrhoea. However, children with NoV were not dehydrated and admitted for a median of 2 days. These results suggest that children with NoV detected are probably brought to the hospital for the treatment of numerous vomiting episodes rather than severe dehydration or diarrhoea. In NoV challenge studies, vomiting was more prevalent than diarrhoea and nearly half of the participants who experienced vomiting did not report diarrhoea symptoms [Reference Kirby30]. Based on the surveillance case definition, NoV cases presenting as only vomiting or treated in the outpatients department will have been missed. Furthermore, a large proportion of children with NoV may not have required medical treatment resulting in an under estimation of the true prevalence of NoV diarrhoea in children <5 years in SA.
NoV GI strains have been detected at similar levels to GIIs in river water in SA [Reference Mans28] but were not seen at comparable levels in hospitalized patients (12% vs. 3%). These results suggest that either NoV GI strains survive longer in the environment or patients shed NoV GI strains at higher titres [Reference Kirby31] or NoV GI strains cause less severe or asymptomatic infections. Furthermore, these cases may be treated at home or at a clinic level, which means that they would not be captured by the study surveillance system.
The exception to the perceived mild nature of GI infections may be in HIV-infected children. The current study indicated increased detection of single GI infections in HIV-infected children compared with HIV-uninfected children (9% vs. 1%) in children admitted for the treatment of diarrhoea. In addition, the median NoV GI C t values in HIV-infected children were also significantly lower compared with HIV-uninfected children (P = 0·05). These results combined could be used as an indicator of NoV GI disease severity in HIV-infected children. Similar differences in disease severity in RV and NoV GII infections in HIV-infected and HIV-uninfected patients have not been reported. However, this may be due to our inability to distinguish subtle differences in clinical severity between the two populations rather than the absence i.e. diarrhoea infections associated with RV and NoV GII strains are severe regardless of immune status.
A study by Groome and Madhi [Reference Groome and Madhi32] estimated that 26% of children admitted with acute gastroenteritis to CHBAH, Johannesburg between March 1998 and October 2000 were HIV infected, based on the prevalence rate in women attending antenatal clinics in the area. While the study did not detect more frequent RV infections in HIV-infected children, the absolute burden of disease in these children was twofold higher than in HIV-uninfected children [Reference Groome and Madhi32]. In addition, HIV-infected children were more likely to be hospitalized for a longer period and had a higher case fatality rate [Reference Groome and Madhi32]. A similar trend was noted in the current study where NoV strains were detected at similar levels in HIV-infected and HIV-uninfected children. However, NoVs were associated with a higher case fatality rate in HIV-infected children compared with HIV-uninfected children.
Population denominators were unavailable for the surveillance sites and; therefore, the incidence and increased risk of NoV associated with HIV infections could not be calculated. However, the mean HIV prevalence among children 0–4 years with NoV detected was 9·7% (43/443) between 2009 and 2013 while the HIV prevalence among children 0–4 years in the general population was 3·3% in 2008 [Reference Shisana33], a threefold increase. These results suggest that HIV infection may be associated with hospitalization of NoV cases in children and further study may be warranted.
Analysis of the environmental features associated with NoV detection found that NoVs were less common in diarrhoeal patients living in informal housing (aOR 0·7; P = 0·021) compared with brick housing or using external water sources (aOR 0·7; P = 0·045) compared with indoor water. The role of continuous environmental exposure to NoV strains in these settings is unclear. Future study including control groups from the same community without diarrhoea would be required to interrogate these findings further.
The study has several limitations that should be considered when evaluating the findings. Missing data were dealt with by pairwise deletion and information selection bias may be present affecting the estimates and associations observed. The comparison groups used for the analysis were not strict control groups with the absence of diarrhoea and may have resulted in an underestimation of the clinical and environmental features associated with NoV infections. While patients who refused to participate in the study were noted, no additional information was gathered from these patients and, therefore, there may be non-participation biases unaccounted for in the analysis. The study enrolled children who were admitted to hospital overnight and was limited to moderate-to-severe diarrhoea. Therefore, any findings are restricted to this category of diarrhoeal infections and should be extrapolated to less severe outcomes with care. Not all the participants included in the study had specimens screened for all enteric pathogens. Limited clinical specimen volumes resulted in reduced screening for parasites. This may have resulted in an underestimation of mixed infections and affected the analysis of single pathogen infections.
Asymptomatic infections and prolonged shedding of enteric pathogens complicates epidemiological evaluations when trying to establish whether a pathogen is associated with an illness. Asymptomatic NoV infections have been described in paediatric patients at frequencies between 11·6% and 49·2% in a recent review [Reference Robilotti, Deresinski and Pinsky6]. An Australian study recorded NoV GII shedding in seven young children for 2–100 days [Reference Kirkwood34]. In addition, NoV shedding in immunocompromised patients has been recorded for up to 898 days [Reference Schorn35]. The current study did not have any data on asymptomatic NoV infections or duration of NoV shedding and the frequency of mixed pathogen NoV cases ranged from 77% to 66%.
However, efforts have been made to translate the faecal NoV viral load measurements or C t values as a proxy measure into disease-attribution cut-offs [Reference Phillips36]. An English study calculated an optimal C t cut-off for children <5 of 30 [Reference Phillips36]. As no South African C t-disease-attribution analysis of the NoV real-time detection assay has been performed, the current study included all specimens positive for NoV irrespective of C t value. An interesting finding was the statistically significant difference in the median C t values between sole pathogen and mixed pathogen cases (27·2 vs. 32·0; P = 0·006). Additional research establishing the C t-disease-attribution cut-off and investigating NoV shedding in SA should be considered.
The study determined the detection rate, clinical characteristics and environmental features associated with NoV detection in hospitalized children <5 years in SA. Furthermore, the study identified NoV GI strains as a potentially serious pathogen in vulnerable HIV-infected patients and demonstrated an association between NoV detection and mortality in this group. Future monitoring of NoV detection rates and variants circulating in the South African population may aid in enumerating diarrhoea burden due to the introduction of new NoV strains.
SUPPLEMENTARY MATERIAL
The supplementary material for this article can be found at https://doi.org/10.1017/S0950268817000668
ACKNOWLEDGEMENTS
The authors acknowledge participants and staff of the sentinel diarrhoea surveillance programme. Meera Chagan (deceased 29 August 2014) for supervision of the Kwa-Zulu Natal site, The South African Field Epidemiology Training Programme.
The sentinel diarrhoea surveillance programme was funded by GlaxoSmithKline (E-Track 200238). The funders were not involved in study design, writing or publication of the paper.
DECLARATION OF INTERESTS
N.A.P. received honoraria from GlaxoSmithKline, Merck and Aspen Pharma. M.J.G. received honoraria from GlaxoSmithKline and Sanofi Pasteur. C.C. received honoraria from Sanofi Pasteur and Pfizer.
ETHICAL STANDARDS
The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national and institutional committees on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008. Ethical approval for the study was obtained from the Human Research Ethics Committee (Medical), University of Witwatersrand (M091018), the Biomedical Research Ethics Committee, University of Kwa-Zulu Natal (BF074/09) and the Faculty of Health Sciences Research Ethics Committee, University of Pretoria (278/2015).






