INTRODUCTION
Reactor-grade graphite is a material fabricated for having high purity, high thermal conductivity, and in so far as possible, high resistance to irradiation damage (density, minimum gas content, nuclear graphite [prepared under the auspices of the Division of Technical Information, United States Atomic Energy Commission; Nichols and Woodruff 1962]).
Any reactor-grade graphite has a pore structure that enables the penetration of aqueous and gaseous phases and therefore radionuclides, which are not intrinsically bound in the graphite (crystalline structure), could be leached. It has been demonstrated that irradiation of graphite produces changes in its structure (irradiation damage; Cobos et al. 2008), which could promote the corrosion of the material and is expected to have influence in irradiated graphite leaching behavior.
The encapsulation of graphite is based on its conversion into IGM (Patent EP2347422 ALD-France) by hot pressing as an alternative waste conditioning option. Besides the usage of a long-term stable inorganic binder matrix, the resulting material is homogeneously filled and therefore consequently mechanical damage would not reopen a sealed pore system. The sealed the pore system could lead to minimizing all relevant effects for disposal time scales. The repository safety assessment could be modified by the leached species. Leached gaseous and aqueous species have to be studied in order to determine the mobility of radiocarbon (14C) in the repository environment.
The leaching behavior of IGM was investigated for two different leachates: deionized water and granitic-bentonite water. Previous studies determined the corrosion progress of the waste-form by mass loss and elemental chemical determinations in the leachates. In particular, the corrosion behavior of glass will be a key parameter for the applicability of IGM as a waste matrix. The corrosion rate is determined on carbon species and nuclides, especially 14C.
A procedure for the fabrication of IGM specimens has been established to obtain densities close to 2.2 g/cm3. The porosity of the material has been tested and it has been proven that the volume does not increase in this waste-form material regarding graphite density (Nichols and Woodruff 1962).
IMPERMEABLE GRAPHITE MATRIX (IGM)
Nuclear graphite can be transformed into a long-term stable IGM, which minimizes graphite porosity, avoiding the penetration of leachant and retarding the release of radionuclides, resulting in a safer waste form.
To manufacture IGM, milled graphite is mixed with suitable a glass powder that has been pressed under vacuum at high temperature. The vacuum is required to avoid gas enclosures in the obtained products, which themselves have densities that have to be above 99% of theoretical density of graphite to be disposed.
An optimized protocol to manufacture the IGM specimens in the hot vacuum press was established through performing a set of experiments including characterization of obtained specimens. IGM requires a leaching resistant borosilicate glass, in order to obtain a long-term stable material. Leaching experiments of IGM specimens were performed with five different glass types used as inorganic binder material because glass may interact with aqueous phases and could be dissolved.
MANUFACTURING IGM SAMPLES BY HOT VACUUM PRESS
ALD-France (FNAG) developed a specific hot vacuum press at lab scale to manufacture the IGM specimens in a CIEMAT control area.
The procedure to prepare the specimens is as follows:
∙ Graphite is ground to obtain graphite powder (particle size ∅≤250 μm).
∙ The obtained graphite powder is mixed with the glass in the appropriate proportion in order to maximize the density as well as the graphite amount in the final product.
∙ The mixture is introduced into the pressing tool that is mounted into the furnace (Figure 1). Previously the tool wall has to be covered with graphite foil to prevent the mixture baking together with the wall of the tool (Figure 2).
∙ The cooled specimen is demolded (extracted) from the tool (Figure 3).

Figure 3 IGM samples.

Figure 1 Pressing tool, 2 cm in diameter.

Figure 2 Inside view of furnace.
The relative amounts of glass and graphite are chosen according to the pore volume of the graphite to be conditioned. A ratio 80:20 graphite:glass is adopted to maximize the graphite conditioned according to the pore volume determined in previous studies (Fachinger et al. Reference Fachinger, Grosse, Seemann and Hrovat2011). The method allows the production of encapsulated graphite without increasing the volume in the repository because the resulting IGM densities are in the same order that the bulk density of unirradiated graphite before grinding.
Leaching Procedure for IGM
Specimens made of graphite mixed with five types of glass were used to perform preliminary leaching experiments in order to study the corrosion behavior of IGM and their mass loss. Al, B, K, Na, and Si were the main components determined in the leachant. The results of the aforementioned leaching studies were used to establish the glass type to use for manufacture of the IGM with better performance.
The IGM encapsulation capacity was studied, manufacturing specimens, for this purpose, with irradiated graphite to check whether the radionuclides contained in it would be able to migrate out of the piece.
Leaching solutions used to investigate the long-term behavior of IGM are deionized water (Milli-Q® water) and granitic-bentonite water; the composition of the latter is shown in Table 1.
Table 1 Granitic-bentonite water composition.

* DL: detection limit.
∙ Deionized water was chosen because it is the more conservative case; in the absence of ions, this leaching solution becomes the best medium for the radionuclides to be leached from the waste form.
∙ Granitic-bentonite water was selected to simulate the conditions of potential Spanish HLW deep geological disposal.
The leaching reactors are high-pressure stainless steel containers with a PEEK (PolyEther Ether Ketone) beaker inside (total volume of the vessel is 120 mL). PEEK avoids misleading the leaching results and is used to substitute Teflon® beakers, due to Teflon®, under high dose rates, contaminate leachant with small amounts of fluoride, which complexes certain radionuclides, as was demonstrated at Jülich with Th (IV) in Teflon® beakers. The leaching process (Carpentiero et al. Reference Carpentiero, Bienvenu, De la Huebra, Dale, Grec, Gallego, Rodriguez, Vanderlinden, Voors, Welbergen, Maz and Fazs2004; ISO 6961-1982(E)) of the IGM specimens was performed at room temperature.
∙ The specimen is suspended in 100 mL of leachant by means of a PTFE (PolyTetraFluoroEthylene) thread and surrounded by at least 1 cm of liquid in any direction (Figure 4).

Figure 4 Leaching containers for deionized water and granitic-bentonite water.
∙ The initial gas phase composition consists of synthetic air supplied from a high-pressure gas bottle.
The leaching steps were selected in consideration of radioactivity concentration and the sensitivity of methods and equipment to be used for measurement. Based on that, it was planned to renew both gas and leachate in six leaching steps after approximately 14, 28, 56, 90, 180, and 360 days from the test start (ISO 6961-1982(E)). The renewal of the leachate prevents the solution from becoming saturated, favoring the release of chemical species.
∙ The gas sample (volatile species) is collected through the extraction valve by means of a gas tight syringe (Figure 5) and immediately injected into the GC-MS system.

Figure 5 Gas sample extraction by a syringe.
∙ The container is opened; the specimen is withdrawn from the leachant, which is reserved for further analyses. The reactor is prepared for another leaching step.
CHARACTERIZATION
The 14C has been separated quantitatively from the leachate by a catalytic furnace (Harvey OX500) using specific trapping solutions. Activity of 14C has been analyzed by liquid scintillation counting (LSC) systems (Packard Tricarb 3110 TR/LL LSC and Quantulus LSC system).
Determination of concentration of organic dissolved species (carboxylic acids) was undertaken with a Dionex® ICS-900 by Thermo Scientific with an AS40 autosampler Ion Chromatography System. The results of the deionized water leachates were obtained with the column AS-11, with a gradient of concentrations (1.5 mM KOH [0–8 min]; 40 mM KOH [27 min], and 1.5 mM KOH [35–40 min]) and a flow of 1 mL/min.
Gamma emitters were measured in the leachate with a Canberra BEGe 3830 HPGe Detector. Typical relative efficiency was ≥34%. The gas and leachate volatile species were measured with an Agilent® GC-MS 7890B system (Agilent® DB624UI 60 m column type for liquid samples and Molsieve 5A Porabond Q column for permanent gases and CO2).
The assays to analyze CO were carried out with a Molsieve 5A column, 1 mL of injection volume, in isothermal conditions (70ºC) and a Split Flow of 50 mL/min (ratio 43.5:1). The assays to analyze alcohols and aldehydes were carried out with a DB-624UI (L=60 m; ID=0.25 mm and F=1.40 μm) column, with the following gradient of temperature: 40ºC (2 min); 1ºC/min to 45ºC (5 min); 1ºC/min to 50ºC (5 min) and 50ºC (2 min) with a Split 100:1. In this case, the technique of headspace sampling was used with 30 s of incubation time at 40ºC.
LEACHING RESULTS AND DISCUSSION
Two replicates of active IGM specimens, labeled A-I and B-I, have been prepared in order to perform leaching studies in the two media considered.
The characteristics and physicochemical properties of IGM specimens, primary waste (i-graphite), and leachant are described in this section. The main properties studied are:
∙ Macroscopic physical characteristics of specimens
∙ pH and electrical conductivity (EC) of leachant
∙ Corrosion rate of 14C and gamma emitters (60Co)
∙ Identification of volatile carbon species in leachate gases
∙ Identification of solved gaseous carbon species in leachant
∙ Identification and concentration of carboxylic acid in the leachant
Characteristics (size and weight) of the specimens of IGM used in the experiments are shown in Table 2.
Table 2 Physical properties.

The initial massic activities of the main nuclides in the specimens with irradiated graphite are indicated in Table 3.
The gamma activities are determined by non-destructive methodologies (gamma spectrometry) using numerical calibration (ISOCSTM calibration software). 14C is separated by a destructive assay system with a Harvey OX500 furnace; using samples <10 mg. Radiocarbon activity was calculated by the average of the measures obtained by LSC of 5 samples.
The pH and EC have been determined before and after each leaching step. Both parameters have not been measurable in the case of granitic-bentonite water due to its high ion content. The pH and the EC increase in the first steps when the leachant is deionized water. It is guessed that this increase is due to the ions leached from the surface of the IGM specimens. The profile of this behavior, in function of leaching time, has been cross-checked with the total ion concentration found in the determination of IGM matrix corrosion behavior, as is described in the section Leaching Procedure of IGM.
In Figure 6 it is observed that the increase in conductivity coincides effectively with the presence of dissolved ions in the first steps.
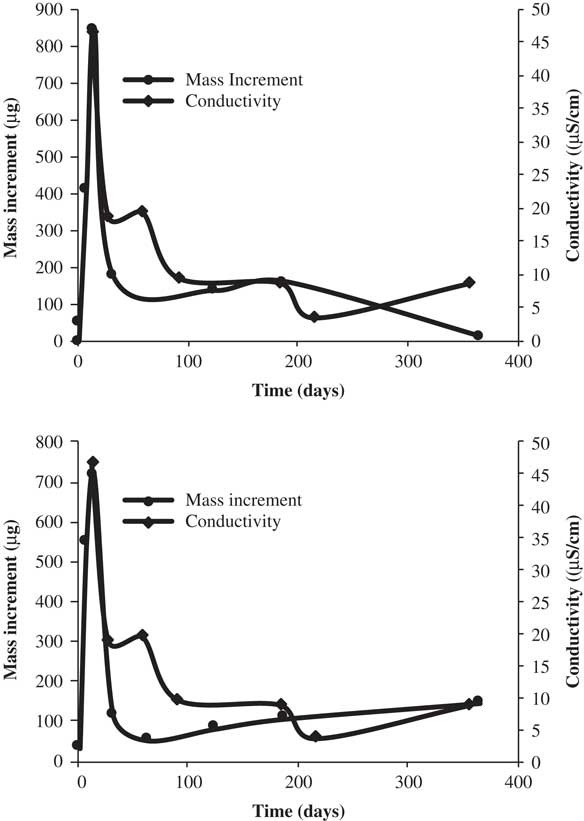
Figure 6 Mass increment versus conductivity in deionized water.
The additional increase of the ion concentration by dissolution from the glass matrix may result in an increase of EC in the leachant that leads to the precipitation of secondary phases. The EC and the presence of ions in dissolution decrease with the time; this demonstrates the matrix integrity over time and its capability of encapsulating the radionuclides, as will be observed in the following study.
Results of Organic Carbon Compound
The results of the deionized water leachates are shown in Table 4.
Table 4 Organic carbon leached concentration ([]) in deionized water.*

* U: Expanded combined uncertainty about 10% at the coverage factor k=1.
The organic carbon species detected in the leachates are formate (HCOO–) (in samples from days 28, 90, and 180) and oxalate (COO)2 = (in day 28).
Ionic species with organic carbon are not detected in the granitic-bentonite water leachant, due to the presence of high concentrations of other anions that mask the detection of carboxylic acids.
14C Results and Gamma Emitters
All obtained values of 14C and 60Co in deionized water are lower than the minimum detectable activity (MDA). Assuming that the maximum amount leached is equal to the MDA, the corrosion rate (Equation 1) can be calculated using these limits as data. The equation is:
where A ss is the accumulative mass amount of leachate per unit surface area (g/m2), ρ is the density of IGM (g/m3), and t is the test time (yr).
The calculated corrosion rate values are the upper limits since in Equation (1) MDAs are assumed as activity concentration (A ss =MDA). The uncertainties of these values are larger due to the counting-rate standard deviation. With these values, authorities could contrast these values with the waste acceptant criteria at repository.
The corrosion rate calculated in this way is for non-porous material. The fluctuations in the data of 14C and 60Co in deionized water are due to the fact that the minimum activity detected by the equipment can vary according to the measurement being made. The corrosion rate for 14C is shown in Table 5. In Figures 7 and 8 the corrosion rate is represented.
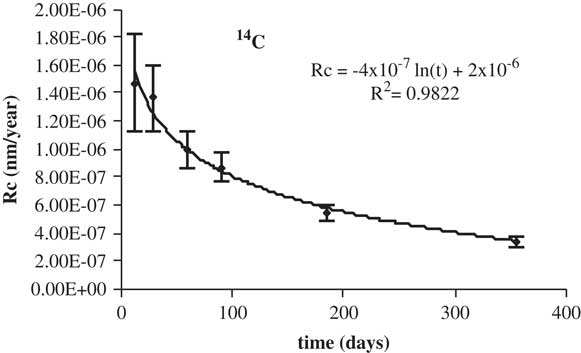
Figure 7 Corrosion rates of 14C in deionized water.
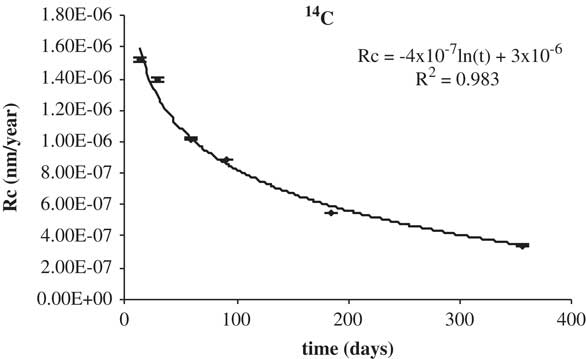
Figure 8 Corrosion rates of 14C in granitic bentonite water.
Table 5 Corrosion rates of 14C.*

* U: Expanded combined uncertainty about 10% at the coverage factor k=1.
It is noted that the experimentally observed trend is the expected one, as the corrosion rate decreases with time to a constant value. The percentage leached of 14C is shown in Table 6. The total amount leached in deionized water as well as in granitic-bentonite water is approximately 3×10–3%. This result would meet, likely, the waste acceptance criteria, in terms of available radiocarbon inventory, of the Spanish near surface repository (C.A. El Cabril). The activity concentration 60Co, in deionized water, is below the MDA. The corrosion rate of 14C is shown in Table 7.
Table 6 Percentage 14C leached in deionized and granitic-bentonite water.

Table 7 Corrosion rates of 60Co.*

* U: Expanded combined uncertainty about 10% at the coverage factor k=1.
The higher concentration beta gamma emitter in irradiated graphite, 60Co, was considered for studying its leaching behavior in both media. The data of the corrosion rate of 60Co versus leaching time in deionized water are shown in Figure 9. The data of the corrosion rate of 60Co versus leaching time in granitic betonite are shown in Figure 10. The corrosion rate decreases with time, as is expected, to a constant value lead by its logarithmic trend. The percentage leached is listed in Table 8.
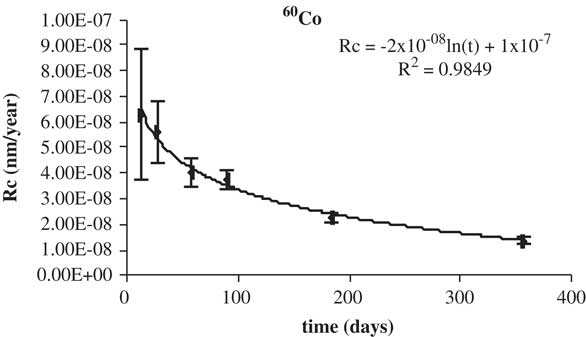
Figure 9 Leaching rates of 60Co in deionized water.
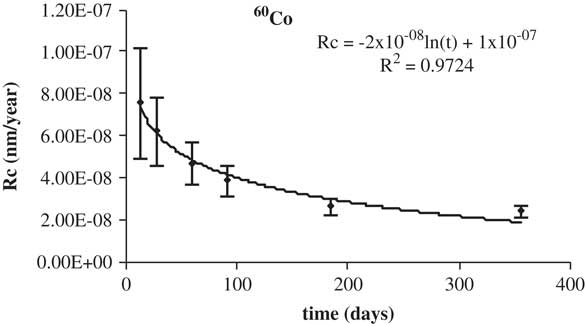
Figure 10 Leaching rates of 60Co in granitic bentonite water.
Table 8 Percentage leached of 60Co in deionized water.*

* U: Expanded combined uncertainty about 10% at the coverage factor k=1.
The low leaching rates indicate the enclosing potential of IGM. The percentage leached of 60Co is lower than 0.3%.
Gas and Leachate Volatile Species by GC-Mass Spectrometry
The assay to analyze CO was performed in isothermal conditions (70ºC) and with a split flow of 50 mL/min. CO has been detected, but it has not been possible to determine the presence of 14C in CO as there was no technique to trap this gas at the laboratory used. Negligible amounts of leachant vaporization have been registered during the leaching time.
The assays to analyze alcohols and aldehydes were carried out with the following gradient of temperature: 40ºC (2 min); 1ºC/min to 45ºC (5 min); 1ºC/min to 50ºC (5 min) and 50ºC (2 min) with a Split 100:1. The technique of head space sampling was used with 30 s of incubation time at 40ºC. Both functional groups have not been detected in any step of the leaching process; the absence of a signal for these organics could be due to there being not enough time to form these molecules or there were no thermodynamic and kinetic conditions to the formation from the materials in the waste form.
For the first and second leaching period with granitic-bentonite water, CO was found in concentrations of 27.9 mg/L and 10.4 mg/L. This can be associated to the carbonate content in the leachate. In the case of deionized water 33.9 mg/L was found in the first step. These results indicate, only at the beginning, it is possible to find CO in the gas phase if the air atmosphere is removed. So for final disposal where the atmosphere will be saturated it is less likely to find an increase in the amount of CO.
CONCLUSIONS
A methodology has been established to manufacture the IGM samples at laboratory scale. The durability of the IGM glass matrix has been validated by leaching experiments. It is possible to close the pore system in irradiated graphite without increasing the volume of the waste. Conductivity and pH are practically constant in any leaching step with granitic-bentonite water, the presence of ions leached from tested IGM specimen is masked by the high ion concentration in the leachant. These parameters (pH and EC) increase, in the case of deionized water, which means a certain amount of ions migrate from IGM into the leachant.
Organic carbon has been found in deionized water as formate (HCOO–) and oxalate (COO2 =). Volatile alcohols and aldehydes in leachates have not been detected at any time of the leaching process by GC-MS. CO was found in the gas phase of the leaching process for the first leaching period with deionized water, 33.9 mg/L and for the first and second leaching period with granitic-bentonite water, where the concentration is 27.9 mg/L and 10.4 mg/L. This may also be related to the carbonate content in the leachate. As the values obtained are below the MDA, it is not possible to establish a correlation between 14C and 60Co leached to establish scaling factor methodology. The percentage leached obtained for 14C and 60Co indicated the sealing capacity of IGM as final waste form.
ACKNOWLEDGMENTS
The work has been carried out by CIEMAT in CAST project, which has received funding from the European Union’s European Atomic Energy Community’s (Euratom) Seventh Framework Programme FP7/2007-2013 under grant agreement no. 604779, the CAST project. The equipment used in the realization of this project has been provided by ALD-France.
























