Introduction
Over 80% of Persons with Dementia (PwD) will develop Behavioral and Psychological Symptoms of Dementia (BPSD), also referred to as neuropsychiatric symptoms, during the course of their disease (Abraha et al., Reference Abraha2017), with 97% of PwD developing at least one symptom over a five-year period (Steinberg et al., Reference Steinberg2008). BPSD is defined as “signs and symptoms of disturbed behavior, mood, thought, or perception” (Kales et al., Reference Kales, Gitlin and Lyketsos2015). The Neuropsychiatric Inventory–Questionnaire (NPI-Q) has categorized the reported signs and symptoms into: delusions, hallucinations, agitation/aggression, depression/dysphoria, anxiety, elation/euphoria, apathy/indifference, disinhibition, irritability/lability, motor disturbance, nighttime behaviors, and disturbance in appetite/eating (Kaufer et al., Reference Kaufer2000). BPSD could induce physical injuries and psychological distress in PwD and their caregivers, and it is identified as a predictor of nursing home placement (Gaugler et al., Reference Gaugler, Kane, Kane, Clay and Newcomer2003). In nursing homes, caregivers have reported to respond to PwD exhibiting BPSD with verbal or physical abuse or to minimize contact with them, thus reducing the quality of care received by PwD (Kales et al., Reference Kales, Gitlin and Lyketsos2015). Nursing home administrators could face increased financial costs due to increased medical and psychological care, security concerns, and staff turnover (Kales et al., Reference Kales, Gitlin and Lyketsos2015). Therefore, effective management of BPSD needs to be identified and applied, especially in the nursing home setting.
Antipsychotic medication has been applied to manage BPSD, which has been shown to have low efficacy and serious side effects, such as increasing fall risk, mortality rate, and stroke occurrence (Gill et al., Reference Gill2007; Seppala et al., Reference Seppala2018). As a result, non-pharmacological interventions have been developed to act as an alternative of antipsychotic medication. This study proposes a hypothesis that new insights could be gained if the non-pharmacological interventions and their clinical trials are also reviewed and assessed from a perspective of ergonomics. Ergonomics studies “the interactions between humans and other elements of a system, and applies theories, principles, data, and methods to design for optimizing human well-being and system performance” (Karwowski, Reference Karwowski2012). Human performance depends on the person’s capabilities and limitations; while the system has its own requirements and affordances. These requirements and affordances need to match with the capabilities and limitations of the target population for the outcome of the system to be reliable. From an ergonomist’s view, non-pharmacological interventions could be regarded as the system. The remaining capabilities of PwD and the requirements of the interventions should match for the outcome of the clinical trial to be reliable. Capability is an umbrella term used in ergonomics to describe one’s ability in sensory, cognition, and movement aspects when interacting with a system (Czaja and Nair, Reference Czaja and Nair2006). One needs certain levels of capabilities to be able to perform certain cognitive and functional activities, namely Activities of Daily Living (ADL) or Instrumental Activities of Daily Living (IADL).
Several systematic reviews have reviewed and assessed the quality of the clinical trials to conclude on the quality of evidence on the effectiveness for each intervention. Abraha et al. found that music therapy and behavioral management techniques are effective in managing BPSD, in general, while the evidence base is weak due to variations in application of the interventions and measurements (Abraha et al., Reference Abraha2017). Brasure et al. reached the same conclusion on the weak evidence base after systematically reviewing non-pharmacological interventions in managing agitation and aggression in PwD (Brasure et al., Reference Brasure2016). Cohen-Mansfield identified that many non-pharmacological interventions have led to a statistically and clinically meaningful improvement in the management of behavioral problems, and stated that the variation in criteria for success, screening procedures, and control procedures together with underreported treatment failures prevent further conclusions to be drawn (Cohen-Mansfield, Reference Cohen-Mansfield2001). A meta-analysis showed that non-pharmacological interventions delivered by family caregivers had an effect size in managing BPSD at least equaling that of antipsychotic medication (Brodaty and Arasaratnam, Reference Brodaty and Arasaratnam2012). However, as the focus of the current review is on capability considerations of PwD when they are interacting with non-pharmacological interventions, caregiver interventions are not included in this review as caregiver interventions do not require capabilities of PwD.
The systematic reviews currently available in the literature have evaluated neither the non-pharmacological interventions nor their clinical trials based on the abovementioned capability matching principle in ergonomics. Specifically, these reviews have not assessed if capabilities of PwD were considered in the non-pharmacological interventions and their clinical trials (Abraha et al., Reference Abraha2017; Brasure et al., Reference Brasure2016; Cohen-Mansfield, Reference Cohen-Mansfield2001). In addition, the availability of resources, severity of cognitive impairment, and levels of comorbidity are different in community and nursing home settings. However, no systematic review on non-pharmacological interventions for BPSD has distinguished between interventions in community settings and nursing home settings. A systematic review on the effectiveness of non-pharmacological interventions for BPSD in nursing home residents is also lacking. Therefore, this study aims to systematically review the non-pharmacological interventions for BPSD in nursing home residents, with a special focus on capability considerations as recommended in ergonomics.
Methods
Search strategy
The literature search was performed in three electronic databases: PsycINFO, EMBASE, and MEDLINE. For the searches, the following sets of search terms were used: (1) dementia, (2) BPSD, and (3) nursing home, with the searches limited to therapy (maximize sensitivity). The first set of terms included “dementia (exploded)”, “dementi*”, “Alzheimer*”. The second set of terms consisted of “neuropsych*”, “behav*”, “behav* problems (exploded)”. The third set of terms involved “nursing home (exploded)”, “nursing care”. The date of the last search is January 8, 2018. The full search strategy is listed in the supplemental digital content (SDC) -1 (published as supplementary material online attached to the electronic version of this paper at https://www.cambridge.org/core/journals/international-psychogeriatrics).
Inclusion criteria
Inclusion criteria for studies relevant for this review were: (1) studies that involved nursing home residents with a diagnosis of dementia; (2) studies that applied non-pharmacological interventions for BPSD which require capabilities of nursing home residents; (3) studies that reported on effects of the interventions on symptoms of BPSD; (4) studies with a pre-post, (quasi) experimental, cross-sectional, randomized controlled, or longitudinal design; and (5) studies written in English and published in a peer-reviewed journal between January 1, 1998 and January 1, 2018.
Intervention categorization
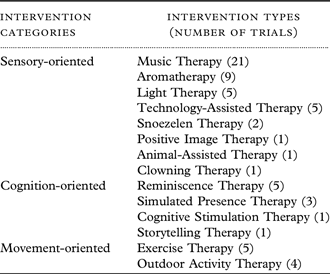
The interventions were categorized according to the capabilities of PwD required to participate. Ergonomics has investigated human capabilities and how these capabilities change with age. The age-related capabilities investigated were in terms of the sensory, cognition, and movement aspects (Freudenthal, Reference Freudenthal1999). As the majority of PwD are over the age of 65, they suffer from capability declines in these three aspects not only due to dementia, but also age. Therefore, the interventions were categorized into sensory-, cognition-, and movement-oriented in this review.
Quality assessment
We evaluated the quality of each trial based on the National Health and Medical Research Council (NHMRC) evidence hierarchy (NHMRC, 2000). In addition, each trial was judged for whether capabilities of PwD were considered. Two criteria were used in this review for rating “capability consideration.” First, we assessed if the intervention investigated in the trial had been designed with the capabilities of PwD in mind; that is, if design guidelines for PwD had been incorporated or PwD had been involved in the design process. When the first criterion was not satisfied, we assessed if the trial only included PwD with adequate capabilities required by the intervention. For example, if PwD with hearing impairments were excluded from a clinical trial on Music Therapy. The clinical trial was rated “Yes” for “capability consideration,” if it satisfied either criterion, and rated “No” when both criteria were not met.
Results
The study selection process, guided by Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA), is shown in Figure 1. The search disclosed 2295 abstracts, of which 101 clinical trials were chosen as potentially relevant; of these, 64 trials met all the inclusion criteria. The summary of the three intervention categories is shown in Table 1. More intervention types were categorized as sensory-oriented, rather than cognition- and movement-oriented. The summary of intervention outcomes is shown in Figure 2. Forty-one trials reported a significant reduction on at least one BPSD-symptom; 20 trials reported no significant reduction while three trials reported that the BPSD-symptoms worsened after the intervention. The “capability considerations” of the clinical trials are summarized and shown in Figure 3. As shown in Figure 3, 28 trials did not consider the capabilities of PwD. The details of each trial are listed in the supplemental digital content (SDC) -2 (published as supplementary material online attached to the electronic version of this paper at https://www.cambridge.org/core/journals/international-psychogeriatrics). The details include: trial type, patient type, intervention, scores in NHMRC and “capability consideration,” and outcomes. Each intervention type is described below in the sequence of sensory-oriented, cognition-oriented, and movement-oriented categories, with the intervention types that were investigated by only one trial described together as “other interventions” in each category. For trials satisfying the first “capability consideration” criterion (i.e. Snoezelen Therapy, Technology-Assisted Therapy, Animal-Assisted Therapy, Clowning Therapy, and Reminiscence Therapy), we included the specific design guidelines used, or features of the intervention that had been modified based on preliminary field testing in the description below.
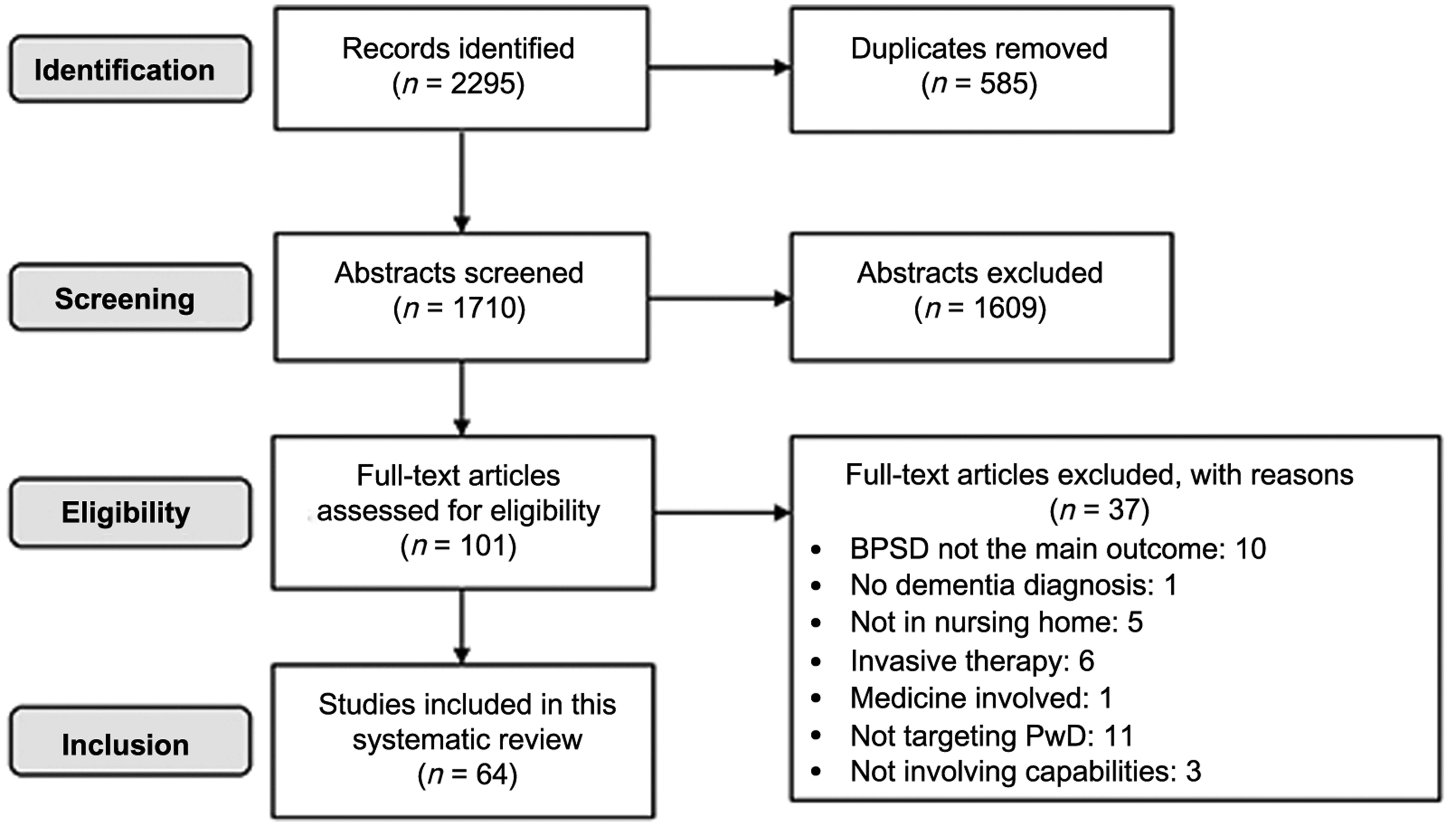
Figure 1. Flow diagram of the systematic review.
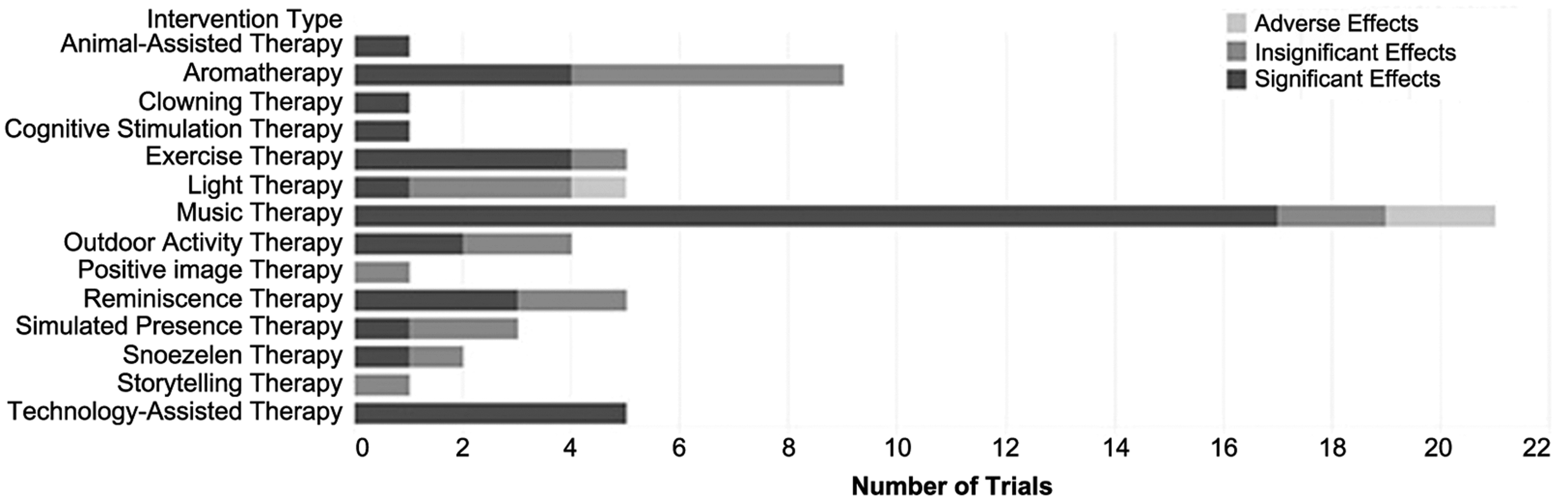
Figure 2. Summary of clinical outcomes of interventions for BPSD in nursing home residents.
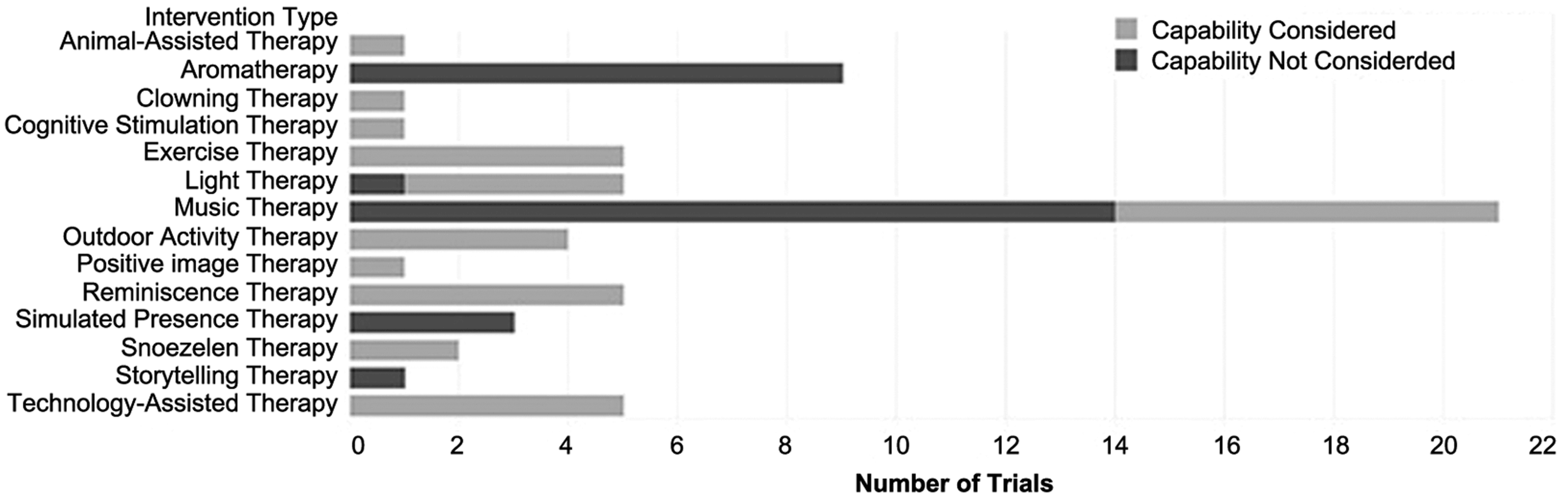
Figure 3. Summary of “capability consideration” of clinical trials on interventions for BPSD in nursing home residents.
Table 1. Summary of the three intervention categories for BPSD in nursing home residents according to capabilities in ergonomics
Sensory-oriented therapy
Music Therapy
Twenty-one trials investigated the effectiveness of Music Therapy; 10 trials are of NHMRC evidence hierarchy II (Clark et al., Reference Clark, Lipe and Bilbrey1998; Garland et al., Reference Garland, Beer, Eppingstall and O’Connor2007; Guétin et al., Reference Guétin2009; Raglio et al., Reference Raglio2008, Reference Raglio2010a, Reference Raglio2010b; Remington, Reference Remington2002; Sung et al., Reference Sung, Chang, Lee and Lee2006; Thornley et al., Reference Thornley, Hirjee and Vasudev2016; Tuet and Lam Reference Tuet and Lam2006), six trials are of hierarchy III-1 (Chang et al., Reference Chang, Huang, Lin and Lin2010; Ledger and Baker, Reference Ledger and Baker2007; Irish et al., Reference Irish2006; Suzuki et al., Reference Suzuki2004, Reference Suzuki, Kanamori, Nagasawa, Tokiko and Takayuki2007; van de Winckel et al., Reference van de Winckel, Feys, De Weerdt and Dom2004), two trials are of hierarchy III-2 (Cooke et al., Reference Cooke, Moyle, Shum and Harrison2010; Nair et al., Reference Nair, Heim, Krishnan, D’Este and Marley2011), and three trials are of hierarchy IV (Ashida, Reference Ashida2000; Ray and Mittelman, Reference Ray and Mittelman2017; Sung and Chang, Reference Sung and Chang2010). The delivery approach, music type, content, session type, duration, frequency of application, and the total intervention time of Music Therapy vary across trials.
The delivery approach can be classified into receptive and active. The PwD only listened to the music in the receptive approach, while the PwD interacted with the music by singing or playing with instruments in the active approach. The music type can be divided into recorded and live. The contents of the music used were either generic music or music preferred by PwD. The session type was either an individual session or a group session. The duration of music ranged from 10 to 60 minutes in the trials, which reported duration. The reported frequency of application varied from once a week to everyday, and the reported total intervention time spanned from two weeks to one year.
The outcomes of Music Therapy are mainly positive. Seventeen trials demonstrated that one or more BPSD symptoms had reduced significantly (Ashida, Reference Ashida2000; Chang et al., Reference Chang, Huang, Lin and Lin2010; Clark et al., Reference Clark, Lipe and Bilbrey1998; Cooke et al., Reference Cooke, Moyle, Shum and Harrison2010; Garland et al., Reference Garland, Beer, Eppingstall and O’Connor2007; Guétin et al., Reference Guétin2009; Raglio et al., Reference Raglio2008, Reference Raglio2010a, Reference Raglio2010b; Ray and Mittelman, Reference Ray and Mittelman2017; Remington, Reference Remington2002; Sung et al., Reference Sung, Chang, Lee and Lee2006; Sung and Chang, Reference Sung and Chang2010; Suzuki et al., Reference Suzuki2004, Reference Suzuki, Kanamori, Nagasawa, Tokiko and Takayuki2007; Tuet and Lam Reference Tuet and Lam2006; van de Winckel et al., Reference van de Winckel, Feys, De Weerdt and Dom2004). Two trials showed that the change in BPSD was insignificant (Irish et al., Reference Irish2006; Ledger and Baker, Reference Ledger and Baker2007), and two trials found that the BPSD symptoms deteriorated after the intervention (Nair et al., Reference Nair, Heim, Krishnan, D’Este and Marley2011; Thornley et al., Reference Thornley, Hirjee and Vasudev2016). Only seven trials considered the auditory capability of the PwD (Chang et al., Reference Chang, Huang, Lin and Lin2010; Garland et al., Reference Garland, Beer, Eppingstall and O’Connor2007; Ray and Mittelman, Reference Ray and Mittelman2017; Remington, Reference Remington2002; Sung et al., Reference Sung, Chang, Lee and Lee2006; Sung and Chang, Reference Sung and Chang2010; van de Winckel et al., Reference van de Winckel, Feys, De Weerdt and Dom2004). One trial mentioned the music was played “sufficient to be heard throughout the common area,” but it did not specify if the music was sufficient to be heard for the researchers or PwD (Nair et al., Reference Nair, Heim, Krishnan, D’Este and Marley2011). Thus, the author decided this trial did not consider the hearing capabilities of PwD.
Aromatherapy
Nine trials have investigated the effectiveness of Aromatherapy; five trials were of NHMRC evidence hierarchy II (Akhondzadeh et al., Reference Akhondzadeh, Noroozian, Mohammadi, Ohadinia, Jamshidi and Khani2003; Ballard et al., Reference Ballard, O’Brien, Reichelt and Perry2002; Fu and Moyle, Reference Fu and Moyle2013; Holmes et al., Reference Holmes, Hopkins, Hensford, MacLaughlin, Wilkinson and Rosenvinge2002; Smallwood et al., Reference Smallwood, Brown, Coulter, Irvine and Copland2001), one trial was of hierarchy III-1 (Snow et al., Reference Snow, Hovanec and Brandt2004), and three trials were of hierarchy III-2 (Lin et al., Reference Lin, Chan, Ng and Lam2007; O’Connor et al., Reference O’Connor2013; Yoshiyama et al., Reference Yoshiyama, Arita and Suzuki2015). The delivery approach, formulation, concentration, frequency of application, duration, and total intervention time of Aromatherapy varied across trials.
The essential oil was delivered to the PwD through massage, spray, or diffuser. The formulation of essential oil used was either Lavender or Melissa officinalis with concentrations varying from 2% to 100%. The reported frequency of application ranged from twice daily to three times a week, and the reported duration ranged from two hours to the whole night. The reported total intervention time spanned from ten days to four months.
The outcomes of Aromatherapy are mixed. Four trials showed the BPSD symptoms were significantly alleviated after the intervention, in which the essential oil was applied dermally or via a high-concentration spray (Akhondzadeh et al., Reference Akhondzadeh, Noroozian, Mohammadi, Ohadinia, Jamshidi and Khani2003; Ballard et al., Reference Ballard, O’Brien, Reichelt and Perry2002; Lin et al., Reference Lin, Chan, Ng and Lam2007; O’Connor et al., Reference O’Connor2013). The remaining five trials, in which the essential oil was delivered by spray in low concentration, found no significant reduction in BPSD (Fu and Moyle, Reference Fu and Moyle2013; Holmes et al., Reference Holmes, Hopkins, Hensford, MacLaughlin, Wilkinson and Rosenvinge2002; Smallwood et al., Reference Smallwood, Brown, Coulter, Irvine and Copland2001; Snow et al., Reference Snow, Hovanec and Brandt2004; Yoshiyama et al., Reference Yoshiyama, Arita and Suzuki2015). None of the trials considered the olfactory capability of PwD.
Light Therapy
Five trials evaluated the effectiveness of Light Therapy; three trials were of NHMRC evidence hierarchy II (Ancoli-Israel et al., Reference Ancoli-Israel2003; Burns et al., Reference Burns, Allen, Tomenson, Duignan and Byrne2009; Riemersma-van der Lek et al., Reference Riemersma-van der Lek, Swaab, Twisk, Hol, Hoogendijk and Van Someren2008) and the other two trials were of hierarchy III-1 (Barrick et al., Reference Barrick2010; Hickman et al., Reference Hickman2007). The brightness, duration, and total intervention time of Light Therapy varied across trials.
The reported brightness of the light ranged from 1000 lux to 10,000 lux in the intervention groups and 100 lux to 300 lux in the control groups. The duration of the Light Therapy varied from 120 minutes to a whole day, and the total intervention time spanned from 10 days to 3.5 years.
The outcomes of Light Therapy are mixed. The clinical trial with the best experimental design found that agitation and depression were significantly reduced in a follow-up ranging from 0.5 to 3.5 years. In this trial, bright light was applied for the whole day with or without melatonin in the intervention group, and dim light with placebo in the control group. This is a 2 x 2 factorial double-blind cluster-randomized trial comparing two light settings in several nursing homes, with a total of 189 participants, of whom 87% had dementia (Riemersma-van der Lek et al., Reference Riemersma-van der Lek, Swaab, Twisk, Hol, Hoogendijk and Van Someren2008). However, three trials found that Light Therapy had no significant effect in reducing BPSD (Ancoli-Israel et al., Reference Ancoli-Israel2003; Barrick et al., Reference Barrick2010; Burns et al., Reference Burns, Allen, Tomenson, Duignan and Byrne2009; Hickman et al., Reference Hickman2007), and one trial reported that the agitation in PwD was worsened (Barrick et al., Reference Barrick2010). One of the five trials did not consider the visual capability of PwD (Ancoli-Israel et al., Reference Ancoli-Israel2003).
Snoezelen Therapy
Snoezelen Therapy, also referred to as Multisensory Therapy, was investigated by two trials both with NHMRC evidence hierarchy lV (Berkheimer et al., Reference Berkheimer, Qian and Malmstrom2017; van Weert et al., Reference van Weert, van Dulmen, Spreeuwenberg, Ribbe and Bensing2005). One trial compared Snoezelen Therapy with Exercise Therapy for three weeks and found that the reduction in agitation was not significant under both intervention types (Berkheimer et al., Reference Berkheimer, Qian and Malmstrom2017). The other trial integrated Snoezelen Therapy into 24-hour care for 18 months, with each resident having a personal care plan, and found significant reduction in apathy, aggression, and depression after the intervention (van Weert et al., Reference van Weert, van Dulmen, Spreeuwenberg, Ribbe and Bensing2005). Snoezelen Therapy is designed with the sensory capabilities of PwD in mind. Declines in sensory capabilities (i.e. hearing, vision, taste, smell, touch) are common in elderly adults, with the rate of decline varying across senses for each person. By stimulating a few senses together in Snoezelen Therapy, a PwD with sensory impairments in less than five senses could still receive stimuli from the therapy via their remaining functional senses. According to the guidelines for designing perceptible information for PwD, different modes (pictorial, verbal, tactile) should be used together to present essential information (Mäki and Topo, Reference Mäki and Topo2009). Hence, all trials applying Snoezelen Therapy have considered sensory capabilities of PwD.
Technology-Assisted Therapy
A Technology-Assisted Therapy is the intervention type in which the roles of caregivers or therapists are replaced by technologies. Five trials were found for this intervention type. One trial explored the effectiveness of a personalized multimedia device (Davison et al., Reference Davison2016). The device can play music and display images, films, and messages that were selected or made by family members of the PwD. This trial was of NHMRC hierarchy level II and showed a significant reduction in agitation in PwD after the intervention. It also reported that assistance was needed and offered for PwD in late stages. This personalized multimedia device is designed with the touch capabilities of PwD in mind. Specifically, researchers found some PwD find touch sensitive icons confusing in preliminary field testing, thus they added traditional buttons affixed to the screen for those PwD with that preference.
Four trials examined the effectiveness of a therapeutic robot (Joranson et al., Reference Joranson, Pedersen and Mork2015a, Reference Joranson, Pedersen, Rokstad and Ihlebaek2015b, Reference Joranson, Pedersen, Rokstad and Ihlebaek2016; Moyle et al., Reference Moyle2017). This robot, with an appearance of a baby seal, was placed on the lap of PwD. The robot generated movement and sound mimicking the baby seal to give somatosensory and auditory stimulations to PwD. The four trials were of NHMRC evidence hierarchy III-1 and reported that agitation and depression in PwD were significantly reduced with no reduction in antipsychotic medication after the intervention. One trial applied the robot in individual sessions (Moyle et al., Reference Moyle2017), and the remaining three trials applied the robot in group sessions (Joranson et al., Reference Joranson, Pedersen and Mork2015a, Reference Joranson, Pedersen, Rokstad and Ihlebaek2015b, Reference Joranson, Pedersen, Rokstad and Ihlebaek2016). This robot is designed with the sensory capabilities of PwD in mind as it stimulates several senses in PwD, which is similar to Snoezelen therapy. Therefore, all trials in this intervention type have considered the capabilities of PwD.
Other sensory-oriented interventions
Three sensory-oriented interventions were evaluated by one trial only. Animal-Assisted Therapy was examined by a trial of NHMRC evidence hierarchy III-1 (Olsen et al., Reference Olsen, Pedersen, Bergland, Enders-Slegers, Patil and Ihlebaek2016). This therapy organized regular dog visits in a group session accompanied by a dog handler, which found depression in PwD was reduced significantly after the intervention. Positive Image Therapy was assessed by a trial of hierarchy II (Chou et al., Reference Chou, Waszynski, Kessler, Chiang and Clarkson2016). This therapy displayed images preferred by PwD with voice prompt during bathing time, which led to fewer behavioral problems. However, the sample size of this trial was too small to calculate the significance. Clowning Therapy was evaluated by a trial of hierarchy IV (Kontos et al., Reference Kontos2016). The elder clowns visited the nursing home regularly to entertain the PwD for 12 weeks and reported that BPSD was reduced significantly after the intervention. In the Animal-Assisted Therapy and Clowning Therapy, several senses of PwD were stimulated, which is similar to Snoezelen Therapy. In the Positive Image Therapy, visual impairment was used as an exclusion criterion in the clinical trial. Therefore, all three trials have considered the sensory capabilities of PwD.
Cognition-oriented therapy
Reminiscence Therapy
Five trials examined the effectiveness of Reminiscence Therapy, which were all of NHMRC evidence hierarchy level II (Haight et al., Reference Haight, Gibson and Michel2006; Haslam et al., Reference Haslam, Haslam, Jetten, Bevins, Ravenscroft and Tonks2010; Lai et al., Reference Lai, Chi and Kayser-Jones2004; Politis et al., Reference Politis, Vozzella, Mayer, Onyike, Baker and Lyketsos2004; Wang, Reference Wang2007). The session type, structure, duration, and total intervention time of Reminiscence Therapy varied across trials. The therapy has been conducted in group or individual sessions via unstructured, semi-structured, and structured activities. The reported duration of one session ranged from 30 to 60 minutes, and the reported total intervention time went from four to eight weeks.
Three trials showed the apathy, agitation, and depression of PwD were significantly reduced after the intervention (Haight et al., Reference Haight, Gibson and Michel2006; Lai et al., Reference Lai, Chi and Kayser-Jones2004; Politis et al., Reference Politis, Vozzella, Mayer, Onyike, Baker and Lyketsos2004), and the remaining two trials showed the reduction was insignificant (Haslam et al., Reference Haslam, Haslam, Jetten, Bevins, Ravenscroft and Tonks2010; Wang, Reference Wang2007). This intervention is designed with the memory and attention capabilities of PwD in mind. In terms of memory, this intervention stimulates the long-term memory, which is relatively intact in PwD, compared to other types of memories. In terms of attention, this intervention involves interactions between caregivers and PwD to keep PwD concentrated during the intervention. These interactions are essential as the capability of inhibiting irrelevant information is declined in PwD. According to the guidelines for designing for PwD, the design should remind PwD about their previous experiences and give feedback immediately, given the short attention span of the PwD (Mäki and Topo, Reference Mäki and Topo2009). Thus, the main cognitive capabilities of PwD were considered in the trials.
Simulated Presence Therapy
Three trials investigated Simulated Presence Therapy, with one trial of NHMRC evidence hierarchy III-1 (Camberg et al., Reference Camberg1999) and two trials of hierarchy IV (Cheston et al., Reference Cheston, Thorne, Whitby and Peak2007; Miller et al., Reference Miller, Vermeersch, Bohan, Renbarger, Kruep and Sacre2001). All trials applied audio recordings to simulate a phone call from a family member of the PwD to engage PwD in conversations.
One trial reported that the response of PwD varied widely from dramatic reduction in distress to apparent indifference (Cheston et al., Reference Cheston, Thorne, Whitby and Peak2007). One trial found that some PwD recognized this phone call is a simulation and refused to listen again (Miller et al., Reference Miller, Vermeersch, Bohan, Renbarger, Kruep and Sacre2001). One trial reported that the “interest” subscale of Positive Affect Rating Scale was significantly increased after the intervention; however, there was no change in BPSD (Camberg et al., Reference Camberg1999).
The main cognitive capabilities of PwD were not fully considered in the intervention. In terms of memory, the phone call could trigger the long-term memory of the PwD by the familiar voices. However, in terms of attention, the interactions between PwD and the audio recordings were limited at the audial level, and this lack of sensory engagement could result in the PwD getting easily distracted during the therapy. Besides, it is difficult for caregivers to check if PwD are distracted. None of the trials have examined if PwD had the adequate capability to inhibit irrelevant information to ensure they can concentrate throughout the therapy. Therefore, these trials have not fully considered the main cognitive capabilities of PwD.
Other cognition-oriented interventions
Two cognition-oriented interventions were examined by one trial only. Cognitive Stimulation Therapy was evaluated by a trial of NHMRC hierarchy II, which found apathy and depression in PwD were reduced significantly after the intervention (Niu et al., Reference Niu2010). Storytelling Therapy was assessed by a trial of hierarchy III-1, which found no significant reduction in BPSD (Houser et al., Reference Houser, George and Chinchilli2014). The Cognitive Stimulation Therapy provides cognitive games to stimulate PwD, and its clinical trial considered the cognitive capabilities of PwD by only including PwD in their early to moderate stage (Niu et al., Reference Niu2010). The Storytelling Therapy prompts a group of PwD to give comments about a picture and uses these comments to form a story, and its clinical trial had no assessment on cognitive capabilities of PwD participants.
Movement-oriented therapy
Exercise Therapy
Five trials examined the effectiveness of Exercise Therapy; four trials were of NHMRC evidence hierarchy II (Hokkanen et al., Reference Hokkanen, Rantala, Remes, Härkönen, Viramo and Winblad2008; Rolland et al., Reference Rolland2007; Telenius et al., Reference Telenius, Engedal and Bergland2015a, Reference Telenius, Engedal and Bergland2015b) and one trial was of hierarchy III-1 (Treusch et al., Reference Treusch, Majic, Page, Gutzmann, Heinz and Rapp2015). The content of the exercise varied across trials from intensive strengthening to dance. Ranking the intensity of the exercise in these trials is difficult due to the lack of detailed exercise description in each trial. The total intervention time ranged from 12 weeks to 12 months in these trials.
Four trials found apathy and agitation in PwD reduced significantly (Hokkanen et al., Reference Hokkanen, Rantala, Remes, Härkönen, Viramo and Winblad2008; Telenius et al., Reference Telenius, Engedal and Bergland2015a, Reference Telenius, Engedal and Bergland2015b; Treusch et al., Reference Treusch, Majic, Page, Gutzmann, Heinz and Rapp2015), while one trial found no significant reduction in BPSD after the intervention (Rolland et al., Reference Rolland2007). All trials in this intervention type considered the movement capabilities of PwD by excluding PwD who were not capable to move independently with or without an assistive device.
Outdoor Activity Therapy
Four trials examined the effectiveness of Outdoor Activity Therapy; three trials were of hierarchy IV (Calkins et al., Reference Calkins, Szmerekovsky and Biddle2007; Luk et al., Reference Luk2011; Vuolo, Reference Vuolo2003) and one trial was of hierarchy II (Connell et al., Reference Connell, Sanford and Lewis2007). The content, duration, frequency of application, and total intervention time varied across trials. The reported content included gardening, horticultural therapy, and walking outdoors, and the duration ranged from 30 to 60 minutes. The reported frequency of application went from daily to twice a week, and the total intervention time spanned from 2 weeks to 12 months.
One trial found a significant reduction in verbal agitation in PwD (Connell et al., Reference Connell, Sanford and Lewis2007), and one trial reported the physically non-aggressive behavior in PwD reduced significantly after the intervention (Vuolo, Reference Vuolo2003). Two remaining trials found no significant reduction in BPSD (Calkins et al., Reference Calkins, Szmerekovsky and Biddle2007; Luk et al., Reference Luk2011). All trials considered the movement capabilities of PwD by only including PwD who were capable to move independently or with an assistive device.
Discussion
As far as we are aware from the current literature, this is the first study to systematically examine the effectiveness of non-pharmacological interventions for BPSD in nursing homes, with an additional assessment criterion based on ergonomics, specifically, capability consideration. The current review investigated whether the capabilities of PwD were considered in the interventions and their clinical trials. The interventions were categorized according to the capabilities of PwD required to participate, which are sensory-, cognition-, and movement-oriented. The quality of evidence for the effectiveness of these interventions is found to be low in general. Sensory-oriented interventions have been explored and evaluated more than cognition- and movement-oriented interventions, with Music Therapy being investigated by the highest number of trials. In terms of capability consideration, the sensory capabilities have been considered less than cognitive and movement capabilities, especially for auditory and olfactory capabilities.
To elaborate on the capability considerations in clinical trials, the trials satisfied the first “capability consideration” criterion were on Snoezelen Therapy, Technology-Assisted Therapy, Animal-Assisted Therapy, Clowning Therapy, and Reminiscence Therapy. These interventions have accommodated capability considerations in them, thus the trials on these interventions have considered the capabilities in PwD. For interventions without capability considerations (i.e. Music Therapy, Aromatherapy, Light Therapy, Positive Image Therapy, Simulated Presence Therapy, Cognitive Stimulation Therapy, Storytelling Therapy, Exercise Therapy and Outdoor Activity Therapy), it is essential for trials to only include PwD with adequate capabilities for the intervention outcomes to be reliable. From this review, clinical trials on Positive Image Therapy, Cognitive Stimulation Therapy, and all movement-oriented interventions have assessed the capabilities of PwD for inclusion. Two-thirds of the clinical trials on Music Therapy, one-fifth of the clinical trials on Light Therapy, and all clinical trials on Aromatherapy, Simulated Presence Therapy, and Storytelling Therapy have not assessed the capabilities of PwD for inclusion. This lack of capability consideration implies that not all participants might have had the capabilities that the interventions required. For instance, not all participants can hear the music in a trial on Music Therapy. Consequently, the effectiveness of these interventions would have been underestimated, and, thus, a lack of capability consideration could reduce the quality of a trial and consequently lower the quality of evidence about the corresponding intervention type.
A few factors could cause the sensory capabilities to be considered less than cognitive and movement capabilities. The sensory capabilities are more difficult to measure, and the PwD cannot actively communicate their sensory experiences due to their cognitive impairments. In contrast, the remaining cognitive capabilities of PwD are relatively well-tracked, as they are assessed regularly to monitor how their dementia progresses. The movement capabilities are crucial for the safety of PwD and, thus, were also assessed carefully in movement-oriented interventions.
To include capabilities of PwD in designing interventions and clinical trials, three approaches should be considered. First, clinical trials should include capability requirements in their in- and exclusion criteria and indicate that the capabilities of PwD participants were adequate to participate in the interventions. This practice should be used as a criterion for evaluating the quality of a trial in systematic reviews. Second, a capability profile should be created for each PwD in terms of sensory, cognition, and movement aspects. The suitable interventions for a PwD can then be identified by matching the capability profile with the capability requirements of the interventions. The capabilities of PwD can be tracked over time with this profile to identify new interventions when the current interventions are no longer suitable due to capability decline. This profile also helps researchers to identify suitable PwD for clinical trials. Third, the interventions themselves should be designed with the capabilities of PwD in mind by incorporating design guidelines for PwD or involving PwD in the design process. An intervention with adequate capability consideration should be able to include as many capability impairments as possible or adapt to the remaining capabilities of PwD. For example, bed-bound exercises could be designed for PwD who are immobile. The third approach is the most fundamental because some capabilities are difficult and costly to measure regularly. Moreover, by designing the interventions with capability considerations, more PwD with capability impairments will be able to participate and, thus, benefit from the interventions. With more participants, the clinical trials on non-pharmacological interventions for BPSD could have a larger sample size, which could improve the quality of evidence. Since ergonomics has a human-centered approach and has investigated age-related capability changes, it would facilitate the intervention design process.
A meta-analysis is impossible due to the following limitations of the clinical trials. Firstly, the outcomes have been measured by a broad range of validated scales and physiological parameters. Over 60 scales have been used with some scales focusing on one aspect of BPSD (e.g., Cohen-Mansfield Agitation Inventory [Cohen-Mansfield, Reference Cohen-Mansfield1997]) while other scales measuring BPSD comprehensively (e.g., Neuropsychiatric Inventory [Kaufer et al., Reference Kaufer2000]). The physiological parameters include heart rate, cortisol level, and skin conductance. Comparing the outcomes of these trials is difficult due to this wide variation in measurement methods. In addition, the design of control groups varies across trials. Some trials applied a placebo activity in the control group (e.g., reading activity as a placebo for Music Therapy [Cooke et al., Reference Cooke, Moyle, Shum and Harrison2010]), while most trials applied “usual care” in the control group. “Usual care” was not clearly described in these trials and further hinders comparison. Moreover, some trials have not accounted for potential confounding factors. For instance, the social interactions between PwD might also contribute to reducing BPSD for Music Therapy conducted in a group session (Sung et al., Reference Sung, Chang, Lee and Lee2006; Suzuki et al., Reference Suzuki2004; van de Winckel et al., Reference van de Winckel, Feys, De Weerdt and Dom2004). The current study demonstrates a lack of standardization and heterogeneity in study designs of nonpharmacological interventions in clinical trials of BPSD. Therefore, improving data homogeneity, study designs, and reporting in future clinical trials is urgent, which is in accordance with previous systematic reviews (Abraha et al., Reference Abraha2017; Brasure et al., Reference Brasure2016; Cohen-Mansfield, Reference Cohen-Mansfield2001).
The link between the effectiveness of non-pharmacological interventions and their capability considerations cannot be drawn due to the abovementioned limitations in clinical trials. These limitations make comparisons between intervention outcomes across clinical trials difficult. A hypothesis is that interventions that consider capabilities in PwD are more effective than interventions that do not. This hypothesis needs to be examined in future clinical trials; that is, by comparing the outcome measurements of the same intervention with and without capability considerations. This comparison can only be carried out when these clinical trials have overcome the limitations.
Apart from capability considerations, treatment parameters could also affect the effectiveness of the interventions, such as the dose, timing, and duration of an intervention. Cohen-Mansfield stated that the effectiveness of an intervention may depend on these parameters, instead of the inherent applicability of the intervention type (Cohen-Mansfield, Reference Cohen-Mansfield2001). As treatment parameters were not commonly reported in clinical trials, together with the abovementioned limitations in these trials, it is impossible to conclude on how these parameters affected the effectiveness of an intervention, which is a remaining challenge for future studies.
A limitation of this review is that it is only focused on if the capabilities of the PwD are considered in the interventions and their clinical trials. We did not include caregiver interventions in the current study, as the focus of the current review is on capability considerations of PwD. The capabilities of caregivers are also vital for the interventions to be carried out reliably, thus to ensure high quality of evidence. For example, if a caregiver can accomplish the non-pharmacological intervention given the time constraints; and if the training is adequate for the caregiver to understand how to operate a device used for the intervention. However, it is not common for clinical trials to report these details, thus, the consideration of the capabilities of caregivers cannot be made in the current review. This review provides a starting point for considering human capabilities in non-pharmacological interventions and the subsequent clinical trials for BPSD in nursing homes. In future trials, it might be worthwhile to consider the capabilities of both PwD and caregivers. The fact that not all interventions were truly based on capabilities of PwD, and that 28 out of 64 trials did not consider capabilities, is also a limitation of the current review. It would be worthwhile for future studies to explore how to assess if capabilities of PwD are truly considered.
Despite the fact that the current quality of evidence is low, the evidence indicates that a wide variety of non-pharmacological interventions for BPSD in nursing homes have been developed and carried out with a few reported adverse effects. The consideration of human capabilities for antipsychotic medications is not as vital as that of non-pharmacological interventions, which indicates that non-pharmacological interventions are more challenging to be implemented. Given their fewer adverse effects than that of medication, future studies focusing on non-pharmacological interventions for BPSD should include capability considerations for PwD to gather more high-quality evidence.
Recommendations for future research
The findings lead to the following five recommendations for future studies:
Capability requirements for PwD should be included in the in- and exclusion criteria of clinical trials on non-pharmacological interventions
Capability requirements should be included as a criterion for assessing the quality of clinical trials on non-pharmacological interventions in systematic reviews
The sensory, cognition, and movement capabilities of each PwD should be assessed regularly over time and recorded in a profile to identify suitable interventions for each PwD and ease the selection process in clinical trials.
Non-pharmacological interventions should be designed with the capabilities of PwD in mind under the guidance of ergonomics.
Clinical trials should be conducted more systematically by establishing consensus on outcome measurements, refining study designs, developing reporting standards, and managing confounding factors.
Conflict of interest
None.
Description of authors’ roles
G. Wang and T. J. M. van der Cammen conceived this study and carried out the systematic review. G. Wang wrote the initial draft of the manuscript. All authors were involved in the critical revisions of the manuscript.
Acknowledgments
This research was supported by the Chinese Scholarship Council (CSC), China.
Supplementary material
To view supplementary material for this article, please visit https://www.cambridge.org/core/journals/international-psychogeriatrics.






