- AI
adequate intake
- DRI
dietary reference intake
- DRV
dietary reference value
- EAR
estimated average requirement
- FW
fresh weight
- LRNI
lower reference nutrient intake
- RNI
reference nutrient intake
The essential mineral elements
An average human individual comprises about 94% C, H and O. Most of the remainder is composed of a few inorganic mineral elements (‘minerals’) that are required for normal bodily functioning. Eight minerals are required in relatively large amounts (100–>1000 mg/d): Ca, Cl, Mg, N, P, K, Na and S. These eight minerals, together with C, H and O account for 99·98% body mass(1, Reference Emsley2). Nine further minerals (trace elements) are required in smaller quantities (<10 mg/d): Cu, Co, iodine, Fe, Mn, Mo, Ni, Se and Zn. Numerous additional trace elements are also present in human subjects, many with proven biochemical roles in some organisms, e.g. arsenic, B, Br, Cd, F, Si, W and V, although their essentiality for human subjects is unclear.
Mineral deficiencies are likely to be widespread
Much of the world's population is likely to be deficient in one or more essential minerals and at increased risk of physiological disorders. Most at risk are those whose dietary energy consumption is continuously below the minimum dietary energy requirement. The minimum dietary energy requirement is a defined level required to maintain health according to a height-normalised acceptable minimum body-weight, assuming light physical activity(3). For the period 2004–2006, the global average minimum dietary energy requirement was 7·6 MJ (1825 kcal) per person per d, with 872·9 million people estimated to be energy-malnourished from a total population of 6·5 billion. Those with energy intakes above the minimum dietary energy requirement are unlikely to be deficient in N, P and S due to the high proportion of these elements present as proteins in many foods. Similarly, Cl and Na deficiencies are unlikely due to their presence in processed food and beverages. In contrast, among populations consuming sufficient or excess energy, many are likely to be deficient in one or more of the major minerals, Ca, Mg or K (the foci of this paper) and in one or more of the micronutrients, notably Cu, Fe, I, Se and Zn(Reference White and Broadley4). Numerous physiological disorders associated with Ca, Mg and K deficiency in human subjects have been recognised(1, 5, 6).
Identifying calcium, magnesium and potassium deficiencies from intake studies
Identifying mineral deficiency risks among those with adequate energy intakes is non-trivial. For the major elements Ca, Mg and K, dietary intake data are used to identify those at risk of mineral deficiency, because (1) Ca, Mg and K are acquired primarily from dietary sources and (2) (bio)assays of Ca, Mg and K status in human subjects can be unreliable due to tight homeostatic control(1, 5, 6).
In the UK, dietary mineral intakes are assessed and planned at a population level using the dietary reference value (DRV) framework. The DRV framework was introduced in 1991 by the Department of Health to replace the previous system of RDA(1). The DRV framework includes an estimated average requirement (EAR), a reference nutrient intake (RNI) and a lower RNI (LRNI) for each mineral (Fig. 1(a)). Half of the group will usually require more of a mineral than the EAR intake level, and half will require less(1). The RNI and LRNI are defined as the intakes of two standard deviations (sd) above and below the EAR, respectively (Fig. 1(a)). The RNI defines an intake that is sufficient, or more than sufficient, for about 97% of a population. If the average mineral intake of a group approximates the RNI, mineral deficiency risk within that group is considered small (e.g. Fig. 1(b)). The LRNI defines an intake that is sufficient only for those few people in the group who have low needs, and therefore intakes below the LRNI carry a very high risk of deficiency(1). For UK adults in the age range 19–64 years, the RNI/EAR/LRNI for Ca is 700/525/400 mg/d, and for Mg is 270/200/150 (for females) and 300/250/190 (for males) mg/d (Table 1). No EAR has been set for K, whose RNI/LRNI is 3500/2000 mg/d (Table 1). For UK adults, the DRV framework assumes reference body weights of 60 and 62 kg for females and 74 and 71 kg for males, for the age range 19–50 and >50 years, respectively, and that mineral intakes among the population are normally distributed.

Fig. 1. The UK dietary reference value framework. (a) Estimated average requirement (EAR), reference nutrient intake (RNI), and lower RNI (LRNI), based on a hypothetical population with an EAR for a nutrient of 100 arbitrary units and an sd of 20. Data are expressed as a probability density distribution (- - -) and as a cumulative probability function (–––). (b) Illustrative nutrient intake among a population whose mean intake equals the RNI (sd=20) and which is normally distributed. The probability density distribution is the grey-shaded area; the cumulative probability function () shows that the risk of intake deficiency is extremely low. Adapted from Department of Health(1).
Table 1. Calcium, magnesium and potassium intakes: dietary reference value framework for UK adults(1) and dietary reference intake system for US adults(5, 6)

RNI, reference nutrient intake; LRNI, lower RNI; EAR, estimated average requirement; AI, adequate intake.
In the USA, dietary intakes are assessed using the dietary reference intake (DRI) system(5, 6). The DRI system also includes an EAR, analogous to the UK EAR and which is used for population-level dietary assessments and policy planning. In addition to population-level assessments, an RDA or an ‘adequate intake’ (AI) level is also specified as a potential target intake level for individuals. The RDA is set at 2 sd above the EAR, assuming that an EAR is available and that the requirement for a mineral is normally distributed among a population. If the requirement for a mineral is known to be skewed, other approaches are used to set the RDA. An AI is specified as an individual target level if there are insufficient data to calculate an EAR. The AI is based on observed or experimentally determined average mineral intakes among healthy people. A summary of US DRI data for Ca, K and Mg intakes is provided for healthy adult females and males (Table 1). There are no US EAR for Ca and K intakes.
Dietary surveys in the UK and USA show that calcium, magnesium and potassium deficiencies are widespread
Since different foods and drinks are consumed day-to-day, estimates of dietary mineral intake are based on food and drink intakes averaged over several days. In the UK, the most recent dietary survey reporting mineral intakes is the National Diet and Nutrition Survey, commissioned by the UK Food Standards Agency. Adults aged 19–64 were asked to record the weights of all foods and drinks consumed over a period of seven consecutive days within and outside of the home(Reference Henderson, Irving and Gregory7, Reference Hoare, Henderson and Bates8). In the USA, the most recent comprehensive dietary surveys are those reported by the US Department for Agriculture (Continuing Survey of Food Intakes by Individuals), and by the US National Center for Health Statistics (Third National Health and Nutrition Examination Survey). The Continuing Survey of Food Intakes by Individuals and Third National Health and Nutrition Examination Survey assessed mineral intakes based on dietary-recall at interview, again among representative population samples(Reference Dwyer, Picciano and Raiten9). Mineral intakes are subsequently estimated from dietary survey data using comprehensive mineral composition tables available for a wide variety of foodstuffs in the UK(10) and in the USA(11).
Ca, Mg and K intakes from all sources among UK(Reference Henderson, Irving and Gregory7) and US(5, 6) population samples are summarised in Fig. 2. For the UK, cumulative frequencies of Ca, Mg and K intakes among the sample are plotted as functions of mineral intakes of Ca, Mg and K, directly as reported (Fig. 2). For the USA, cumulative intake frequencies are reported in separate age bins, 19–30, 31–50 and 51–70(5, 6). Therefore, to enable comparison with UK intakes, US Ca, Mg and K intakes were estimated for all females and all males (aged 19–70) using weighted means(5, 6). Subsequently, the number of UK and US adults at risk of Ca, Mg and K deficiency was estimated by comparing reported intakes directly with UK DRV. Deficiency risk is defined here in simplistic terms as an intake ⩽UK LRNI. For UK data, the percentile of the sample at each DRV is reported directly. For US data, the proportion of the population at or below UK DRV thresholds was interpolated from reported cumulative frequency distributions. The use of the UK LRNI to estimate Ca, Mg and K deficiency risks among both UK and US adults is justified, since there is no US LRNI or EAR for Ca or K, and since US RDA and AI are not intended to guide policy-level decision-making directly(5, 6).

Fig. 2. Ca, Mg and K intakes among UK (•) and US (○) adults reported in dietary intake surveys(5–Reference Hoare, Henderson and Bates8), as cumulative probability functions. The UK dietary reference value framework terms lower reference nutrient intake (LRNI), estimated average requirement (EAR) and reference nutrient intake (RNI), and the US dietary reference intake system terms RDA or adequate intake (AI) (for the age group 31–50) are indicated with dotted lines. UK intake data are reported directly(Reference Henderson, Irving and Gregory7, Reference Hoare, Henderson and Bates8), US intake data are interpolated from flanking percentile groups(5, 6).
Many UK and US adults are at risk of Ca, Mg and/or K deficiency (Fig. 2; Table 2). At highest risk of Ca deficiency, i.e. intakes <UK LRNI, are 25·4 million adults (i.e. about 9% of the estimated UK and US combined population of 288 million adults), with 25·3 million (about 9%) and 41·4 million (about 14%) at similarly high risk of Mg and K deficiency, respectively. There are large differences in deficiency risks between genders, with females at increased risk of Ca, Mg and K deficiency (Table 2). For Ca, 5 and 16% of UK and US females, respectively, have intakes below the UK LRNI, compared to 2 and 3% of UK and US males, respectively. For Mg, 13 and 12% of UK and US females, respectively, have intakes below the UK LRNI, compared to 9 and 5% of UK and US males, respectively. For K, 19 and 27% of UK and US females, respectively, have intakes below the UK LRNI, compared to 6 and 3% of UK and US males, respectively. Notably, dietary intakes of Ca, Mg, and K show much greater variation among males (Fig. 2). While care must be taken not to over-extend this analysis, it is noteworthy that most UK and US adults are likely to have intakes below the UK RNI of one or more of Ca (43%), Mg (58%) and K (76%). The UK RNI is explicitly not intended to be used as an individual target intake. However, the UK RNI is 20–40% less than the US RDA for Ca, Mg and K and the US RDA is explicitly, ‘ … intended to be used as a goal for daily intake by individuals’(6). Thus, while intakes below the UK RNI are not indicative of dietary deficiency risk, dietary intakes of Ca, Mg and K are likely to be suboptimal for a majority of the UK and US adult population.
Table 2. UK and US adults at risk of sub-optimal Ca, Mg and K intake based on dietary surveys(5–Reference Hoare, Henderson and Bates8)
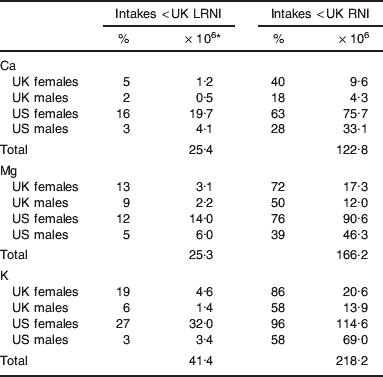
RNI, reference nutrient intake; LRNI, lower RNI.
* Assuming 48 and 240 million UK and US adults, respectively, 50:50 males:females.
Why are calcium, magnesium and potassium deficiencies widespread?
There are several possible causes of sub-optimal Ca, Mg and K intakes among UK and US adults. In general, very few UK and US adults are likely to be energy malnourished involuntarily. The UK and USA are ranked 16th and 1st, respectively, among countries in terms of dietary energy availability per person (US, 16·0 MJ/d; UK, 14·4 MJ/d), from food production and trade data(3). However, energy availability is not equivalent to energy consumption. In the UK, the energy intakes reported in the National Diet and Nutrition Survey are 6·9 and 9·7 MJ/d for females and males, respectively(Reference Hoare, Henderson and Bates8). Energy intake in the UK is therefore much less than the energy availability. In addition to significant under-reporting of energy intakes, this reflects food wastage and cultural factors such as the intentional restriction of energy intake to lose weight. During the National Diet and Nutrition Survey, 24 and 10% of females and males, respectively, reported they were dieting to lose weight(Reference Hoare, Henderson and Bates8). Those dieting to lose weight are likely to be at increased risk of Ca, K and Mg intake deficiencies. However, all dietary survey data must be interpreted with extreme care since significant under-reporting of energy intake is likely to be widespread, especially among overweight and obese adults. For example, among healthy UK adults not restricting their energy intake intentionally during the National Diet and Nutrition Survey, under-reporting approximates 25% of energy needs in both sexes(Reference Rennie, Coward and Jebb12), consistent with observations of daily energy expenditure of modern human subjects in the range of 10·2–12·6 MJ/d(Reference Westerterp and Speakman13). The following calculations have not been adjusted for energy-under-reporting, and should therefore be interpreted with due care. However, it is noteworthy that social desirability may also lead to over-reporting of foods perceived to be healthy, including horticultural crops, among some groups(Reference Vuckovic, Ritenbaugh and Taren14, Reference Ovaskainen, Paturi and Reinivuo15).
The UK and USA have diverse diets compared to most countries. In terms of dietary diversity, defined as the contribution of non-starchy foods to total dietary energy consumption, the UK and US are ranked 25th and 2nd, respectively(3). Thus, 68% (UK) and 74% (USA) of energy is estimated to come from non-starchy sources. However, more detailed analysis of dietary surveys reveals that cultural and economic factors increase the risk of Ca, Mg and K deficiencies among UK adults(Reference Henderson, Irving and Gregory7, Reference Hoare, Henderson and Bates8). Three-quarters of Ca intake comes from milk and dairy products (46% for females; 41% for males), and from cereal sources (28% for females; 32% for males) (Fig. 3). The high contribution of cereals to UK Ca intakes is due to UK legislation requiring processed wheat flour to be fortified with Ca and Fe, and the ‘B vitamins’ thiamin and nicotinic acid(16). Thus, processed flour must contain Ca within the range 235–390 mg/100 g flour, with exceptions for wholemeal flour, self-raising flour with Ca >200 mg/100 g, and wheat malt flour. In the absence of fortification, cereal grains are naturally low in Ca. Furthermore, the husks and bran of these grains, which contain greater concentrations of Ca, Mg and K, are removed during milling. Meat and meat products (females=5%, males=7%), vegetables excluding potatoes (females=5%, males=4%), and potatoes and savoury snacks (females=2%, males=1%) account for relatively little Ca intake. Intake of Mg (27%) and K (14%) from cereals is also relatively high. Conversely, horticultural produce contributes more Mg and K than Ca to the average UK adult diet. Vegetables excluding potatoes (females=9%, males=7%), potatoes and savoury snacks (10%), and fruit and nuts (females=8%, males=5%) contribute approximately one-quarter of daily Mg intake. Vegetables excluding potatoes (females=11%, males=9%), potatoes and savoury snacks (18%) and fruit and nuts (females=9%, males=6%) contribute approximately one-third of daily K intake.

Fig. 3. Sources of Ca, Mg and K intake among UK adults as a proportion of mean intake, as reported in the National Diet and Nutrition Survey(Reference Henderson, Irving and Gregory7, Reference Hoare, Henderson and Bates8).
The intake of horticultural crop products (vegetables, fruit and nuts) is well below the UK policy levels. The UK Government recommends that each adult consumes five or more portions of fruit and vegetables (excluding potatoes) daily(Reference Hoare, Henderson and Bates8). However, 85% female and 87% male UK adults consume less than this amount, with a mean/median intake of 2·9/2·4 (females) and 2·7/2·2 (males) portions per day(Reference Hoare, Henderson and Bates8). The average UK adult consumes just 134 g (females) and 137 g (males) of vegetables and vegetable dishes, 124 g (females) and 87 g (males) of fruit (excluding juices), and 96 g (females) and 117 g (males) of potatoes, excluding savoury snacks, per day(Reference Hoare, Henderson and Bates8). Despite low overall consumption, the Ca, Mg and K concentration of specific horticultural crops can be high(10, 11) (Table 3). For example, kale contains 130 mg Ca/100 g fresh weight (FW), 34 mg Mg/100 g FW and 450 mg K/100 g FW. Other leafy Brassica are also good sources of Ca, Mg and K, although mineral losses can occur when leaves are boiled (Table 3). Potatoes are a good dietary source of K and Mg, but do not tend to deliver high quantities of Ca to the diet. Similarly, onions and carrots provide a good dietary source of K, but typically deliver less Ca and Mg than Brassica leaves.
Table 3. Ca, Mg and K concentrations of edible portions of selected leaf, tuber and root crops from UK(10) and US(11) food composition tables, and ranges of Ca, Mg and K concentration identified from the largest representative cultivar screen for each crop

The average quantities of Ca, Mg or K delivered to the diet per unit weight of different horticultural food groups can thus be calculated using data reporting the percentage Ca, Mg or K contributed to diet by different food groups(Reference Henderson, Irving and Gregory7), and data reporting the weight of different food groups consumed(Reference Hoare, Henderson and Bates8). Across all vegetables (excluding potatoes) and averaged across sexes, the average concentrations of Ca, Mg and K delivered to the diet are 29·9 mg Ca/100 g FW, 15·8 mg Mg/100 g FW and 219·7 mg K/100 g FW. For potatoes, the equivalent values are 12·8 mg Ca/100 g FW, 508·2 mg K/100 g FW and 25·4 mg Mg/100 g FW. Since slightly differing product categories are used to report the percentage Ca, Mg or K contributed to diet by different food groups(Reference Henderson, Irving and Gregory7), and to report the weight of different food groups consumed(Reference Hoare, Henderson and Bates8), ‘vegetables (excluding potatoes)’(Reference Henderson, Irving and Gregory7) and ‘vegetables and vegetable dishes’(Reference Hoare, Henderson and Bates8) are treated as equivalent categories in this current analysis. Similarly, ‘potatoes, including savoury snacks’(Reference Henderson, Irving and Gregory7) and ‘potatoes, excluding savoury snacks’(Reference Hoare, Henderson and Bates8) are also treated as equivalent categories. Despite this potential source of error, the contribution of Ca, K and Mg per gram of ‘vegetables’ and ‘potatoes’ are within the ranges of selected leaf, tuber and root crops in UK and US food composition tables, and show that leafy-Brassica, onion, carrots and potato are suitably representative horticultural crops (Table 3).
These baseline data on dietary delivery of Ca, Mg and K via horticultural produce can be used to explore the impact of dietary diversification through increased consumption of horticultural produce, and the potential for horticultural biofortification strategies, i.e. enhancing the mineral content of horticultural produce through breeding and agronomy(Reference White and Broadley4, Reference White and Broadley17), on dietary intakes of Ca, Mg and K at a population level. Before dietary diversification or biofortification is considered, it is appropriate to identify physiological and evolutionary constraints to increasing the Ca, Mg and K in edible portions of crops, thereby to determine the most suitable crops and crop parts for potential intervention.
Why do crop plants differ in calcium, magnesium and potassium concentration?
The concentration of Ca, Mg and K in plant shoot tissues is typically in the low percentage (w/w) dry weight range. However, extraordinarily wide ranges (Ca, 0·02–12·2%; Mg, 0·03–3·2%; K, 0·08–15·6%) have been reported in surveys of terrestrial plant species(Reference Watanabe, Broadley and Jansen18). Furthermore, there are distinct general constraints to Ca, Mg and K distribution in plants, due to physiological and evolutionary attributes of plants linked to the functional roles of these elements. These constraints affect the delivery of minerals to human diets via edible crop parts. Low concentrations of Ca, Mg and K in all crops can also arise when grown in soils of low mineral phytoavailability(Reference White and Broadley4).
Ca is required by plants in large quantities for structural roles in the cell wall and membranes, as a counter-cation for anions in the vacuole, and – critically in terms of its distribution in plant cells – for coordinating responses to developmental cues and environmental challenges through changes in cytosolic Ca2+ concentration(Reference White and Broadley19, Reference Karley and White20). The gross movements of Ca in plants are dominated by extracellular apoplastic fluxes in the transpiration stream, with less movement of Ca in the symplast, including the phloem-symplast, which is critical for delivering water and solutes from leaves to fruits, roots, seeds (including cereal grains) and tubers(Reference Karley and White20–Reference White, Bradshaw and Finlay23). Consequently, except for roots and transpiring leaf tissues, horticultural and cereal products tend to have low Ca concentrations. By contrast, Mg is present at relatively high concentrations in most tissues. About 75% of leaf Mg appears to be associated with protein synthesis through its roles in ribosomal structure and function and enzyme activation, and between 15 and 20% is associated with chlorophyll, with the remainder in the vacuole(Reference Karley and White20). Unlike Ca, Mg is mobile in the phloem and is present at high concentrations in rapidly growing, phloem-fed tissues including fruit, seeds, roots and tubers. Most horticultural and cereal products therefore have high Mg concentrations. K is also present at relatively high concentrations in plants, reflecting its natural abundance and its biophysical (charge-balance and osmotic) and biochemical (enzyme activation) functions(Reference Karley and White20). Like Mg, K is also translocated readily within the phloem, and is present at high concentrations in fruit, seeds, roots and tubers.
In addition to physiological constraints, tissue concentrations of Ca, Mg and K differ markedly between equivalent tissues of plant species growing in the same environment due to evolutionary factors(Reference Watanabe, Broadley and Jansen18, Reference White and Broadley19, Reference Thompson, Parkinson and Band24–Reference Broadley, Bowen and Cotterill26). For example, a considerable proportion of the genetic variation in shoot Ca and Mg, and to a lesser extent K, concentrations occurs at the family level or above. Therefore, the phylogeny of food choice affects Ca, Mg and K intakes. A striking example of evolutionary differences in shoot mineral composition can be seen in commelinoid monocot families, including cereal and grass species within the Poaceae. This group of families have characteristically lower shoot Ca (and higher Si) concentrations than most non-commelinoid monocots and eudicot species, due to differences in their cell wall chemistry and cation exchange capacity(Reference White and Broadley19). In general, the ability of many plants to accumulate Ca and Mg is correlated, and phylogenetic variation in shoot Mg concentrations resembles that for Ca(Reference Watanabe, Broadley and Jansen18, Reference Broadley, Bowen and Cotterill26). However, species from families within the Caryophyllales order, including the Amaranthaceae (e.g. amaranth, beets, chards, quinoa and spinach) have a tendency towards high leaf Mg concentrations and therefore higher leaf Mg:Ca quotients than most other angiosperms. For example, Ca/Mg quotients for spinach (Spinacia oleracea) were typically 5-fold lower than the Ca/Mg quotients for Brassica oleracea (Brassicaceae) grown under a variety of nutritional conditions(Reference Broadley, Hammond and King27). The important issue of bioavailability of Ca, Mg and K in edible crops is outside the scope of this paper. However, there is notable phylogenetic variation in antinutritional factors among different plant species(Reference White and Broadley4). Thus, most vacuole- and endoplasmic-reticulum-located Ca and Mg is water soluble in many plant species. However, the Caryophyllales families accumulate large quantities of oxalate, while other groups such as cereals produce considerable quantities of phytate. Oxalate and phytate can considerably reduce the bioavailability of Ca and Mg to monogastrics, including human subjects.
What happens to dietary calcium, magnesium and potassium intakes when more vegetables are consumed, or when horticultural produce is biofortified?
The effects of increased consumption of vegetables and horticultural biofortification on Ca, Mg and K intakes among the UK population are now considered. Due to the myriad of potential dietary scenarios, just three changes to horticultural consumption and mineral composition are simulated for illustrative purposes. The scenarios are: (1) two additional portions of vegetables (excluding potatoes) per day; (2) biofortification of all horticultural produce (vegetables, potatoes and fruit) so that Ca, Mg and K concentrations are 50% greater than at present across all produce; and (3) two additional portions of biofortified vegetables combined with Ca, Mg and K biofortification (+50%) of all horticultural produce. All three scenarios assume a baseline average mineral concentration per portion of vegetables (excluding potatoes) of 23·9 mg Ca, 175·8 mg K and 12·6 mg Mg/80 g FW, and current consumption rates of horticultural produce among the UK adult population as described previously(Reference Henderson, Irving and Gregory7, Reference Hoare, Henderson and Bates8). No adjustment for energy intakes is made since the additional energy intake of two portions of vegetables (excluding potatoes) is relatively small.
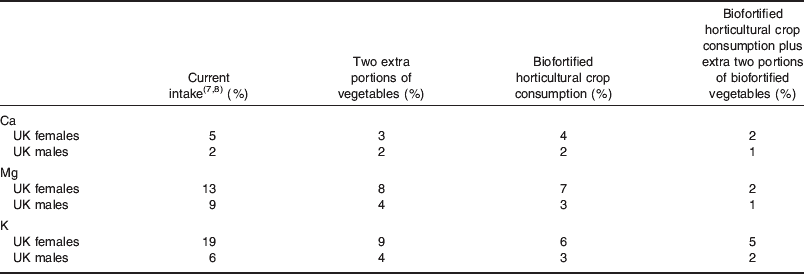
Despite contributing little energy to many UK diets, dietary intakes of Ca, Mg and K would increase to levels above the LRNI for millions of UK adults if two additional portions of vegetables (excluding potatoes) were consumed per day and/or whether horticultural biofortification strategies were adopted (Fig. 4; Table 4). Specifically, two additional portions of vegetables (excluding potatoes) per day would bring Ca intakes above the LRNI for a further 0·5 million UK adults. A horticultural biofortification strategy alone would bring Ca intakes above the LRNI for a further 0·3 million UK adults. A combined strategy of increased vegetable consumption and biofortification would bring Ca intakes above the LRNI for almost 1 million additional UK adults, i.e. about 60% of those with deficient Ca intakes. Biofortifying horticultural produce by 50% with Mg and K has a greater effect on dietary Mg and K intakes than increased vegetable consumption alone. An additional two portions of vegetables (excluding potatoes) per day would bring Mg and K intakes above the LRNI for a further 1·4 million and 3 million UK adults, respectively. A horticultural biofortification strategy alone would bring Mg and K intakes above the LRNI for a further 2 million and 4·1 million UK adults, respectively. A combined strategy of increased vegetable consumption and biofortification would bring Mg and K intakes above the LRNI for almost 4 million and 4·2 million additional UK adults, respectively, i.e. about 75% of those with deficient Mg intakes and about 70% of those with deficient K intakes. A healthy diverse diet that includes five portions of vegetables and fruit per day clearly lowers the risk of Ca, Mg and K deficiency considerably. However, since long-running policies to increase consumption of vegetables and fruit are not reflected in UK dietary habits, it may be appropriate to combine policies to promote dietary diversification alongside strategies to biofortify horticultural produce, in order to optimise Ca, Mg and K intakes among the population.

Fig. 4. Simulated Ca, Mg and K intake among UK adults following hypothetical dietary diversification and biofortification. The solid lines are simulated intakes based on sample means and sd reported in the UK National Diet and Nutrition Survey(Reference Henderson, Irving and Gregory7, Reference Hoare, Henderson and Bates8), assuming a normal distribution. The four dotted lines indicate the effect of increased consumption of vegetables (excluding potatoes) by up to four, 80 g fresh weight, portions per day. The left-hand dashed lines represent the effect of biofortifying all edible horticultural products (vegetables, potatoes and fruit) by 50%. The right-hand dashed line represents the same biofortification strategy plus an additional two, 80 g fresh weight, portions of vegetables (excluding potatoes).
Table 4. Effect of extra vegetable consumption and biofortification on proportion of UK adults with Ca, Mg and K intakes < lower reference nutrient intake
Is a 50% increase in the calcium, magnesium and potassium composition of edible horticultural products a realistic biofortification target?
Increasing the Ca, Mg and K concentrations of the edible portions of horticultural crops can be achieved through genetic and agronomic biofortification(Reference White and Broadley4). Genetic biofortification can employ either conventional breeding or genetic manipulation-based approaches. Conventional breeding requires natural genetic variation in the genepool to be present and heritable for Ca, Mg and K concentrations in edible portions of crops. While heritable natural genetic variation has been demonstrated, horticultural crops with greater mineral concentrations have apparently not yet been bred conventionally, yet a 50% increase appears a feasible breeding target(Reference White and Broadley4) (Table 3). For example, leaf or shoot Ca and Mg concentration varies by >50% among varieties and accessions of B. oleracea (Reference Broadley, Hammond and King27–Reference Kopsell, Kopsell and Lefsrud30), Brassica rapa (Reference Wu, Yuan and Zhang31), spinach (S. oleracea)(Reference Grusak, Cakmak, Broadley and White32), onion (Allium cepa)(Reference Rodríguez Galdón, Oropeza González and Rodríguez Rodríguez33) and chickpea (Cicer arietinum)(Reference Ibrikci, Knewtson and Grusak34). Within each subtaxa of B. oleracea (N.B. cabbage, kale, broccoli and cauliflower represent different subtaxa), shoot Ca, Mg and K concentrations ([Ca]shoot) varies by 1·5- to 2-fold(Reference Broadley, Hammond and King27, Reference White, Hammond and King35) (Table 3). In addition, a significant fraction of the variation in [Ca]shoot, [Mg]shoot and [K]shoot in B. oleracea is heritable and can be associated with specific chromosomal loci, opening up targeted (marker-assisted) breeding opportunities(Reference Broadley, Hammond and King27, Reference White, Hammond and King35). In roots, up to 8-fold variation in Ca and 4·6-fold variation in Mg occurred among 600 cassava genotypes (Manihot esculenta)(Reference Chávez, Sánchez and Jaramillo36), a significant variation also reported for carrot (Daucus carota)(Reference Nicolle, Simon and Rock37) (Table 3). There are fewer published data for fruit, although >2-fold variation in fruit Ca concentrations has been reported among plantain (Musa spp.)(Reference Davey, Stals and Ngoh-Newilah38) and plum (Prunus domestica)(Reference Nergiz and Yildiz39) varieties. To date, the only published transgenic strategy for increasing [Ca]shoot, [Mg]shoot or [K]shoot in crops followed the discovery that Arabidopsis thaliana plants overexpressing genes encoding vacuolar Ca2+/H+ antiporters had [Ca]shoot greater than those of wild-type plants(Reference Hirschi40, Reference Hirschi, Miranda and Wilganowski41). Subsequently, increased [Ca]shoot, and potentially dietary Ca delivery, has been shown to be possible through the expression of genes encoding AtCAX1 lacking its autoinhibitory domain (sCAX1), a modified AtCAX2 (sCAX2), or AtCAX4, in the edible portions of carrot(Reference Park, Kim and Pike42, Reference Morris, Hawthorne and Hotze43), lettuce(Reference Park, Elless and Park44), potato(Reference Park, Kang and Kim45) and tomato(Reference Park, Cheng and Pittman46, Reference Chung, Han and Giovannoni47). Shoots of plants overexpressing calreticulin, a major Ca2+-binding protein in the endoplasmic reticulum, also have higher [Ca]shoot than wild-type plants(Reference Wyatt, Tsou and Robertson48) and may be a potential target for genetic manipulation.
A more immediate biofortification strategy for Ca, Mg and K is to fertilise crops with these elements, above levels required directly for crop growth. The effectiveness of this approach will depend on soil conditions that determine the phytoavailability of Ca, Mg and K already in the soil, and fertiliser choice. Ca is typically present in the soil solution at millimolar concentrations, sufficient for adequate crop growth. However, crop Ca deficiencies can arise on highly weathered tropical soils, and on acidic, sodic or saline soils where Ca2+ uptake is inhibited by Al3+, Na+ and other cations(Reference Marschner49). In horticultural crops, Ca deficiency disorders also occur when Ca becomes temporarily unavailable to developing tissues(Reference Ho and White22). K is typically present at lower concentrations than Ca in the soil solution and crop Mg deficiency is more widespread, especially on acidic soils due to inhibition of Mg2+ uptake by Al3+ and Mn2+ on alkaline soils, where MgCO3 formation and excess Ca, K and Na also reduce Mg availability to crops, and during intensive crop production without concomitant Mg fertilisation(Reference Mengel and Kirkby50, Reference Römheld and Kirkby51). Most K in soils is unavailable to crops since it occurs in mineral or ‘non-exchangeable’ K forms bound to clay minerals. Soil solution K is typically in the micromolar range, and is buffered by exchangeable forms of K bound to the edges of clay minerals. Soils deficient in K typically include those of low clay contents including old, highly weathered soils, sandy soils and organic soils and peats(Reference Mengel and Kirkby50).
While agronomic biofortification has been deployed for the micronutrients Se(Reference Broadley, White and Bryson52, Reference Broadley, Alcock and Alford53) and Zn(Reference Cakmak54), attempts to increase crop Ca, Mg and K composition for human nutrition have not yet been reported. Experimental evidence indicates that a 50% target for Ca, Mg and K biofortification with fertilisers is probably conservative, since many plant species will accumulate Ca, Mg and K to levels well above those required for growth, especially when these elements are supplied in nutrient solutions. For example, [Ca]shoot varies several-fold in plants adapted to both Ca-rich and Ca-poor habitats, without effect on the growth, when different concentrations of Ca are supplied(Reference Jefferies and Willis55). Mg concentrations vary several-fold in sugar beet (Beta vulgaris) shoots and roots, and in A. thaliana shoots, when Mg is withheld from plants that were well supplied with Mg previously(Reference Hermans, Johnson and Strasser56, Reference Hermans and Verbruggen57). Similarly, [K]root varies in lettuce (Lactuca sativa) by up to 5-fold when K is removed from a previously supraoptimal supply(Reference Burns, Walker and Moorby58). Translational experiments using commercial cultivation conditions and fertilisers are now required. Common Ca fertilisers include lime (CaO and CaCO3), gypsum (CaSO4), Ca phosphates and Ca(NO3)2. The liming of soil increases the pH of the soil solution and provides a Ca source in the topsoil, while water-soluble gypsum provides Ca throughout the soil profile. Foliar and fruit applications of soluble Ca fertilisers, used commonly in horticultural crops to prevent Ca-deficiency disorders, could potentially also be used for biofortification. In general, Mg is supplied to crops as a surface application of MgSO4 (Epsom salts or kieserite), which is readily phytoavailable, or as MgCO3 or MgO forms that behave as slower-release fertilisers and can be incorporated into the subsoil(Reference Metson59, Reference Draycott and Allison60). Foliar applications of MgSO4 are also common on some crops and again, could be used for biofortification. Most K is supplied to crops as KCl (muriate of potash) or K2SO4, but it can also be applied in KNO3 or phosphate forms(Reference Mengel and Kirkby50).
Summary
Addressing the ‘hidden hungers’ of mineral nutrient malnutrition is a major challenge for the nutrition and agriculture sectors throughout the world. In this paper, we have reviewed dietary intake survey data from the UK and USA, which shows that 9% of all UK and US adults consume quantities of Ca and Mg below the UK LRNI and are therefore at risk of deficiency. For K, this figure increases to 14%. Since global malnutrition is likely to affect at least 15% of the population, it seems a reasonable assertion that 25% or more of the world's population is likely to be at risk of deficiency in one or more of the three major nutrients, Ca, Mg or K. Other elements likely to be deficient in diets globally include Se, I, Fe, Zn and Cu. Options to address these hidden hungers include direct fortification of food, dietary education and diversification, and biofortification through crop breeding or altered agronomy. Despite low energy intake from horticultural produce generally, increased vegetable consumption and biofortification through breeding and fertiliser use would greatly improve dietary intakes of Ca, Mg and K among UK adults, a situation which is likely to be similar in the USA and elsewhere. Biofortification is a conceptually simple strategy which requires no change in legislation, but which requires translational research from the agriculture and nutrition sectors.
Acknowledgements
M.R.B. is currently funded by BBSRC through an Agri-Food Industry Partnering Award (BB-G013969-1) part-funded by Yara (UK) Ltd. P.J.W. is funded by the Scottish Government Rural and Environment Research and Analysis Directorate. The study was conceived jointly; M.R.B. conducted the data analyses; both authors contributed to writing the paper. The authors declare no conflict of interest. This paper is dedicated to the memory of their inspirational mentor and friend, Dr Duncan J. Greenwood (1932–2010).










