Ergothioneine is a naturally occurring, betaine amino acid found in many foods. A derivative of histidine (2-mercapto-histidine trimethylbetaine)(Reference Barger and Ewins1,Reference Heath and Toennies2) , ergothioneine, was first isolated in 1909 from the ergot fungus Claviceps purpurea, from which its name was derived(Reference Tanret3). Structurally ergothioneine is a tautomer, with both thione and thiol forms (Fig. 1). Unusual among the thiol antioxidants, at physiological pH, ergothioneine exists primarily in its thione form and has a very high redox potential(Reference Cheah and Halliwell4). These unique properties mean that ergothioneine is much more resistant to autooxidation in comparison with other thiols such as glutathione and is a very effective antioxidant and cytoprotectant, with metal chelation properties as well(Reference Cheah and Halliwell4–Reference Borodina, Kenny and McCarthy6). An increasing body of evidence suggests ergothioneine may be an important dietary nutrient for the prevention of a variety of inflammatory and cardiometabolic diseases(Reference Borodina, Kenny and McCarthy6,Reference Cheah and Halliwell7) ; and ergothioneine has alternately been suggested as a vitamin(Reference Paul and Snyder5), ‘longevity vitamin’(Reference Ames8) and nutraceutical(Reference Borodina, Kenny and McCarthy6).

Fig. 1. Chemical structure of ergothioneine. A histidine-derived amino acid, ergothioneine, exists as a tautomer with both thione and thiol forms. As indicated by the length of the reaction arrows, at physiological pH, the thione structure predominates.
Although ergothioneine is present in plants and animals, evidence for ergothioneine biosynthesis, to date, is limited to bacteria and fungi(Reference Jones, Doyle and Fitzpatrick9,Reference Stampfli, Blankenfeldt and Seebeck10) . More recent genetic and structural approaches have built on early work done in the 1950s(Reference Melville, Horner and Otken11–Reference Melville, Genghof and Inamine13) and demonstrated that ergothioneine biosynthesis has independently emerged at least three times in the molecular evolution of mycobacteria, anaerobic archaebacteria and cynaobacteria(Reference Stampfli, Blankenfeldt and Seebeck10). The typically low levels of ergothioneine found in plants have been presumed to be acquired through their roots from soil fungi or bacteria as part of mycorrhizal symbiosis. This has been demonstrated interestingly in an achlorophyllous plant, Gastrodia elata, whose lifecycle is dependent on the presence of symbiotic fungi(Reference Park, Lee and Kim14). However, as has long been opined, absence of evidence is not evidence of absence(15). The systematic investigation of ergothioneine biosynthesis in plants using genetic approaches is ongoing(Reference Cole and Smirnoff16), and new data may yet challenge the presumption that plants do not synthesise ergothioneine.
Mushrooms are typically the richest source of ergothioneine in the human diet, with amounts of ergothioneine varying wildly depending on strain and growing conditions(Reference Halliwell, Cheah and Tang17). Differences in cultivation practices, including cultivation substrates(Reference Tsiantas, Tsiaka and Koutrotsios18), and soil health and tillage methods(Reference Beelman, Richie and Phillips19), likely explain much of the large variation observed in the ergothioneine contents of both mushrooms and other foods from different places of production (Tables 1 and 2). Divergent sample preparation and analytical methods likely also contribute to the variable ergothioneine concentrations reported to date. Fermented foods can also be significant sources of ergothioneine (Table 1), with the concentration of ergothioneine dependent on the different species of bacteria used in fermentation(Reference Choi, Lee and Park20). In addition, spirulina, the dried biomass of cyanobacteria (Arthrospira platensis) sold commonly as a dietary supplement, contains relatively high amounts of ergothioneine(Reference Pfeiffer, Bauer and Surek21). It is interesting to observe that ergothioneine is found in high amounts in certain plants, mushrooms and spirulina, that have been used and investigated for their medicinal properties(Reference Park, Lee and Kim14,Reference Venturella, Ferraro and Cirlincione22,Reference Furmaniak, Misztak and Franczuk23) .
Table 1. Ergothioneine content in fermented foods and mushrooms

wwt, wet weight; dwt, dry weight; UV-Vis, ultraviolet–visible spectrometry.
Table 2. Ergothioneine content in foods

dwt, dry weight; wwt, wet weight.
Absorption, transport and tissue distribution
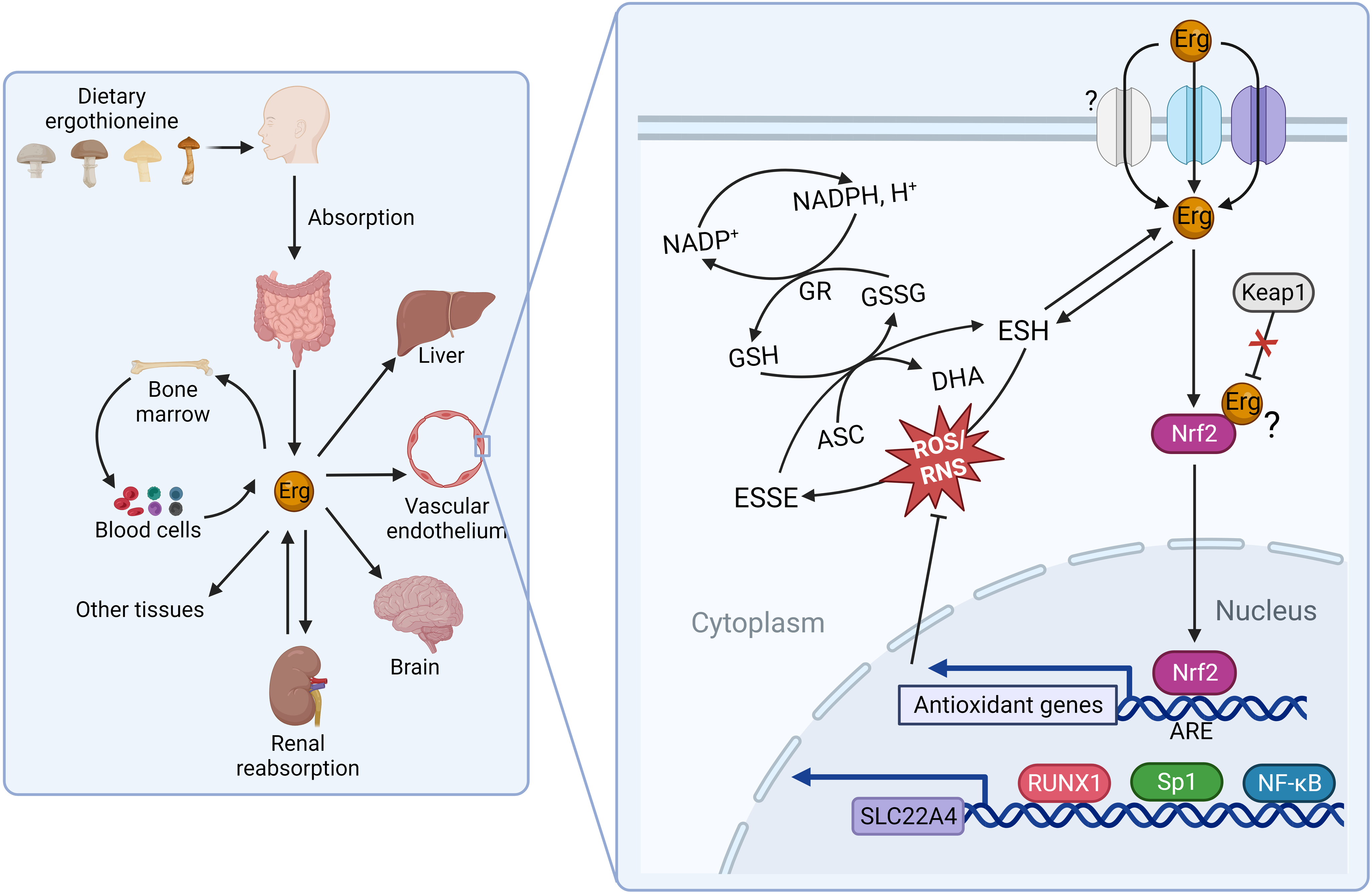
The seminal identification of a mammalian membrane transporter for ergothioneine by Grundemann and colleagues in 2005(Reference Gründemann, Harlfinger and Golz24) provided a mechanism by which ergothioneine is acquired from the diet, and further evidence for an essential role for ergothioneine in vivo. Genetic knockout of solute carrier 22A member 4 (SLC22A4), part of the large solute carrier 22A (SLC22A) family, in both mice(Reference Kato, Kubo and Iwata25) and zebrafish(Reference Pfeiffer, Bach and Bauer26), resulted in dramatic reduction of ergothioneine to undetectable concentrations in many tissues (e.g. liver ergothioneine was 121 ± 25 in wild-type mice v. <2·13 μg/g in SLC22A4 knockout mice)(Reference Kato, Kubo and Iwata25), and increased susceptibility to oxidative stress and inflammation, although organisms remained viable. Nomenclature can be challenging as the SLC22A family is comprised of organic cation (OCT), organic anion (OAT) and ‘novel organic cation’ (i.e. cation and zwitterion; OCTN) transporters. SLC22A4 was originally termed OCTN1(Reference Tamai, Yabuuchi and Nezu27) and is found referred to as both OCTN1 and the ergothioneine transporter in the literature. With the recent de-orphaning of SLC22A15 as a second ergothioneine transporter expressed highly in the brain(Reference Yee, Buitrago and Stecula28), and likelihood of further ergothioneine transporters yet to be characterised (see Fig. 2 for overview and outstanding questions related to ergothioneine membrane transport), we will use the HUGO Gene Nomenclature Committee (HGNC)-approved human gene nomenclature here.

Fig. 2. Physiological transport of ergothioneine in vivo. (a) Ergothioneine is transported across cell membranes by the solute carrier family 22 member’s 4 and 15 (SLC22A4 and SLC22A15) in a Na-dependent manner. Intracellularly, ergothioneine inhibits oxidative and DNA damage through multiple pathways. Whether ergothioneine is transported into the mitochondria is unknown. How ergothioneine is exported from cells is also unknown. (b) Uptake of ergothioneine from the diet into enterocytes is mediated by SLC22A4 expression on the apical membrane. The basolateral transporter is unknown. (c) Ergothioneine is rapidly cleared from plasma. SLC22A4 is highly expressed in granulocytes, monocytes and nucleated erythroid precursors, but not mature erythrocytes. (d) Although ergothioneine accumulates in mouse liver, SLC22A4 is not expressed on hepatocytes. In humans, while SLC22A4 is highly expressed in fetal liver, it is only detected in very low amounts in adult liver. (e) Some data suggest that SLC22A4 is expressed in liver non-parenchymal cells(Reference Sugiura, Kato and Shimizu35) and is upregulated in activated hepatic stellate cells in mice(Reference Tang, Masuo and Sakai46). (f) Ergothioneine is avidly retained through renal reabsorption mediated by SLC22A4 expression at the apical membrane of proximal tubular cells in the kidney. The basolateral transporter is unknown.
Differentially expressed between tissues, SLC22A4 is notably highly expressed both on the apical membrane of the small intestine where it functions in a pH-dependent fashion to take ergothioneine up from the diet(Reference Harwood, Zhang and Pathak29) and on the apical membrane of proximal tubular cells in the kidney where it functions in renal reabsorption(Reference Nakanishi, Fukushi and Sato30). Although, like other SLC22 family members, SLC22A4 is capable of transporting multiple substrates, the transport efficiencies for other confirmed substrates are orders of magnitude lower than that of ergothioneine, and increasing evidence from independent laboratories demonstrates SLC22A4 is highly specific for ergothioneine(Reference Yee, Buitrago and Stecula28,Reference Tschirka, Kreisor and Betz31,Reference Tucker, Cheah and Halliwell32) . In humans, SLC22A4 is also very highly expressed in nucleated erythroid precursor cells, bone marrow and fetal (but not adult) liver(Reference Gründemann, Harlfinger and Golz24,Reference Tamai, Yabuuchi and Nezu27) ; and experimental data are consistent with a role for ergothioneine in erythroid proliferation and differentiation(Reference Nakamura, Sugiura and Kobayashi33). In addition, SLC22A4 is expressed highly in circulating neutrophils and monocytes, suggestive of a requirement for ergothioneine’s antioxidant function in these cells, which are predisposed to oxidative stress(Reference Gründemann, Hartmann and Flögel34).
Several lines of evidence suggest that there are other ergothioneine transporters yet to be characterised(Reference Gründemann, Hartmann and Flögel34). Perhaps most notably, a basolateral transporter responsible for the efflux of ergothioneine in polarised cells such as enterocytes and proximal tubules cells of the liver has yet to be identified. Secondly, plasma concentrations of tritiated ergothioneine were reported to increase in response to oral administration of ergothioneine in SLC22A4 knockout mice, albeit not as highly as in wild-type mice(Reference Sugiura, Kato and Shimizu35). Although transport by SLC22A15 cannot be ruled out as an explanation, it is (at least in humans) expressed at very low levels in the small intestine(Reference Yee, Buitrago and Stecula28) and has a much lower transport efficiency for ergothioneine in comparison with SLC22A4, so unlikely to explain the kinetics reported by Sugiura and colleagues(Reference Sugiura, Kato and Shimizu35). The authors suggest the plasma increase was because the knockout mice were lacking the efficient liver uptake observed in the wild-type mice. However, it is not clear how ergothioneine could have crossed the enterocyte in the absence of SLC22A4 or another transporter. Lastly, the question of whether or not there is a mitochondrial (or other subcellular location)-specific transporter for ergothioneine is unresolved(Reference Kerley, McCarthy and Kell36). While early reports of mitochondrial localisation of SLC22A4 are disputed(Reference Gründemann, Hartmann and Flögel34,Reference Kerley, McCarthy and Kell36) , recent data from a rat model of pre-eclampsia showed ergothioneine supplementation decreased mitochondria-specific H2O2 in vivo (Reference Williamson, McCarthy and Manna37), and the question of mitochondrial targeting of ergothioneine remains plausible based on previous in vitro data(Reference Paul and Snyder5).
In both animals and humans, avid absorption and retention of ergothioneine have been observed(Reference Tang, Cheah and Yew38,Reference Cheah, Tang and Yew39) . Regulatory safety approval for ergothioneine supplementation in humans has only occurred quite recently (2016 in Europe(Reference Turck and Bresson40) and 2018 in the USA(41)), and to date only one study has examined ergothioneine supplementation in humans(Reference Cheah, Tang and Yew39). In this pharmacokinetic study, forty-five healthy humans received placebo, 5, or 25 mg encapsulated ergothioneine/d for 7 d and were followed up for an additional 4 weeks. The data show that ergothioneine was rapidly absorbed and largely retained by the body, with large increases in plasma ergothioneine levels and only minimal increases (<4 %) in urinary excretion observed(Reference Cheah, Tang and Yew39). In mice, daily oral administration of a high dose of ergothioneine (70 mg/kg/d) for 28 d showed that while ergothioneine primarily accumulated in liver and whole blood, levels also increased in multiple tissues including kidney and brain(Reference Tang, Cheah and Yew38). In both studies(Reference Tang, Cheah and Yew38,Reference Cheah, Tang and Yew39) , the putative metabolites of ergothioneine (Fig. 3; chemistry reviewed in detail by Servillo(Reference Servillo, Castaldo and Casale42)), the oxidative degradation products hercynine (desulfurated ergothioneine) and ergothioneine sulfonate, as well as its methylated form, S-methyl-ergothioneine, were measured. In humans, hercynine and S-methyl-ergothioneine levels correlated with ergothioneine levels in blood, but ergothioneine sulfonate levels were at the lower limits of detection(Reference Cheah, Tang and Yew39).

Fig. 3. The putative metabolites of ergothioneine. (a) Hercynine. (b) Ergothioneine sulphonate. C. S-methyl-ergothioneine.
Interestingly, while SLC22A4 is highly expressed in rat(Reference Wu, George and Huang43) and mouse liver(Reference Tamai, Ohashi and Nezu44), in humans, although highly expressed in fetal liver(Reference Tamai, Yabuuchi and Nezu27), it is typically barely detectable in adult liver(Reference Cheah and Halliwell7,Reference Taubert, Jung and Goeser45) . In the human pharmacokinetic study from Cheah and colleagues(Reference Cheah, Tang and Yew39), while plasma levels of ergothioneine decreased when supplementation was withdrawn, levels in whole blood continued to increase in a dose–response fashion reaching maximal levels 3 weeks after withdrawal of supplement, which were sustained at 4 weeks follow-up. Whether or not bone marrow, where both SLC22A4 and SLC22A15 are highly expressed(Reference Yee, Buitrago and Stecula28), or another extra-hepatic tissue is the site of ergothioneine retention in humans remains an open question. Likewise in mice, questions also remain about what cell type in liver SLC22A4 are expressed in and where ergothioneine accumulates. SLC22A4 has been reported to be expressed in non-parenchymal cells(Reference Sugiura, Kato and Shimizu35), and to be upregulated in activated hepatic stellate cells(Reference Tang, Masuo and Sakai46). However, these cell types are far fewer in number in the liver than hepatocytes, which represent 70–80 % of liver and are responsible for first-pass metabolism; a fact difficult to reconcile with the efficient hepatic uptake reported in mice administered intravenous ergothioneine(Reference Sugiura, Kato and Shimizu35,Reference Behof, Whitmore and Haynes47) .
Biological roles
Extensive in vitro data (reviewed in detail by Cheah and Halliwell(Reference Cheah and Halliwell4) and Borodina and colleagues(Reference Borodina, Kenny and McCarthy6)) strongly suggests ergothioneine functions as an antioxidant and acts intracellularly as a cytoprotective agent. Ergothioneine reacts almost instantaneously with hydroxyl radicals in vitro (Reference Asmus, Bensasson and Bernier48) and also scavenges a diverse range of additional reactive oxygen and nitrogen species(Reference Cheah and Halliwell4,Reference Borodina, Kenny and McCarthy6) . Ergothioneine deactivates singlet-oxygen species at higher rates than other thiols(Reference Rougee, Bensasson and Land49), and it has been hypothesised that the primary function of ergothioneine may be to prevent damage at intracellular sites of high singlet-oxygen generation(Reference Pfeiffer, Bach and Bauer26). Redox repair of the oxidised forms (ergothioneine disulfide and 5-oxo-ergothioneine) of ergothioneine can be rapidly provided by ascorbate(Reference Asmus, Bensasson and Bernier48) or can be achieved enzymatically by either glutathione reductase in presence of glutathione, or the selenoenzyme thioredoxin reductase(Reference Jenny, Mose and Haupt50).
While the historical study of dietary antioxidants bears cautionary lessons(Reference Halliwell51), in support of a critical antioxidant role for ergothioneine in vivo, the genetic knockout of SLC22A4 in Caenorhabditis elegans increased oxidative damage and reduced lifespan(Reference Cheah, Ong and Gruber52). In addition, knockout of SLC22A4 in zebrafish(Reference Pfeiffer, Bach and Bauer26) and mice(Reference Kato, Kubo and Iwata25) also resulted in increased susceptibility to oxidative stress and inflammation for both organisms. Notably, under basal conditions, high expression of SLC22A4 is typically observed in cells with routinely high amounts of oxidative stress such as granulocytes, bone marrow cells, intestinal and ocular tissues(Reference Cheah and Halliwell7). However, accumulation of ergothioneine and increased expression of SLC22A4 in other (injured) tissues have been observed in animal models of liver fibrosis(Reference Tang, Masuo and Sakai46), fatty liver disease(Reference Cheah, Tang and Ye53) and chronic kidney disease disease(Reference Shinozaki, Furuichi and Toyama54); as well as in humans with Crohn’s disease(Reference Taubert, Jung and Goeser45). Prompting the hypothesis that the accumulation of ergothioneine is an adaptive mechanism to minimise oxidative damage(Reference Halliwell, Cheah and Drum55).
Initial reports that a gain-of-function polymorphism (L503F variant, rs1050152) in SLC22A4 that increased transport efficiency of ergothioneine was associated with increased risk of Crohn’s disease(Reference Peltekova, Wintle and Rubin56) were subsequently shown to have been confounded by the SLC22A4 gene being in linkage disequilibrium with the interferon regulatory factor 1 (IRF1) gene, which was the locus conferring Crohn’s disease susceptibility(Reference Huff, Witherspoon and Zhang57). Similarly, an initial report that an intronic SNP (rs2268277) in SLC22A4 was associated with rheumatoid arthritis(Reference Tokuhiro, Yamada and Chang58) did not replicate in independent populations(Reference Kuwahara, Ikari and Momohara59). In this case, the rs2268277 SNP is located at a runt-related transcription factor 1 (RUNX1) binding site located in intron 6 of the SLC22A4 gene(Reference Tokuhiro, Yamada and Chang58). Reporter gene assay data suggests RUNX1 has a stronger suppressive effect on the minor rs2268277 allele(Reference Tokuhiro, Yamada and Chang58).
In addition, multiple other SNP have been found in the SLC22A4 gene, with functional characterisation of eight non-synonymous SNP in Chinese and Indian populations of Singapore finding four of the variants had reduced transporter activity(Reference Toh, Cheung and Murray60). A large difference in basal concentrations of ergothioneine in whole blood was observed in a pharmacokinetic study by Cheah and colleagues(Reference Cheah, Tang and Yew61), who also noted that participants with the highest basal levels of ergothioneine also appeared to take up more of the supplemented ergothioneine. However, whether or not polymorphisms in SLC22A4 affect ergothioneine tissue concentrations and/or are linked to disease risk in some individuals remains an open question that should be examined in much larger genetic cohorts (e.g. the Biobank cohort with n 500 000(Reference Bycroft, Freeman and Petkova62)).
Along with RUNX1, transcription of the SLC22A4 gene has also been shown to be regulated by the NF-κB transcription factor, and the inflammatory cytokines IL-1 β and TNF-α (Reference Maeda, Hirayama and Kobayashi63), data suggestive of ergothioneine accumulation being part of an orchestrated cellular immune defence. Moreover, multiple in vitro (Reference Hseu, Lo and Korivi64,Reference Ko, Kim and Ahn65) and in vivo (Reference Salama and Omar66,Reference Dare, Channa and Nadar67) studies suggest that ergothioneine activates the transcription factor nuclear factor erythroid 2-related factor 2 (Nrf2), which triggers the cellular antioxidant defence system. Recent molecular docking and dynamic simulation data predict ergothioneine binds Nrf2 directly, preventing Nrf2 degradation(Reference Dare, Channa and Nadar67). These data underscore the pleiotropic cytoprotective effects of ergothioneine beyond its redox activity.
Healthy ageing and the prevention of cardiometabolic disease
In addition to the discussed antioxidant activities, ergothioneine has been demonstrated to chelate divalent cations(Reference Motohashi, Mori and Sugiura68) and protect against gamma(Reference Motohashi, Mori and Sugiura69) and UV(Reference Obayashi, Kurihara and Okano70) radiation with dermato-protective effects observed in human dermal fibroblasts and keratinocytes(Reference Hseu, Lo and Korivi64,Reference Obayashi, Kurihara and Okano70,Reference Hseu, Vudhya Gowrisankar and Chen71) , prompting the use of ergothioneine in some skincare formulas. Beyond cosmetic concerns, the potential anti-ageing effects of ergothioneine in the brain have also been of significant research interest, with in vivo data from animal models showing that ergothioneine protects neurons from damage by cisplatin(Reference Song, Chen and Liao72), β-amyloid(Reference Yang, Lin and Wu73), and age-related learning and memory deficits(Reference Song, Lin and Chen74). In humans, blood levels of ergothioneine start declining linearly with the age after 60 years(Reference Cheah, Feng and Tang75). A number of small human case–control studies have found lower ergothioneine levels in older adults with mild cognitive impairment(Reference Cheah, Feng and Tang75), dementia(Reference Teruya, Chen and Kondoh76) and Parkinson’s disease(Reference Hatano, Saiki and Okuzumi77), in comparison with age-matched healthy individuals. In support of these data, in a prospective elderly cohort in Singapore (n 470, mean age 73), lower baseline ergothioneine levels were associated with poorer baseline cognitive performance and faster rates of decline in function in multiple cognitive domains over 5 years of follow-up(Reference Wu, Kan and Cheah78). Whether or not ergothioneine supplementation (25 mg given three times a week for 52 weeks) may be beneficial in delaying or reversing cognitive decline is the subject of an ongoing clinical trial in elderly individuals with mild cognitive impairment(Reference Cheah, Mahendran and Halliwell79).
Separately, in a larger, longer-term prospective Swedish cohort (n 3236 participants with median follow-up of 21·4 years), higher plasma levels of ergothioneine were associated with significantly lower risk of coronary disease, cardiovascular mortality and overall mortality (hazard ratios per 1 sd increment of ergothioneine were 0·85, 0·79 and 0·86, respectively)(Reference Smith, Ottosson and Hellstrand80). These data re-enforce preclinical studies that suggest the antioxidant and anti-inflammatory activities of ergothioneine interfere with atherogenesis and protect vascular and microvascular endothelial cells from oxidative stress and hyperglycaemia(Reference Servillo, D’Onofrio and Balestrieri81). Interestingly, in a meta-analysis of prospective cohort studies (n 601 893 participants) mushroom consumption was associated with lower risk of all-cause mortality (pooled risk ratio: 0·94; 95% CI: 0·91, 0·98)(Reference Ba, Gao and Muscat82).
Increased reactive oxygen species are a hallmark feature of the pathogenesis of multiple cardiometabolic diseases, including atherosclerosis, diabetes, fatty liver and CVD. Early work in perfused rat heart preparations suggested that ergothioneine protected against ischemia-induced (oxidative) myocardial damage(Reference Arduini, Eddy and Hochstein83), which was supported by later studies showing ergothioneine protects against reperfusion injury in rat liver(Reference Bedirli, Sakrak and Muhtaroglu84) and intestinal(Reference Sakrak, Kerem and Bedirli85) ischemic reperfusion models. Although an older study of perfused rabbit hearts showed no reduction in the damage caused by reperfusion following ischaemic insult(Reference Cargnoni, Bernocchi and Ceconi86), more recent work in diabetic rats showed that 6 weeks of ergothioneine supplementation resulted in decreased biomarkers of cardiac injury, lipid peroxidation and inflammation(Reference Dare, Elrashedy and Channa87).
Ergothioneine is taken up by endothelial cells via SLC22A4(Reference Li, Yang and Sit88), where it has been demonstrated to limit reactive oxygen species production and damage from a variety of insults, including hyperglycaemia, through multiple mechanisms(Reference Li, Yang and Sit88,Reference D’Onofrio, Servillo and Giovane89) . Decreased expression of adhesion molecules, such as endothelial-leucocyte adhesion molecule-1 (E-selectin), intercellular adhesion molecule-1 and vascular cell adhesion molecule-1, has been observed in human aortic endothelial cells cultured with ergothioneine(Reference Martin90). Moreover, reduced adhesion of monocytes, a key initiation step in atherosclerosis, to the human aortic endothelial cells was observed(Reference Martin90). The cytoprotective (reduced reactive oxygen species and reduced cell senescence) effects of ergothioneine on endothelial cells exposed to hyperglycaemic conditions have been shown to be dependent on sirtuin 1 and sirtuin 6 activities and their cellular targets(Reference D’Onofrio, Servillo and Giovane89), which notably include NF-κB, the aforementioned transcriptional regulator of SLC22A4(Reference Maeda, Hirayama and Kobayashi63).
Beyond vascular endothelial cells, supplementing the drinking water of diabetic rats with ergothioneine for 7 weeks improved multiple markers of liver injury(Reference Dare, Channa and Nadar91). Specifically, marked reductions in liver weights and TAG contents were observed, alongside reductions in liver biomarkers of lipid peroxidation (malondialdehyde content) and inflammation (TNF-α and transforming growth factor beta, TGF-β1). In addition, the ergothioneine-supplemented rats had much lower serum concentrations of the liver enzymes alanine aminotransferase, aspartate aminotransferase and alkaline phosphatase. Ergothioneine supplementation attenuated diabetes-related alterations in sirtuin-1 and NF-κB mRNA expression in the liver, as well as the sterol regulatory element-binding transcription factor 1 and fatty acid synthase, the rate-limiting enzyme in fatty acid synthesis(Reference Dare, Channa and Nadar91). Separate work from the same investigators showed benefit from ergothioneine taken alone or in combination with metformin for improving markers of kidney injury (hypertrophy, serum creatinine, blood urea nitrogen and urine albumin and protein)(Reference Dare, Channa and Nadar67). Notably, the reduction of lipid peroxidation with ergothioneine supplementation has been reported in multiple injury models(Reference Dare, Channa and Nadar67,Reference Bedirli, Sakrak and Muhtaroglu84,Reference Sakrak, Kerem and Bedirli85,Reference Dare, Channa and Nadar91) and is of relevance to non-alcoholic fatty liver disease (NAFLD) pathogenesis.
Closely associated with cardiometabolic disease, NAFLD is an independent risk factor CVD(Reference Mantovani, Csermely and Petracca92), and a prevalent co-morbidity of type 2 diabetes(Reference Moore93), with recent estimates suggesting 47–64 % of individuals with type 2 diabetes have NAFLD globally(Reference Younossi, Golabi and de Avila94). While ergothioneine has been demonstrated to be protective in a number of other in vivo models of liver injury(Reference Tang, Masuo and Sakai46,Reference Salama and Omar66,Reference Kawano, Cho and Haruna95,Reference Deiana, Rosa and Casu96) , including the aforementioned model of diabetic liver damage(Reference Dare, Channa and Nadar91); to date, only one study has examined SLC22A4 and ergothioneine in a preclinical model of NAFLD(Reference Cheah, Tang and Ye53). In this study, guinea pigs fed diets with either moderate or high levels of fat and cholesterol diets, increased liver expression of SLC22A4 mRNA, and had increased liver ergothioneine contents after 2 and 6 months of diet. This was hypothesised by the authors to be protective, as oxidative biomarkers (liver F2-isoprostane and protein carbonyl contents) were not different between dietary groups(Reference Cheah, Tang and Ye53).
In short, sufficient preclinical and epidemiological data exist to hypothesise that ergothioneine may play an important role in the prevention of cardiometabolic disease (CVD, type 2 diabetes and NAFLD) and promotion of healthy ageing. Recent regulatory safety approval for ergothioneine supplementation in humans has facilitated the requisite human intervention trials required to test such hypotheses. In addition to the aforementioned trial investigating the effects of ergothioneine supplementation on cognitive decline(Reference Cheah, Mahendran and Halliwell79), we have recently published our ErgMS study protocol(Reference Tian, Cioccoloni and Sier97), which aims to investigate the effects of ergothioneine supplementation in middle-aged adults with metabolic syndrome. Designed as a three-arm randomised, double-blind, placebo-controlled intervention trial, the ErgMS study will supplement participants with placebo, 5 or 30 mg/d ergothioneine for 12 weeks, taking measurements of metabolic syndrome risk factors, serum markers of oxidative stress (lipid peroxidation), inflammation, blood platelet function and liver function at baseline, and after 6 weeks and 12 weeks of supplementation(Reference Tian, Cioccoloni and Sier97).
Future horizons
With many questions about ergothioneine function in humans outstanding, it is a fascinating time to be considering the horizons for ergothioneine-related research. As highlighted, given differences between humans and experimental models, there remain multiple unresolved questions about the molecular biology of ergothioneine transport. Membrane transporters, including the solute carrier superfamily, are notoriously difficult to study experimentally, and it seems probable that we have more to learn about how hydrophilic ergothioneine traverses polarised cells, as well as subcellular organelles. Related to transporter biology, whether the liver acts as the site of ergothioneine retention in humans, as in mice, is uncertain since SLC22A4 mRNA is barely detectable in adult human liver. Indeed, knowledge of rate and patterns of induction of SLC22A4 expression in human tissues under different (e.g. ageing and disease) circumstances is limited. Whether or not polymorphisms in SLC22A4 adversely affect an individual’s ergothioneine status and/or are linked to disease risk is also unknown. In addition, experimental confirmation that ergothioneine directly interacts with Nrf2 in a variety of tissues would further underscore the centrality of ergothioneine in cellular antioxidant defence.
Ergothioneine has alternately been suggested as a vitamin(Reference Paul and Snyder5), ‘longevity vitamin’(Reference Ames8) and a nutraceutical(Reference Borodina, Kenny and McCarthy6). In weighing up the relative semantics of these terms, it is worth revisiting seminal discussions of essential v. Conditionally essential nutrients. Classic feeding studies established nutrients as essential if, when removed from a purified diet, growth failure, failure to maintain nitrogen balance or illness occurred. Deficient nutrients were then fed back incrementally to establish minimum requirements. By the early 1980s, on the basis of such studies, thirteen vitamins, twelve minerals, nine amino acids, one fatty acid and three electrolytes were considered essential for healthy humans(Reference Chipponi, Bleier and Santi98). In parallel, conditionally essential nutrients were defined as those that may be synthesised in adequate amounts endogenously but could become rate-limiting under clinical conditions of stress – whether from anabolic processes (e.g. growth, pregnancy and lactation), infection or trauma(Reference Grimble99). Critically, conditionally essential nutrients, when supplied exogenously, correct a clinically relevant outcome measure(Reference Grimble99,Reference Mischley100)
In the context of clinical nutrition, Grimble in 1993 proposed five criteria to define conditionally essential nutrients in humans, whereby their deficiency results in either: failure to maintain growth or nitrogen balance, organ dysfunction, delayed recovery, metabolic or clinical abnormalities(Reference Grimble99). Metabolic and clinical abnormalities encompass obesity and cardiometabolic disease, and it is interesting to note that in CVD therapy, positive data are accumulating for therapeutic benefit from a number of potential conditionally essential nutrients, including amino acids (e.g. arginine, carnitine and coenzyme Q10, among others)(Reference Kendler101). In contrast, currently, there are no data in relation to ergothioneine intervention in humans. Such data will be critical for determining whether ergothioneine might be a conditionally essential micronutrient required for healthy ageing.
Conclusions
Ergothioneine is a dietary antioxidant acquired from food and retained with tissue specificity in the body through the activity of the SLC22A4 membrane transporter. Genetic knockout of SLC22A4 in multiple organisms has been shown to increase organism susceptibility to oxidative stress, damage and inflammation. High plasma ergothioneine levels have been associated with significantly reduced cardiovascular mortality, and overall mortality, risks in humans. Conversely, low levels of ergothioneine have been associated with poorer cognitive performance and faster rates of cognitive decline in elderly individuals. Although a confluence of data suggests that ergothioneine acts as a powerful, pleiotropic cytoprotectant agent, and supplemental ergothioneine is already marketed direct to consumers for its anti-ageing and anti-inflammatory effects, controlled human intervention trials are just beginning to directly investigate the effects of ergothioneine supplementation in humans. Such data are required to assess whether ergothioneine is a dietary micronutrient required for healthy ageing and the prevention of cardiometabolic disease in humans.
Acknowledgements
This research received no specific grant from any funding agency, commercial or not-for-profit sectors.
X. T. and J. B. M. contributed to the review concept and design. X. T. extracted data, created figures and tables, and contributed to an initial manuscript draft. J. B. M. wrote the final manuscript, which all authors critically reviewed for intellectual content and approved. Fig. 1 and 3 were created with ACD/ChemSketch, version 2021.1.3, Advanced Chemistry Development, Inc., Toronto, ON, Canada, www.acdlabs.com, 2022. Fig. 2 was created with BioRender.com.
All authors declare that they have no competing interests.