Introduction
Hunting in tropical forests provides nutrition and income for local communities and is deeply rooted in social and cultural traditions (Bennett & Robinson, Reference Bennett, Robinson, Robinson and Bennett2000; Secretariat of the Convention on Biological Diversity, 2011). However, wildlife harvest has reached unsustainable levels in many places because of the increasing human population and demand for wild meat (Peres, Reference Peres2000; Bennett et al., Reference Bennett, Eves, Robinson and Wilkie2002), improved hunting technologies and increased access to forests (Bennett et al., Reference Bennett, Eves, Robinson and Wilkie2002; Fa & Brown, Reference Fa and Brown2009). Besides causing species declines or extinctions (Cullen et al., Reference Cullen, Bodmer and Valladares Pádua2000; Corlett, Reference Corlett2007; Peres & Palacios, Reference Peres and Palacios2007), overhunting can affect the ecological functionality of ecosystems (Cullen et al., Reference Cullen, Bodmer and Valladares-Padua2001; Wright et al., Reference Wright, Stoner, Beckman, Corlett, Dirzo and Muller-landau2007; Peres et al., Reference Peres, Emilio, Schietti, Desmoulière and Levi2016), as well as local communities that depend on the consumption of wild meat for subsistence (Milner-Gulland et al., Reference Milner-Gulland and Bennett2003). The importance of hunting in household economies varies depending on socio-economic factors such as level of education (Nielsen & Meilby, Reference Nielsen and Meilby2013), wealth, household size (Foerster et al., Reference Foerster, Wilkie, Morelli, Demmer, Starkey and Telfer2012), livestock ownership (Loibooki et al., Reference Loibooki, Hofer, Campbell and East2002) and age (Melo et al., Reference Melo, Silva, Souto, Alves and Schiel2014). Unsustainable hunting is a major concern both for wildlife conservation and with respect to the well-being of the people that subsist on wild meat (Redford, Reference Redford1992; Nasi et al., Reference Nasi, Taber and Van Vliet2011).
In Brazil, hunting is illegal (Federal Law 9605/98—Law of Environmental Crimes and Decree 6514/2008), and penalties are more severe for hunting inside protected areas and for the hunting of threatened species (Art. 29 Federal Law 9605/98). Nonetheless, hunting is widespread (Carvalho & Morato, Reference Carvalho and Morato2013; El Bizri et al., Reference El Bizri, Morcatty, Lima and Valsecchi2015) and has been cited as a major threat to wildlife in the protected areas of the Atlantic Forest (Chiarello, Reference Chiarello2000; Canale et al., Reference Canale, Peres, Guidorizzi, Gatto and Kierulff2012; Schiavetti et al., Reference Schiavetti, Magro and Santos2012), the Amazon (Carvalho & Pezzuti, Reference Carvalho and Pezzuti2010) and other regions in the north-east of the country (Fernandes-Ferreira et al., Reference Fernandes-Ferreira, Mendonça, Albano, Ferreira and Alves2012, Reference Fernandes-Ferreira, Mendonça, Cruz, Borges-Nojosa and Alves2013). Subsistence is the main reason for hunting, particularly in indigenous and traditional communities (Peres & Nascimento, Reference Peres and Nascimento2006; Hanazaki et al., Reference Hanazaki, Alves and Begossi2009; Barbosa et al., Reference Barbosa, Nobrega and Alves2011; Minzenberg & Wallace, Reference Minzenberg and Wallace2011). However, recreational activity and trade are also motivations for hunting (Chiarello, Reference Chiarello2000; Alves et al., Reference Alves, Mendonça, Confessor, Vieira and Lopez2009; Fernandes-Ferreira et al., Reference Fernandes-Ferreira, Mendonça, Albano, Ferreira and Alves2012; El Bizri et al., Reference El Bizri, Morcatty, Lima and Valsecchi2015). Threatened mammals have been captured for the pet trade (Fernandes-Ferreira et al., Reference Fernandes-Ferreira, Mendonça, Albano, Ferreira and Alves2012; Nascimento et al., Reference Nascimento, Schiavetti and Montaño2013) or killed for consumption (Castilho et al., Reference Castilho, Martinez, Giné, Ribeiro and Schiavetti2013; Melo et al., Reference Melo, Silva, Souto, Alves and Schiel2014; Morcatty & Valsecchi, Reference Morcatty and Valsecchi2015), traditional medicine (Alves, Reference Alves2009; Ribeiro et al., Reference Ribeiro, Pereira, Docio, Alarcon, Schiavetti, Costa Neto and Alves2010), or in retaliation for wildlife-related damage (Carvalho & Morato, Reference Carvalho and Morato2013).
In an attempt to mitigate threats to threatened species in Brazil, National Action Plans have been developed to identify and prioritize conservation actions (ICMBio, 2014). The Atlantic Forest receives special attention because it is considered to be a hotspot for biodiversity (Myers et al., Reference Myers, Mittermeier, Mittermeier, Fonseca and Kent2000). Major threats to species of the Atlantic Forest are the increasing human population and associated activities, such as deforestation, hunting and illegal wildlife trade (ICMBio, 2010). In 2010 a National Action Plan was created to protect threatened mammals of the Central Atlantic Forest, with the aim of increasing the population viability of its target species and improving the quality of their habitats (ICMBio, 2010). For the southern Bahia region one of the main goals of this Action Plan is to decrease hunting pressure on target species within key conservation areas (ICMBio, 2010).
Our research aims to improve understanding of the practice of hunting by local people in the southern Bahia region. Recording information on sensitive and illegal behaviour such as hunting inside and around protected areas is challenging, especially when residents are aware of the illegality of the activity. Novel approaches have emerged to obtain more accurate information about such sensitive behaviours (Nuno & St John, Reference Nuno and St John2015). We tested a relatively recently developed method of indirect questioning for eliciting sensitive information (the randomized response technique), as a means of obtaining an estimate of the prevalence of illegal hunting of species of concern (Solomon et al., Reference Solomon, Jacobson, Wald and Gavin2007; St. John et al., Reference St. John, Edwards-Jones, Gibbons and Jones2010; Razafimanahaka et al., Reference Razafimanahaka, Jenkins, Andriafidison, Randrianandrianina, Rakotomboavonjy, Keane and Jones2012). This was the first use of this technique in Brazil in the context of wildlife conservation, and we explore its suitability for use in this context.
A number of studies have explored the prevalence of, and motivations for, hunting by rural people; for example, Nuno et al. (Reference Nuno, Bunnefeld, Naiman and Milner-Gulland2013) used an indirect questioning method to explore bushmeat hunting in the Serengeti, Tanzania, as did Harrison et al. (Reference Harrison, Baker, Twinamatsiko and Milner-Gulland2015) in the Bwindi area in Uganda. Adding to this literature, our study was the first integrated assessment of the factors affecting hunting of threatened mammals in the Bahia region. We explored the relationship between socio-economic factors and hunting prevalence to understand whether particular sectors of society are more or less dependent on hunting. This type of information not only supports the implementation of Brazil's National Action Plan (ICMBio, 2010) but also adds new insights to improve general understanding of the factors affecting hunting behaviour among people living in and around protected areas.
Study area
The research was conducted in three protected areas (Una Biological Reserve, Una Wildlife Refuge and Serra das Lontras National Park) and a buffer zone (Fig. 1). The protected areas cover a total area of 53,240 ha and are located mostly in the municipalities of Una and Arataca, in southern Bahia. There are c. 970 private properties in the four areas.
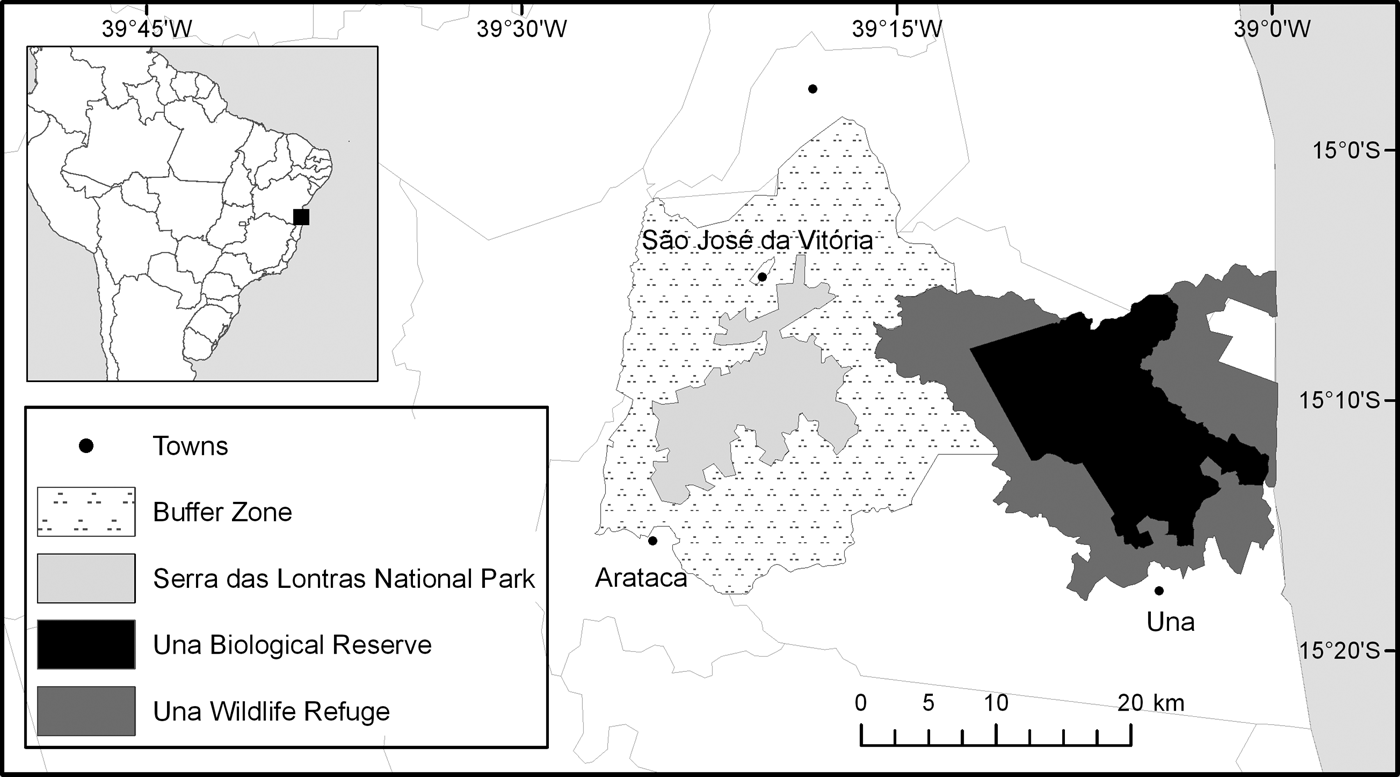
Fig. 1 The study area comprising three protected areas and a buffer zone in southern Bahia, Brazil.
Una Biological Reserve (IUCN category Ia) comprises one of the largest remnants of Atlantic Forest in southern Bahia (Schroth et al., Reference Schroth, Faria, Araujo, Bede, Van Bael and Cassano2011). It was established in December 1980 and expanded from 11,400 to 18,500 ha in December 2007. At the same time, in December 2007, the Reserve's original buffer zone was designated Una Wildlife Refuge (23,404 ha, IUCN category III). Serra das Lontras National Park (11,336 ha, IUCN category II) was established in June 2010 and comprises one of the last remnants of montane Atlantic Forest in north-eastern Brazil (Schroth et al., Reference Schroth, Faria, Araujo, Bede, Van Bael and Cassano2011). The Park has a buffer zone of 58,796 ha. The law of the National System of Conservation Units (Art. 10 and 11 Federal Law 9.985/2000) states that all privately owned areas inside Una Biological Reserve and Serra das Lontras National Park should be expropriated, whereas private land ownership is allowed within Una Wildlife Refuge, as long as land use is compatible with the conservation goals of the Reserve. If this condition is not met, this land must also be expropriated (MMA, 2000). According to the Chico Mendes Institute for Biodiversity Conservation (ICMBio), which manages all federal protected areas, expropriation of private properties within the boundaries of Serra das Lontras National Park has not yet been initiated. In the longer established Una Biological Reserve, 51% of properties have been expropriated, but no expropriations have occurred in the Wildlife Refuge.
Methods
Target species
We focused on mammal species that occur in southern Bahia, especially the threatened species listed in the National Action Plan for the Conservation of Central Atlantic Forest Mammals as well as mammals that are commonly hunted by local communities in the Atlantic Forest region (Table 1).
Table 1 Focal species in our study of hunting of mammals in protected areas in southern Bahia, Brazil (Fig. 1), with their conservation status on the IUCN Red List, and whether or not they were included in interviews using the randomized response technique.
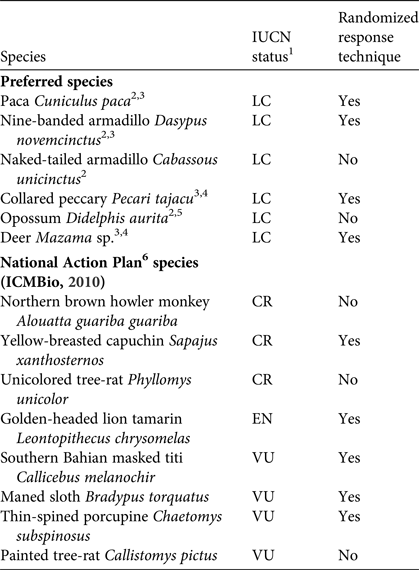
1LC, Least Concern; VU, Vulnerable; EN, Endangered; CR, Critically Endangered
2 Pereira & Schiavetti (Reference Pereira and Schiavetti2010)
3 Flesher & Laufer (Reference Flesher and Laufer2013)
4 Cullen et al. (Reference Cullen, Bodmer and Valladares Pádua2000)
5 Hanazaki et al. (Reference Hanazaki, Alves and Begossi2009)
6 National Action Plan for the Conservation of Central Atlantic Forest Mammals
Data collection
Data were collected during October 2012–May 2014. We conducted 351 interviews within the three protected areas and the buffer zone, covering 36% of the properties located within the four areas. These comprised direct semi-structured interviews with rural residents in all four areas, and interviews using the randomized response technique with a different sample of respondents in Una Wildlife Refuge and the buffer zone. When possible, interviews were performed in the presence of a local field assistant who introduced us and helped us to connect with the respondents. We assured respondents of the confidentiality of their responses, and all interviews were conducted with their consent (State University of Santa Cruz—Ethics Committee CAAE: 03600412.0.0000.5526). Authorization to perform research inside protected areas was provided by the Chico Mendes Institute for Biodiversity Conservation (SISBIO-ICMBio: 34574-1).
To select a random sample of interviewees, we obtained data from the Chico Mendes Institute containing georeferenced locations of properties inside the protected areas and in part of the buffer zone. For both interview methods we used a random number generator to choose properties to sample from this list. However, in several cases selected properties were unoccupied or we were unable to locate the resident, and therefore we also conducted opportunistic sampling, including additional properties to increase the sample size. On arrival at a property we interviewed the person responsible for it or anyone who was available, including both landowners and workers. Only one person per property was interviewed.
Direct interviews
We approached 170 respondents, of whom 169 agreed to be interviewed. Of these, 128 were male and 41 were female. This high response rate was probably because our initial approach focused on non-sensitive subjects. However, we sensed that most people were not comfortable talking about hunting, as they knew that this activity was illegal. In 28 interviews, respondents denied association with hunting but provided information that suggested an association. Despite this we assumed they were non-hunters.
The interview was organized into three parts (Supplementary Table S1):
Part I: Socio-economic information on respondents, including their gender, age, level of formal education, duration of residence, number of people living at home, receipt of government assistance (Bolsa Família, a programme of supplementary income offered to low-income families) and presence of livestock (chickens, pigs, sheep, ducks and geese). Previous research findings on the influence of socio-economic factors on hunting activities are described in Supplementary Table S2.
Part II: Respondents’ perceptions of wildlife and hunting activities in the villages. We used photographs of the target species to verify recognition and perception of occurrence by residents.
Part III: Information about hunting (whether the respondent hunted inside protected areas, their motivations, and which species were hunted). If the respondent was a woman we asked her to answer this part of the interview on behalf of her partner or the household head, as appropriate. The responses suggested that all members of a household had a good awareness of the hunting behaviour of the other household members. As we did not probe for a deep understanding of motivations and techniques, but focused on factual information, we felt that the responses from each type of respondent were representative of the household as a whole. For respondents who were answering on behalf of their partners, their age, level of formal education and duration of residence were usually correlated with their partners’ characteristics, and the rest of the socio-economic variables were at the household level. We considered two forms of hunting: opportunistic capture (an individual captures and kills a wild animal if they encounter it near their house or during work) and active hunting (pre-meditated hunts using traps, and active searching using firearms and dogs). Consequently, to verify the prevalence of hunting we asked two questions: (1) Do you hunt (actively) within the property or nearby? (2) Do you capture a wild animal if you find it opportunistically? We also showed photographs of threatened species to verify if respondents had consumed these species. We had a lower number of responses to these questions because this was the last part of the interview and we did not have enough time to ask these questions to all respondents: 45 respondents answered the questions related to the golden-headed lion tamarin Leontopithecus chrysomelas and the southern Bahian masked titi Callicebus melanochir and 55 respondents answered the question related to the maned sloth Bradypus torquatus.
Randomized response technique
The randomized response technique was developed by Warner (Reference Warner1965) to eliminate evasive answers and response bias in sensitive questions, guaranteeing confidentiality of responses (Gavin et al., Reference Gavin, Solomon and Blank2010). Studies have shown that estimates of illegal behaviour based on this technique are higher compared to those based on direct questioning, and therefore it is a useful technique for assessing sensitive behaviour such as illegal resource use (Solomon et al., Reference Solomon, Jacobson, Wald and Gavin2007; St. John et al., Reference St. John, Edwards-Jones, Gibbons and Jones2010; Razafimanahaka et al., Reference Razafimanahaka, Jenkins, Andriafidison, Randrianandrianina, Rakotomboavonjy, Keane and Jones2012).
We used the randomized response technique to investigate consumption of mammals, focusing on four species known to be preferred, and five threatened species, all of which are illegal to hunt anywhere in Brazil (Table 1). We also included chicken as a control. The approach was adapted from Solomon et al. (Reference Solomon, Jacobson, Wald and Gavin2007). Based on a pilot study, we used an approach known as the ‘two unrelated questions’ technique; one question is related to the sensitive behaviour and the other question is related to a non-sensitive behaviour. Firstly, we asked the respondent to identify which of nine wildlife species they recognized from photographs. Then we separated out the recognized species, to be used in the interview. For each species we showed two cards to the respondent, one with a picture of the animal and one with a picture of a coin, explaining the questions that each card represented, and then placed them inside identical envelopes. Secondly, we gave a coin to the respondent to flip before selecting an envelope. The respondent selected an envelope and answered only yes or no to the question inside, without telling us which card they were looking at. If they selected the photograph of an animal, they would answer yes if they had eaten that animal in the previous 12 months, or no otherwise. If they selected the coin card, they would answer yes if they had got heads when they flipped the coin, and no for tails. The interviewer did not know which card the respondent had chosen, ensuring anonymity. In addition, we obtained socio-economic information from respondents.
Of the 202 people approached for the randomized response technique interviews (a non-overlapping sample with the direct interview survey), 20 (10%) did not wish to participate. Two of them refused because they did not understand the method and the others refused to participate before the method was explained, suggesting the sensitivity of the subject was the main reason for refusal. For people who found it difficult to understand the method, we explained it carefully until they felt satisfied to participate. Overall, we received 182 responses to the randomized response technique interviews.
Data analysis
We used a generalized linear model (logistic analysis of covariance) to verify whether hunting in a location (binary response) was affected by respondents’ demographic characteristics (explanatory variables; Supplementary Table S3). We used Pearson correlation coefficients to explore associations among the explanatory variables. As there was no strong correlation between variables they were all included in the model. We used ANOVA for model simplification to find the minimal adequate model that also had the lowest Akaike information criterion value. We used Pearson's χ2 (contingency tables) tests to explore associations between respondents’ perceptions and locations, and simple logistic regression to explore the relationship between age and respondents’ perceptions of changes in wildlife abundance.
To estimate the proportion of people consuming the species investigated using the randomized response technique, we used the following equation (Fox & Tracy, Reference Fox and Tracy1986):
and to calculate the estimated variance for the population surveyed we used:
where X = estimated proportion of respondents consuming the species, λ = recorded proportion of ‘yes’ responses, π = known proportion of non-sensitive behaviour (= 0.5, i.e. flipping heads in the coin toss), P = probability of selecting the sensitive question (= 0.5) and n= number of respondents. We performed regression modelling using a customized link function (Keane, Reference Keane2014) to explore associations between the estimates of consumption obtained from the randomized response technique and socio-economic variables.
We conducted fewer interviews in Una Biological Reserve and Serra das Lontras National Park because of differences in numbers of residents and challenges in accessing some properties. We therefore grouped the Reserve with Una Wildlife Refuge and the Park with the buffer zone, based on the proximity of the areas and the history of management actions for the protected areas. Socio-economic characteristics of respondents are described in Supplementary Table S3.
Results
Perceptions of wildlife abundance and links to hunting
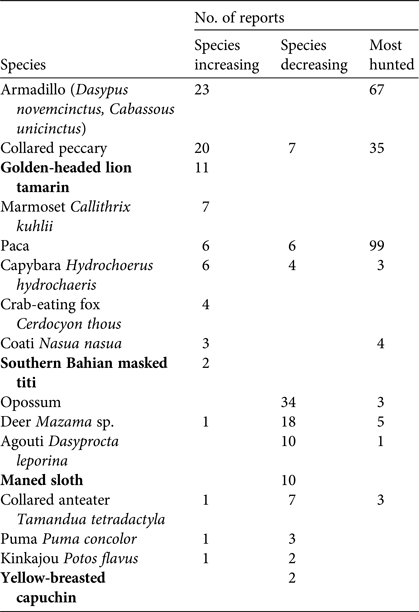
Among the targeted species (Table 1), armadillos (Dasypus novemcinctus and Cabassous unicinctus), collared peccaries Pecari tajacu and pacas Cuniculus paca were the most recognized, and the ones respondents were most likely to say were present in the study area. The northern brown howler monkey Alouatta guariba guariba and the painted tree-rat Callistomys pictus were the least frequently recognized species, suggesting their absence or lower occurrence in the area (Supplementary Table S4). It was not possible to obtain information on the unicolored tree-rat Phyllomys unicolor because residents were unable to identify the species reliably. Fifty-four percent of residents said that overall wildlife abundance was increasing compared to 10 years previously, whereas 46% said that it was decreasing. Perceptions of change in abundance were unrelated to respondents’ age or location. Several species were reported by respondents to have disappeared or decreased in abundance, and most respondents (70%) cited overhunting as the main reason. The reasons given for increased abundance were rapid reproduction or a decrease in hunting of the species (e.g. the golden-headed lion tamarin). Respondents reported that pacas and armadillos were the most hunted species in the region (Table 2). Species believed by respondents to have disappeared more than 10 years previously were the lowland tapir Tapirus terrestris, the northern brown howler monkey, the white-lipped peccary Tayassu pecari, the northern muriqui Brachyteles hypoxanthus and the giant armadillo Priodontes maximus. Fifty-six percent of respondents perceived a reduction in hunting activities in their villages and surrounding areas in recent years and 44% said that hunting had not changed in prevalence. More respondents in Una Biological Reserve–Una Wildlife Refuge perceived a decrease in hunting compared to respondents in Serra das Lontras National Park and the buffer zone (χ2 = 4.26, df = 1, P = 0.038).
Table 2 Numbers of interview respondents who reported wild species to be increasing (n = 51) or decreasing (n = 97) in number, or among the most hunted species (n = 116), in three protected areas and a buffer zone in southern Bahia, Brazil (Fig. 1). Threatened species are in bold.
Prevalence of hunting and motivations
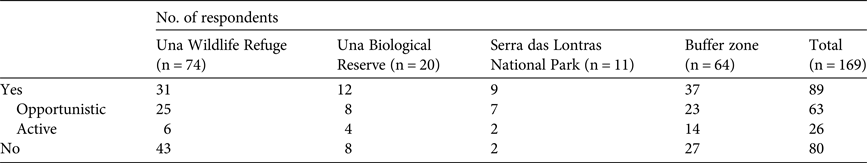
Thirty-seven percent of respondents said that they hunted opportunistically, 16% said they hunted actively and 47% said they did not hunt. The highest level of active hunting was in the buffer zone (22% of respondents said they were active hunters; Table 3). Active hunters used hunting dogs (50%), traps (46%) and hunting platforms (12%). The primary stated motivation for hunting was consumption (92% of responses). However, wild meat was an occasional complement to diets rather than being consumed regularly. In general discussion many respondents stated that wild meat was essential for subsistence in the past, but not anymore. Although most hunters said they killed because they enjoyed a particular animal's meat, some respondents said they hunted out of necessity, because of a lack of animal protein. Most opportunistic hunters stated that they did not consider themselves to be hunters. Besides hunting for consumption, 19% of respondents who had hunted had used wild animals for medicinal purposes, 18% had hunted in retaliation for damage to crops or livestock and 6% said that hunting was a recreational activity (19% among active hunters). According to respondents the species used most in popular medicine are pacas, porcupines (especially the Bahia hairy dwarf porcupine Coendou insidiosus), the black-and-white tegu Tupinambis merianae and armadillos. We were unable to identify anyone involved in hunting for commercial purposes. However, some respondents provided information about prices of wild meat, suggesting the existence of an illegal local trade (Supplementary Table S5).
Table 3 Numbers of interview respondents in three protected areas and a buffer zone in southern Bahia, Brazil (Fig. 1), who stated that they hunted in protected areas of the southern Bahian Atlantic Forest, the numbers of these who hunted opportunistically and actively, and the numbers who stated they did not hunt.
Associations between hunting and socio-economic factors
Of the initial set of seven explanatory variables, the minimal adequate model explaining whether someone stated that they hunted, in response to a direct question, included only level of formal education (Table 4; for details of models see Supplementary Table S6); respondents educated to primary level hunted more than those with either no education or with other levels of education.
Table 4 Minimal adequate model (logistic analysis of covariance) showing effects of level of education on whether a respondent stated they hunted (either actively or opportunistically). The baseline level of education is high-school/college.
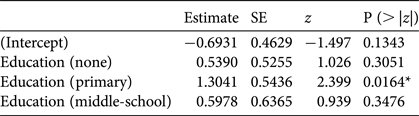
*P < 0.05
Wild meat consumption
Using the randomized response technique, the estimate of whether respondents had consumed chicken in the previous 12 months was not significantly different from 100%. Estimates of consumption prevalence were non-zero for armadillos (62 ± SE 14%), pacas (48 ± SE 14%) and peccaries (26 ± SE 14%). For the other species, including all the threatened species, the confidence intervals for consumption overlapped zero (Fig. 2). Wide confidence intervals are a common issue with the randomized response technique, given the probabilistic nature of the method, requiring a large sample size. However, the confidence intervals give an upper bound on the likely prevalence of consumption of these less-consumed species.
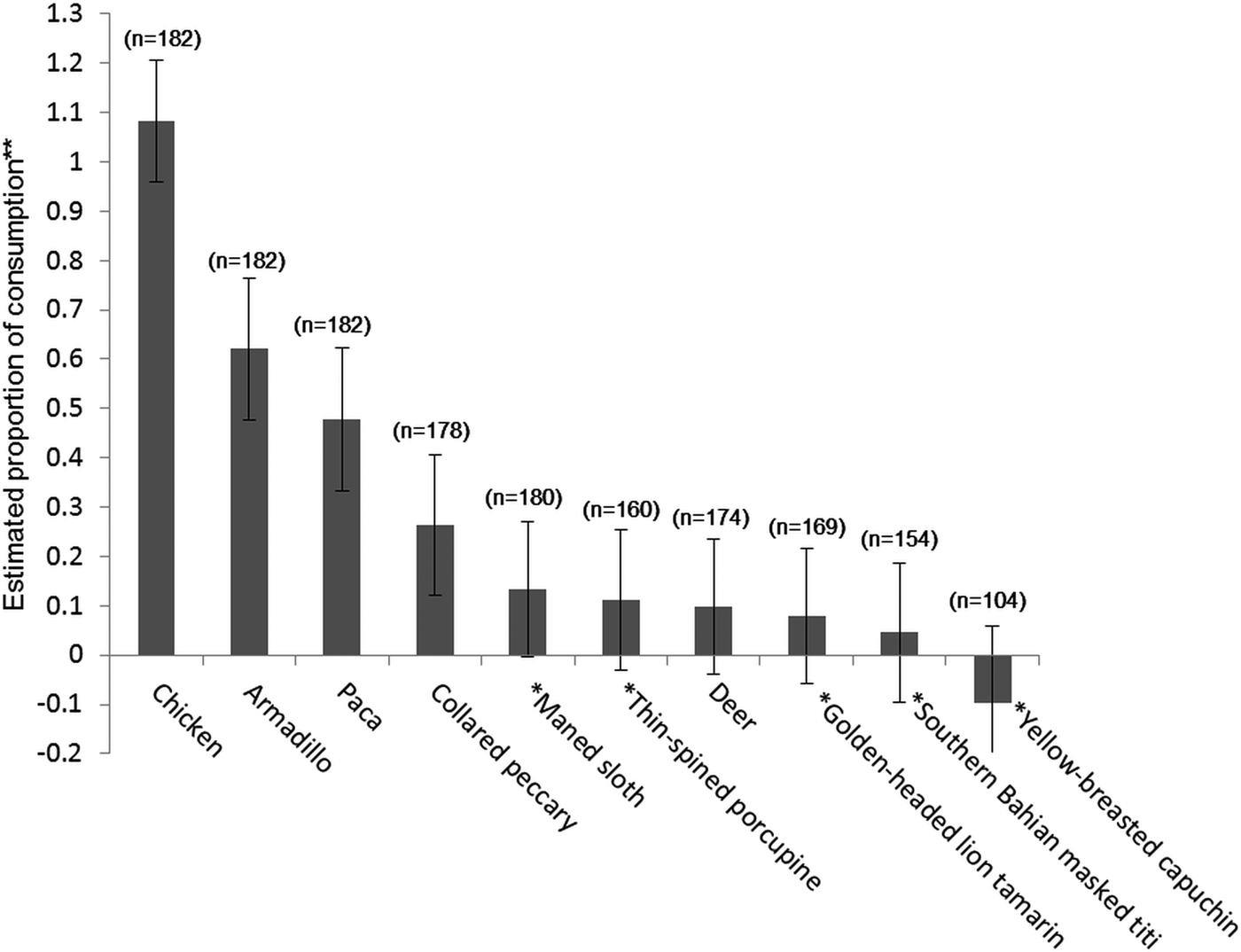
Fig. 2 Estimates of species consumption (with standard error bars) in and around protected areas in southern Bahia, Brazil (Fig. 1), in the 12 months prior to interviews conducted using the randomized response technique. The number of respondents who recognized the species and answered the question is indicated in parentheses. Threatened species are indicated with an asterisk (*). ** Estimated proportion ×100: estimated % of respondents that have consumed the species (negative values and values above 100% are due to the probabilistic nature of the method).
With respect to socio-economic factors affecting the estimated proportion of consumption based on the randomized response technique, the data were sufficient for generalized linear models for only three species (paca, nine-banded armadillo and collared peccary), and only one had a significant result: the estimated consumption of collared peccary tended to be higher for older people (Table 5; for details of candidate models see Supplementary Table S7).
Table 5 Minimal adequate models (logistic analysis of covariance) showing factors affecting the estimated proportion of consumption of paca, armadillo and collared peccary, using the RRT results.
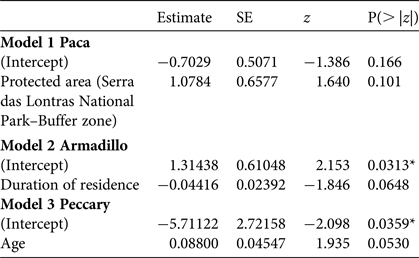
*P < 0.05
In the direct interviews, respondents cited several species that were preferred for consumption (Table 6). Few people said that they consumed any of the threatened species; for example, 96% of the 45 respondents who answered the question about whether they ate monkeys said they did not, and 87% of 55 people said they did not eat sloths. The reasons threatened species were not eaten included taboos or cultural norms, or because they were non-preferred species. Some respondents did not consume sloths and monkeys because they look like people. Others said they did not consume sloths because ‘they are like women’, meaning they have the same menstrual cycle as women. People also said they did not consume porcupines (including the thin-spined porcupine Chaetomys subspinosus) because the meat is considered to be remosa, which means that it is not good for consumption by sick people or pregnant women. Consumption of these less desirable species was often said to be related to opportunistic situations, because of a need for protein and their ease of capture when encountered opportunistically.
Table 6 Preferred wild species for consumption among interview respondents (n = 74) in three protected areas and a buffer zone in southern Bahia, Brazil (Fig. 1). Threatened species are in bold.

Discussion
Although legal subsistence hunting by indigenous and traditional communities has been widely studied in Brazil (Hanazaki et al., Reference Hanazaki, Alves and Begossi2009; Pereira & Schiavetti, Reference Pereira and Schiavetti2010; Barbosa et al., Reference Barbosa, Nobrega and Alves2011), especially in the Amazon region (Peres, Reference Peres2001; Peres & Nascimento, Reference Peres and Nascimento2006; Minzenberg & Wallace, Reference Minzenberg and Wallace2011), illegal hunting in strictly protected areas has been poorly investigated. People are aware that hunting is illegal, which makes research on the subject difficult (Alves & Souto, Reference Alves and Souto2011). We found that using a variety of techniques was a valuable strategy for collecting information about illegal hunting and to triangulate our results. Our respondents were sometimes uncomfortable with the subject matter, but nonetheless through direct interviews we were able to obtain detailed information about hunting, and explore respondents’ perceptions and knowledge. However, direct interviews are prone to non-responses and false answers (Gavin et al., Reference Gavin, Solomon and Blank2010; Mgawe et al., Reference Mgawe, Mulder, Caro, Martin and Kiffner2012). It may be that the proportion of people involved in hunting, and especially those actively involved, is underestimated in our results. The randomized response technique was also a valuable approach, as it guaranteed confidentiality to our respondents and facilitated our investigation of species consumption. The technique works well when behaviours are relatively widespread and not too sensitive, as was the case for some of the species we investigated. However, the method has drawbacks, including the complex explanations required, especially if the targeted population is illiterate (St. John et al., Reference St. John, Edwards-Jones, Gibbons and Jones2010; Razafimanahaka et al., Reference Razafimanahaka, Jenkins, Andriafidison, Randrianandrianina, Rakotomboavonjy, Keane and Jones2012). In addition, a large sample size is necessary to produce precise estimates (Lensvelt-Mulders et al., Reference Lensvelt-Mulders, Hox and Van Der Heijden2005). Our sample size was relatively small, resulting in large standard errors, which affected our ability to estimate consumption of the less widely consumed species. Other indirect questioning techniques, such as the unmatched count technique (Nuno et al., Reference Nuno, Bunnefeld, Naiman and Milner-Gulland2013), which are easier for respondents to understand, would also be worth piloting in this landscape.
Hunting in protected areas
Our results suggest that mammals are hunted in and around protected areas in the southern Bahia region. Most people who hunted did so occasionally, and predominately to complement their diet, although also for medicinal reasons, recreation and retaliation. Consumption of wild meat is common in Brazil, both for subsistence (Peres, Reference Peres2000; Hanazaki et al., Reference Hanazaki, Alves and Begossi2009; Barbosa et al., Reference Barbosa, Nobrega and Alves2011) and because rural residents appreciate its flavour (Fernandes-Ferreira et al., Reference Fernandes-Ferreira, Mendonça, Albano, Ferreira and Alves2012). We also obtained some indirect evidence of commercial hunting for the illegal wild meat trade. Our results suggest that the majority of hunting occurring in the area is opportunistic. People who capture wildlife opportunistically probably do not consider themselves to be hunters because they do not perceive hunting for personal consumption as a profession but as a part of everyday life. Controlling this type of hunting may be a greater challenge than controlling targeted hunting, as it offers an unexpected opportunity for people to supplement their diet (Gardner & Davies, Reference Gardner and Davies2014) and because it is also related to peoples’ feelings and attitudes about their relationship with wildlife. Our finding that hunting occurs in protected areas within the study area was supported by researchers from other projects meeting hunters and finding traps and hunting platforms in Una Biological Reserve. We also found evidence of hunting (traps and dogs) during our fieldwork in all areas, but more frequently in the buffer zone.
Respondents were split on whether hunting had increased or decreased and whether species abundance had changed. The perception of a decrease in hunting was higher in Una Biological Reserve–Una Wildlife Refuge. The absence of law enforcement in Serra das Lontras National Park and the buffer zone, and the Park's more recent designation as a protected area, could be contributing to the perception that hunting was higher and not decreasing there. Law enforcement in the region is irregular and infrequent. During 2010–2013 the Chico Mendes Institute for Biodiversity Conservation recorded 19 seizures related to hunting (seizure of hunting equipment and animal body parts) in Una Biological Reserve and Una Wildlife Refuge in 22 patrol days. However, there were no law enforcement events during this period in Serra das Lontras National Park or the buffer zone, because of a lack of financial and human resources. In addition, after Una Biological Reserve was established, environmental educational activities and technical support for agriculture were provided for rural residents living in areas adjacent to the protected area, which are now designated as the Wildlife Refuge (IBAMA, 1997). Santos & Blanes (Reference Santos and Blanes1999) reported a reduction in hunting levels around the Reserve after 3 years of the environmental education programme (initiated in 1995), which disseminated information about forest conservation and the objectives of the protected area, property rights, environmental laws, and new alternatives for land use. The reasons why respondents were divided on whether species abundances were increasing or decreasing are not clear. It may be a reflection of local environmental variation, such as in presence of roads, human population density and size of forest fragments. Alternatively there may have been differences in how respondents interpreted the question. Further research is needed to understand these responses better.
With respect to socio-economic factors, we found a relationship between educational level and hunting, with people with primary education hunting more than illiterate people (who were generally older) or those with other levels of education. We also found that older respondents consumed collared peccary more than younger respondents. Without more contextual information and validation, however, these relatively weak associations cannot be taken as a basis for policy. Other confounding factors may be responsible for the relationships observed. With respect to hunting legality, according to the Law of Environmental Crimes, hunting is illegal in Brazil except ‘in case of necessity to satisfy the hunger of a person or his family, protection of crops and cattle from predatory or destructive animals, and for animals identified as harmful’ (provided there has been previous authorization; Art. 37 Federal Law 9605/98). However, Decree 6514/2008, which defines administrative infractions against hunting in Brazil, considers the activity to be illegal, without exceptions. As the Chico Mendes Institute is responsible for addressing administrative infractions in protected areas and buffer zones, hunting is illegal inside the study area. According to protected area managers, when a case of hunting to satisfy hunger is observed during a law enforcement event, this information is registered and is considered by the body responsible for taking action with respect to the infraction. Despite only a few respondents stating that they hunt occasionally because of a lack of animal protein, protected area managers need to take this into account, and further work should investigate who these occasional hunters are, and what proportion of residents hunt to satisfy hunger.
Wild meat consumption
Our finding that pacas, armadillos and collared peccaries were both the most consumed species and the most preferred wild meats confirms the findings of other studies in the region (Pereira & Schiavetti, Reference Pereira and Schiavetti2010; Flesher & Laufer, Reference Flesher and Laufer2013) and nationally (Cullen et al., Reference Cullen, Bodmer and Valladares Pádua2000). We also found that consumption of threatened species was low in the study area (too low for the randomized response technique to be able to estimate prevalence robustly). Some species, particularly monkeys, sloths and porcupines, are not prized for consumption and are not easy to hunt unless encountered opportunistically. However, according to records provided by the Chico Mendes Institute, one of the men fined during a law enforcement event in Una Biological Reserve declared that he had hunted yellow-breasted capuchins Sapajus xanthosternos. The southern Bahian masked titi and the golden-headed lion tamarin offer little meat in return for effort invested and therefore are probably not killed frequently. Taboos can also reduce hunting pressure on threatened and endemic species (Colding & Folke, Reference Colding and Folke1997; Jones et al., Reference Jones, Andriamarovololona and Hockley2008) but are not always sufficient; our respondents suggested that when there was a need for animal protein and an opportunity for an easy hunt (e.g. a maned sloth crossing a road), non-preferred species usually protected by taboos were still hunted.
Although the most preferred, and most consumed, species are relatively common in the study region, hunting can still lead to declines. Cassano et al. (Reference Cassano, Barlow and Pardini2012) did not find pacas, peccaries or deer (Mazama sp.) in camera traps in the study region, and suggested that hunting may have caused these species to become uncommon. Species that should occur in the region were either reported to have disappeared or were unknown to our respondents, suggesting that there may have been hunting-related extirpations in the past. These include, among others, the northern brown howler monkey, which is probably locally extinct (Flesher & Laufer, Reference Flesher and Laufer2013; Neves et al., Reference Neves, Clark, Melo, Rylands, Talebi, Schwitzer, Mittermeier, Rylands, Taylor, Chiozza and Williamson2014).
Conclusions
Reducing hunting in protected areas is challenging and complex and requires an integrated approach. Our findings suggest that illegal hunting is occurring within and around protected areas in the study area but that people mostly want to hunt common species for their own consumption. The relatively low level of consumption of threatened species suggests that reducing hunting pressure on threatened species, one of the goals of the National Action Plan in southern Bahia (ICMBio, 2010), is not an impossible task. Management efforts in Una Biological Reserve and Serra das Lontras National Park should prioritize a fair expropriation process for residents, whereas in Una Wildlife Refuge and the buffer zone it is necessary to take an approach to wildlife management that involves local people, such as an expanded education programme to disseminate information about the conservation status of threatened species and the rules governing hunting (Milner-Gulland et al., Reference Milner-Gulland and Bennett2003; Gandiwa, Reference Gandiwa2011). It is also necessary to take account of local needs for protein, even if this involves only a small portion of residents. Furthermore, government and conservation institutions must allocate more financial resources to implement the National Action Plan and protect the threatened species in the study region through effective protected area management.
Acknowledgements
This study was funded by the Brazilian National Council for Scientific and Technological Development (CNPq), the Centre for Research and Conservation of the Royal Zoological Society of Antwerp (funded by the Flemish Ministry for Economy, Science and Innovation) and the State University of Santa Cruz. We thank the Bahia State Research Support Foundation for providing a PhD scholarship to LCC, and CNPq for providing a PhD sandwich scholarship to LCC to spend time working at the Interdisciplinary Centre for Conservation Science in the UK, and a research fellowship to AS. We are grateful to the managers of the protected areas; to Camila Cassano, Natalia Hanazaki, Rômulo Alves, Romari Martinez and the anonymous reviewers for their valuable comments; and to the rural communities in the study area for their willingness to participate in the interviews.
Biographical sketches
Luciana Castilho is interested in social-ecological research, investigating the use of natural resources, and particularly wild fauna, in protected areas, and its implications for wildlife conservation and the welfare of local people. Kristel De Vleeschouwer is a primate ecologist studying factors affecting the persistence of golden-headed lion tamarin populations in degraded and anthropized landscapes in south Bahia, and working towards science-based conservation planning for the species. E. J. Milner-Gulland leads the Interdisciplinary Centre for Conservation Science at Oxford University (http://www.iccs.org.uk) and has a particular interest in understanding the motivations of local resource users. Alexandre Schiavetti’s research interests include planning and management of protected areas, especially for solving conflicts between users of natural resources and biodiversity conservation.










