INTRODUCTION
Salmonella is the second most frequently reported foodborne pathogen in the EU member states and Norway, with an incidence of 31·1 cases/100 000 in 2007. However, in recent years the notification rate in the EU has been decreasing [1]. Salmonella enterica serovar Enteritidis (S. Enteritidis) and Salmonella enterica serovar Typhimurium (S. Typhimurium) are the most common causes of non-typhoid salmonellosis in humans, accounting for 81% of all notified cases in the EU in 2007 [1, Reference Heymann and Thuriaux2]. The notification of Salmonella infections shows a typical peak during summer and autumn, and the-age specific incidence among notified cases is known to be highest in the first years of life [1, Reference Tauxe, Pavia, Evans and Brachman3].
In Norway, almost 80% of notified cases are travel-related and the level of domestically acquired salmonellosis is low. This epidemiological pattern is similar to the situation in Sweden, Finland and Iceland, but differs from most European countries [1, Reference Kapperud, Lassen and Hasseltvedt4]. Of domestically acquired cases, S. Typhimurium is the most commonly reported serovar (accounting for 30–40%), and the only serovar demonstrated to be present in Norwegian wildlife [Reference Refsum5]. S. Enteritidis is overall the most commonly reported serovar in Norway, reflecting the fact that the majority of the cases are infected abroad. Norwegian Salmonella control programmes have documented that, so far, live cattle, swine and poultry, as well as domestically produced food products of animal origin in Norway are virtually free from Salmonella. S. Enteritidis has been detected in Norwegian poultry only once [Reference Hofshagen, Heier and Hauge6].
Thailand and the Canary Islands in Spain are among the most popular tourist destinations of Norwegians. The Canary Islands have been popular for decades, while Thailand has experienced increasing popularity as the travel activity has recently been dominated more by charter tourism in addition to the more independent backpackers. The numbers of notified travel-associated salmonellosis cases may reflect travel activity rather than the actual risk of disease in various foreign countries. To investigate risk of disease during travel, denominator data on number of travellers is needed [Reference Ekdahl7].
The aims of this article were to present the epidemiology of notified human cases of salmonellosis among Norwegian travellers to two popular charter tourist destinations, the Canary Islands and Thailand during the 15-year period 1994–2008. We also present trends in risk of salmonellosis among Norwegian tourists to the Canary Islands 2000–2008 and Thailand 1997–2008, using travel statistics as denominator data. The findings were intended to serve the public health purpose of informing prevention messages to travellers and to assess the effects of the EU Salmonella Control Programme.
METHODS
Surveillance data
Human cases of salmonellosis have been notifiable to the Norwegian Surveillance System for Communicable Diseases (MSIS) since 1975. For each notified case, the database contains information from the doctor seeing the patient, the local medical microbiological laboratory identifying the bacteria, and the National Reference Laboratory for Enteropathogenic Bacteria located at the Norwegian Institute of Public Health. We classify a case as ‘infected abroad’ if the case was abroad during the incubation period. Information on travel history has been recorded in MSIS since 1982, but new routines of notification introduced in 1995 improved the reporting of country of infection. In this study, data on all cases of salmonellosis notified to MSIS during the years 1994–2008 were included.
Denominator data
The Civil Aviation Administration in Norway (Avinor) provided data on the number of passengers by month on direct flights (both charter and scheduled flights) from Norwegian airports to destinations in the Canary Islands. The data were comparable for the years 2000–2008 only. The Tourist Authority of Thailand collects data on number of travellers visiting the country by nationality, and we obtained data on Norwegian travellers for the years 1997–2008. These data were available on the website of the Thailand Tourist Authority [8]. The denominator data used in this article were all aggregated data and did not contain any personal data or information on gender and age.
Statistical analyses
Surveillance data included in the study were extracted from the MSIS database by Microsoft SQL Server and the pivot table function in Microsoft Office Excel 2003. Calculations, graphs and tables were performed in Microsoft Office Excel 2003. Risks were given as the number of notified travel-related salmonellosis cases/100 000 travellers, and calculated as attack rates among travellers using surveillance data as numerators and number of travellers to the Canary Islands and Thailand, respectively, as denominators. Confidence intervals (95% CI) for the risks were calculated using EpiSheet.
RESULTS
A total of 22 813 cases of human salmonellosis were notified to MSIS during the period 1994–2008. The annual average number of cases was 1501 (median 1492) with a minimum of 1022 cases in 1995 and a maximum of 1941 cases in 2008. Of the 22 813 cases, 18 002 (79%) acquired the infection abroad, 3579 (16%) were domestic cases, while information on country of infection was missing in 1232 cases (5%).
Norwegians have acquired salmonellosis in 148 foreign countries in the study period. Spain was the most commonly reported country, accounting for 22% (3927 cases) with the Canary Islands accounting for 1923 cases (8%) of the total number of imported cases, followed by Thailand (1767 cases; 8%).
The Canary Islands
Annually, the median number of notified cases returning from the Canary Islands was 113 (range 56–251 cases), peaking with 251 cases in 1998 and 246 cases in 2001, followed by a gradual decrease to 81 cases in 2008 (Fig. 1).
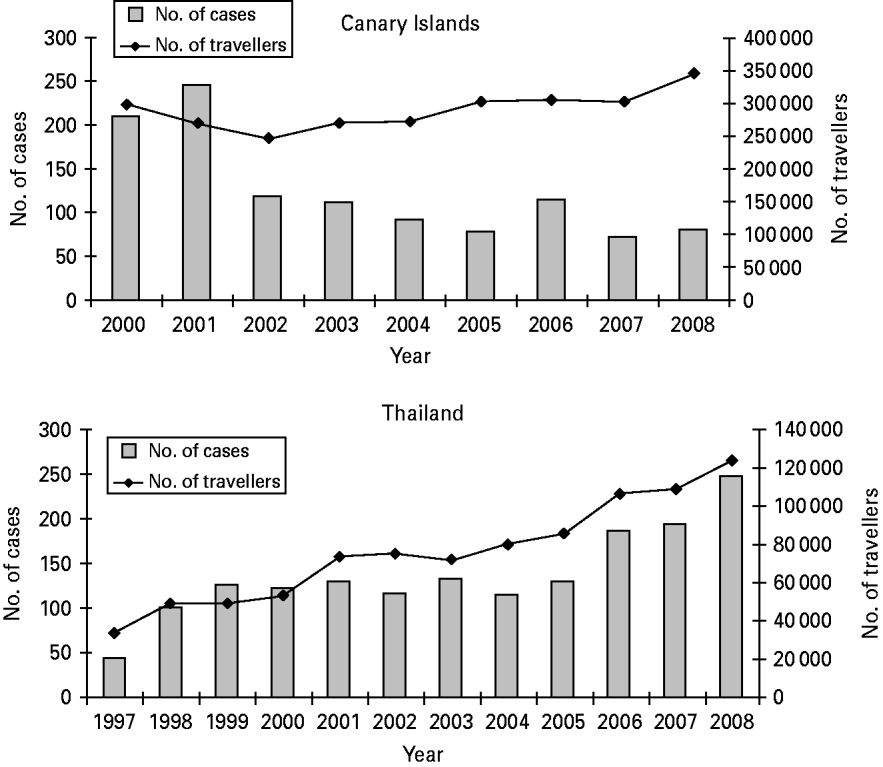
Fig. 1. Number of notified imported cases from, and Norwegian travellers to, the Canary Islands and Thailand by year, Norway, 1997–2008.
The majority (58%) of the cases imported from the Canary Islands were women, and the predominant age group was 40–59 years, accounting for 41% of the cases.
Over the 15-year period, most cases (68%) were reported during the cold months, November to April, with a peak of 326 cases in November. Fewer than 50 cases were reported with disease onset in May (48 cases) and June (39 cases). For July–October, the number of cases with symptom onset varied between 99 cases in September and 170 cases in October.
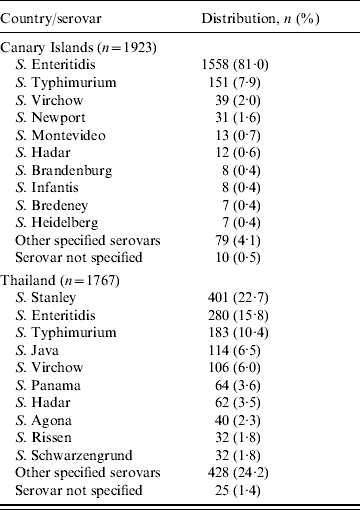
S. Enteritidis was the dominating serovar among the cases who visited the Canary Islands, accounting for 1558 cases (81%) followed by S. Typhimurium (151 cases, 8%) and S. Virchow (39 cases, 2%) (Table 1). S. Enteritidis became more dominant over the time period, from 54% (30 cases) in 1994 to 91% (70 cases) in 2005. S. Typhimurium isolates proportionally ranged from 1% (one case) in 2003 to 21% (15 cases) in 2007. Figure 2 presents the distributions of the five most common serovars from the Canary Islands by year.
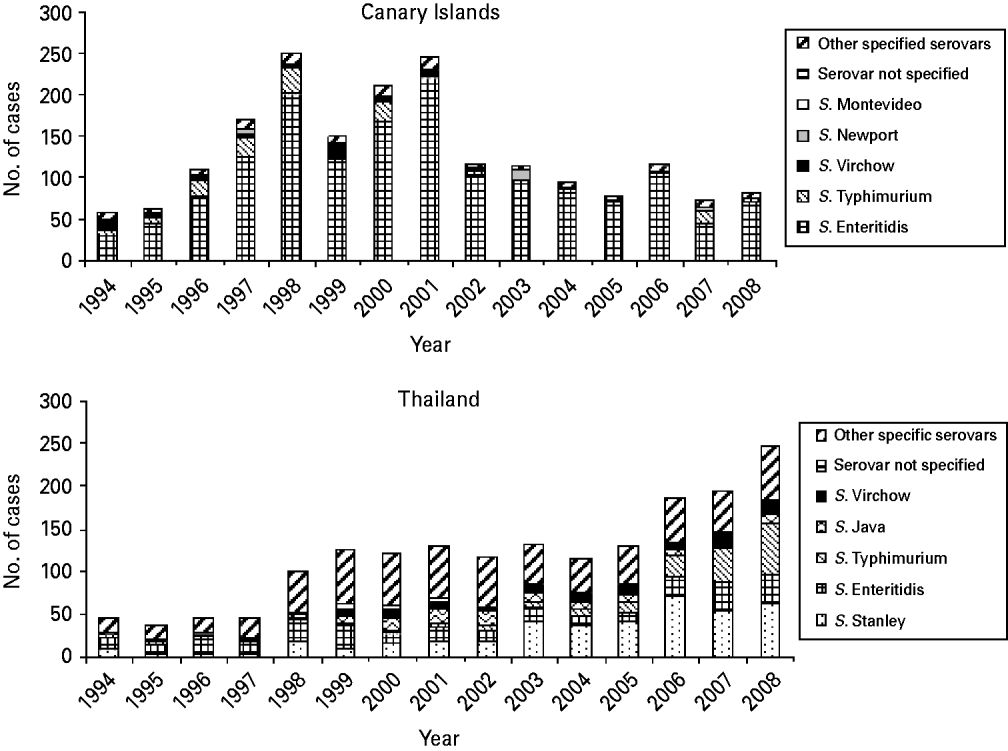
Fig. 2. Distribution of the five most commonly reported serovars for imported cases from the Canary Islands (n=1923) and Thailand (n=1767) by year, Norway, 1994–2008. The peak in cases from the Canary Islands in 1998 was partly due to an outbreak of S. Enteritidis infections in Norwegian tourists to Lanzarote, involving more than 30 notified cases.
Table 1. Distribution of the 10 most commonly reported serovars in cases infected in the Canary Islands and Thailand during the period 1994–2008
Annually, an average of 291 157 passengers (median 300 510) departed from Norwegian airports to destinations in the Canary Islands during 2000–2008. The number of travellers ranged from 247 837 in 2002 to 345 987 in 2008, thus showing an increase of 39·6%. However, this increase in travel activity was not paralleled with an increase in number of reported cases (Fig. 1). The average annual risk (attack rate) of being notified with salmonellosis after travelling to the Canary Islands was 42·9/100 000 travellers during the period 2000–2008, decreasing from 90·0/100 000 travellers in 2001 to 23·4/100 000 travellers in 2008 (Table 2). This decrease was largely due to a gradual decrease in risk of being notified with S. Enteritidis, from 82/100 000 (95% CI 72–93/100 000) travellers in 2001 to 21/100 000 (95% CI 16–26/100 000) in 2008. The risk of being notified with S. Typhimurium remained more stable over the period with the risk varying between 1 and 2 per 100 000 travellers except for the top years 2000 and 2007 with 8/100 000 (95% CI 6–12/100 000) and 5/100 000 (95% CI 3–8/100 000) travellers, respectively.
Table 2. Risk (attack rate per 100 000 Norwegian travellers by year) with 95% confidence intervals (CI) of salmonellosis acquired in the Canary Islands (2000–2008) and Thailand (1997–2008)
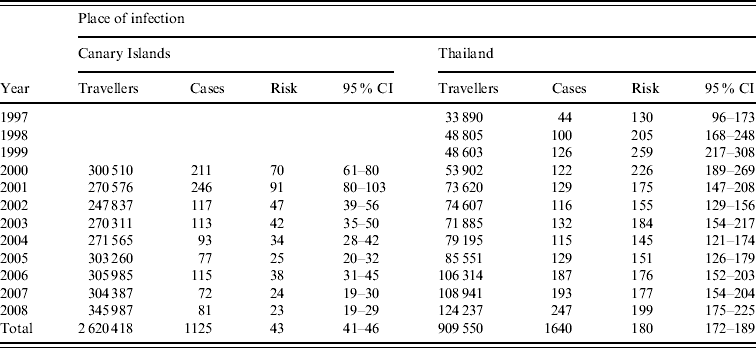
Comparable travel data was available only for 2000–2008 to the Canary Islands and for 1997–2008 to Thailand.
Thailand
The annual number of reported cases increased steadily over the period with a range from 36 cases in 1995 to 247 cases in 2008 (median 122 cases). The number of cases reported increased markedly after 2005 (Fig. 1).
Of the cases imported from Thailand, the majority (53%) were men and most (43%) cases were in the 20–39 years age group.
Most (69%) cases were reported during the cold months of November–April, with a peak of 258 cases in March, followed by 206 cases in April. The summer months had the lowest numbers of cases, with 73 cases in May, 55 cases in June and around 130 cases in July and August.
S. Stanley was the most common serovar representing 23% (401 cases), followed by S. Enteritidis with 16% (280 cases) and S. Typhimurium with 10% (183 cases) (Table 1). S. Stanley became more dominant during the second half of the 15-year period, ranging from only three cases (7%) in 1996 to 71 cases (38%) in 2006. S. Enteritidis was the most common serovar during the first half of the period, ranging from 41% (18 cases) of the isolates in 1996 to 11% (15 cases) in 2000. However, after 2005 there has been a clear increase in both S. Enteritidis and S. Typhimurium isolates, with S. Enteritidis ranging from 7·8% and S. Typhimurium 9·3% in 2005, to 14·2% and 24·3%, respectively in 2008 (Fig. 2).
In 2008, S. Stanley and S. Typhimurium constituted 26% each (61 and 60 cases, respectively) of the isolates of travellers returning from Thailand.
The travel activity from Norway to Thailand more than tripled over the 15-year period, from 33 890 travellers in 1997 to 124 237 in 2008. The annual average over the period was 75 796 travellers. As Figure 1 shows, the number of cases from Thailand corresponded well with number of Norwegian travellers to the country. Thus, the trend in risk of acquiring salmonellosis as a Norwegian traveller to Thailand has been rather stable around 150–200/100 000 travellers, except for a peak in 1999 with a risk of 259/100 000 travellers (Table 2).
DISCUSSION
We have presented the epidemiology of salmonellosis cases notified in Norway after travel to two popular charter destinations, the Canary Islands and Thailand, for the 15-year period 1994–2008.
Our results show a considerable reduction in the number of notified salmonellosis cases, as well as in the risk of acquiring the disease, among Norwegian tourists to the Canary Islands over the years after 2002.
The travel activity from Norway to Thailand almost doubled over the 5-year period 2003–2008, paralleled with an increase in numbers of notified salmonellosis cases related to travel to Thailand. Due to this correlated increase in both travellers and cases, the risk of Salmonella infection for a Norwegian tourist to Thailand was rather stable. The risk of acquiring salmonellosis was at least four times higher during travel to Thailand than to the Canary Islands.
The two datasets on travel activity used as denominator data in the calculations of risks are not directly comparable as they are of different origins, covering different time spans and do not measure the same travel activity. We used the total figures of travellers on direct flights between Norwegian airports and destinations in the Canary Islands. Hence, the dataset did not capture those who changed flights in other countries. The dataset for Thailand is more complete, based on registrations of all tourists entering the country, covering all means of transport. With this distinction in mind, the calculated risk of salmonellosis by travel to the Canary Islands is probably overestimated compared to the calculated risk by travel to Thailand. It should also be noted that the dataset we used for Thailand covered Norwegian nationals, not Norwegian residents. The denominator data will also include Norwegian nationals who do not necessarily live in Norway and, therefore, would not be able to be a notified case in Norway. However, this limitation does not influence trends in risk over time within each of the destinations separately, as both datasets are regarded as comparable over the years covered by the chosen time spans. We suggest our results should be interpreted as rough estimates used in the study of trends, rather than attempts to assess the true differences in risk of disease.
As the datasets on travel activity are aggregated only, we have not been able to consider the impact of factors such as differences in length of stay and age profiles of the travellers to the two destinations. It is likely that travellers to Thailand are younger than those travelling to the Canary Islands, which our findings of different age distributions among the cases to the two destinations may reflect. Younger travellers are more likely to eat cheaper food, and take greater risks in their choice of food. As a consequence, our finding of differences in risk of salmonellosis by travelling to the Canary Islands compared to Thailand may partly be due to differences in the tourist groups' lengths of stay, habits and behaviour rather than merely reflecting differences in the local true risks of disease.
It is well known that surveillance data on infectious diarrhoeal diseases cover only a fraction of all cases [Reference Wheeler9]. Therefore, our study suffers from detection bias as it was based on surveillance data. Data on travel-related cases may be particularly prone to this kind of bias as many patients may be past the disease when they return home so that they are not diagnosed and reported in Norway. However, this seems less important in the study of trends, as there have not been any changes in notification routines, except an improved reporting of country of infection since 1995. As a consequence of the detection bias, the results should be interpreted as the epidemiology of the notified cases and the risk of being notified with salmonellosis after travel to the Canary Islands and Thailand, rather than the true risk and epidemiology of the disease.
We do not know the reasons for the reduced risk over time of salmonellosis among Norwegian tourists to the Canary Islands. However, our results are in accordance with the European Food Safety Authority (EFSA) documentation of a steady decrease in numbers of notified human cases of salmonellosis in Spain from 8558 cases in 2003 to 3658 cases in 2007, as well as in the prevalence of Salmonella in Spanish poultry during recent years [1, 10]. Furthermore, we found that the decrease in risk was largely due to a decrease in S. Enteritidis while S. Typhimurium remained stable. This finding is in line with data from Spain showing a decrease in notified S. Enteritidis cases after 2003 and a paralleled stable or even increasing notification of S. Typhimurium cases [11]. These changes may be ascribed the implementation of the new Salmonella control programmes in the EU in 2003 [12]. Our finding of a shift from EU countries (Spain, Greece) to non-EU countries (Thailand, Turkey) accounting for the majority of the travel-related salmonellosis cases notified in Norway, could also be interpreted as a possible consequence of the new control programmes in the EU. However, the shift could also be the result of a possible change in travel habits of Norwegians in favour of non-EU countries. Future studies with denominator data are needed to explore these changes in risk. Changes in local epidemiology, improved hygiene at the tourist resorts or improved health knowledge among tourists are all plausible explanations.
The serovar distribution of the Norwegian cases returning from Thailand is more diversified than that of the cases from the Canary Islands. Concurrent with the increase in travel activity and notified cases, the serovar distribution among the cases returning from Thailand has changed towards a more ‘European distribution’ with an increasing dominance of S. Enteritidis and S. Typhimurium, although S. Stanley was most commonly isolated during the latter half of the study period.
Other studies have documented greater diversity in the isolation of serovars among Thai nationals [Reference Bangtrakulnonth13, Reference Boonmar14], which may reflect different foods and other reservoirs for infections [Reference Bangtrakulnonth13].
A possible explanation of the change in serovar distribution among Norwegian tourists to Thailand is that it reflects a similar change in serovar distribution among Thai nationals. However, when we compared the serovar distribution reported among Thai nationals during 1993–2002 (dominated by S. Weltevreden, S. Enteritidis, S. Anatum and S. Derby) [Reference Bangtrakulnonth13] to the distribution among Norwegian tourists to Thailand during the same period (dominated by S. Enteritidis, S. Stanley and S. Paratyphi B var. Java) they did not correspond. Studies on foods in Thailand during the mid 1990s showed that S. Weltevreden was most commonly isolated in frozen shrimps, S. Derby and S. Anatum in ready-to-eat Thai food and S. Enteritidis in frozen chicken meat [Reference Boonmar14]. A study in farm animals, farm workers and children with diarrhoea in Northern Thailand in 2000–2003 found that the most frequently isolated serovar was S. Weltevreden in chickens and humans, and S. Rissen in pigs. S. Enteritidis was not isolated from humans in that study, and only in a small proportion of isolates from chickens [Reference Padungtod and Kaneene15]. Except for the isolation of S. Enteritidis in frozen chicken meat, these findings of common serovars in Thai foods are not reflected in parallel dominance of the same serovars among Norwegian tourists notified with salmonellosis from Thailand over the same period of time. It should also be noted that S. Enteritidis was not isolated from humans during the years 2000–2003 in the latter study, during a time when that very serovar became increasingly dominant in Norwegian tourists returning from the country.
This discrepancy between serovars of Salmonella causing infections in Norwegian tourists and the serovars most commonly found in Thai foods and dominating the reported Salmonella infections in Thai nationals could be a result of a distinct tourist distribution. This explanation is supported by Ekdahl et al., who presented a serovar distribution dominated by S. Enteritidis (15·1%) followed by S. Stanley (11·1%) among Swedish tourists returning from East Asia for the period 1997–2003 [Reference Ekdahl7]. Infections and outbreaks among tourists may not always affect the local resident and therefore not be detected by the local public health authorities. Therefore the observed shift towards a more European distribution pattern among Norwegian tourists may be the result of a change in food habits and lifestyle among tourists visiting Thailand, moving towards a more Western European style as the destination grew in popularity and became more dominated by charter activity than independent backpackers.
The seasonal variability in the number of notified cases observed in tourists returning from the Canary Islands and Thailand shows a peak in the winter months and a decline during summer and autumn and differs clearly from the previously described seasonal variation [1, Reference Tauxe, Pavia, Evans and Brachman3]. This could be partially explained by different seasonality of different serovars, especially regarding the seasonality of the Thailand cases since there has been a shift in serovar distribution. However, we suggest that this diverging seasonal variability in number of notified cases is merely a result of the seasonal variation in travel activity rather than reflecting the true risk of disease [Reference Ekdahl7].
The risk of acquiring salmonellosis is far greater during travel to Thailand or the Canary Islands than at home in Norway. Norwegian travellers need to be informed about the increased risk and basic hygienic and food safety habits. It seems especially relevant to inform travellers about the risk in new travel destinations such as Thailand. However, more research is needed to understand which particular foodstuffs and behaviours are associated with infection so that a more tailored prevention message can be delivered.
CONCLUSIONS
The Canary Islands and Thailand are important tourist destinations for Norwegians, accounting for a large proportion of reported Salmonella infections in Norway. The risk of salmonellosis among Norwegian travellers in the Canary Islands has decreased steadily and is at the end of the study period 23/100 000 travellers, while the risk in Thailand increased up to 199/100 000 travellers in 2008. The example of the Canary Islands indicates that it is possible through a Salmonella control programme to reduce the risk of salmonellosis, while the different epidemiology in Thailand indicates that more research needed to understand the spread of salmonellosis there. Overall, future research using denominator data is needed to further understand changes in risk of travel-associated salmonellosis.
DECLARATION OF INTEREST
None.






