The consumption of a plant-based (PB) diet has been associated with a reduced risk of type 2 diabetes, CVD and other cardiometabolic risk factors, some cancers and all-cause mortality(Reference Chiavaroli, Nishi and Khan1–Reference Dinu, Abbate and Gensini5). The recent report from the Eat-Lancet Commission recommends a global shift towards PB diets, emphasising an increased intake of PB foods such as fruits, vegetables, wholegrains, legumes and nuts and a reduced intake of animal-derived foods, for both health and environmental sustainability(Reference Willett, Rockström and Loken6). While food-based dietary guidelines have traditionally provided guidance to consume fruit and vegetables and starchy staples (i.e. PB foods) as the bulk of the diet, ongoing updates to food-based dietary guidelines globally are placing even more emphasis on PB foods(Reference Gonzalez Fischer and Garnett7,8) . Nonetheless, almost all countries still recommend the consumption of animal-derived foods, along with other food groups, in recognition of the important contribution of animal-derived foods towards providing high-biological value protein, bioavailable n-3 and a range of micronutrients, including riboflavin, niacin, vitamin B6, vitamin B12, iron and zinc(Reference Herforth, Arimond and Alvarez-Sanchez9–Reference Swanson, Block and Mousa12).
The term ‘plant-based diet’ encompasses a wide spectrum of dietary patterns which emphasise plant products, such as fruits and vegetables, wholegrains, legumes, nuts and seeds and PB alternatives and limit or exclude animal-derived products(Reference Satija and Hu13,14) . However, there is huge variability in PB definitions between studies and as the popularity of PB diets grow, PB terminology is also evolving as PB diets are being described as ‘plant-centred’, ‘plant-predominant’, ‘plant-rich’, ‘plant-focused’, ‘plant-forward’, etc.(Reference Remde, DeTurk and Almardini15–Reference Graça, Truninger and Junqueira18).
In the Western world, media sources, consumer bodies and vegan and vegetarian societies are reporting a shift towards an increase in PB consumers(Reference Alcorta, Porta and Tárrega19); however, data from national food consumption surveys continue to show that 98–99 % of people in all population groups still consume meat(Reference Bates, Lennox and Bates20–22). While these figures may not be reflective of other PB diets that include small amounts of meat and/or dairy, generally the number of PB diet consumers remains largely unknown and difficult to elucidate due to the large variation in definitions of PB diets.
While it has been acknowledged that not all PB diets are necessarily healthy, few studies have differentiated between ‘healthful’ and ‘unhealthful’ PB diets(Reference Satija, Bhupathiraju and Rimm23,Reference Kim, Caulfield and Rebholz24) . Furthermore, concerns remain regarding the nutritional adequacy of some restrictive PB diets, such as vegan diets with respect to some key micronutrients such as vitamin D and B12, which are only naturally occurring in animal-derived products(Reference Bakaloudi, Halloran and Rippin25). Simultaneously, the global market for PB alternative foods and beverages is growing rapidly(26,27) ; however, the dietary quality of ultra-processed PB alternative products is under scrutiny, with some studies showing that PB alternative foods may contain higher sodium and many do not contain key micronutrients, such as vitamins D, B12, iron or zinc, that would traditionally be found in their animal-source counterparts(Reference Wickramasinghe, Breda and Berdzuli28–31).
While there is a general consensus that consuming a PB diet confers health and environmental benefits, there remains a significant challenge in understanding the nutritional role of PB diets due to the variations in definitions and the paucity of studies reporting nutrient intake from PB diets. This review aims to summarise the definitions of PB diets globally and to investigate the nutritional role of PB diets in adults.
Methods
Inclusion/exclusion criteria
The present paper includes a review of definitions of PB diets from peer-reviewed literature, position statements and vegan and vegetarian society websites. Furthermore, this review includes intervention or large observational studies of adults (≥18 years) that report nutrient intakes from those consuming a PB diet compared to a general omnivorous/baseline diet(Reference Fagerland32,33) . This review includes studies that were published in English and post the year-2000.
Search strategy
To search for PB definitions, an electronic search was conducted in PubMed and Web of Science. A search of the grey literature was also conducted which included UK vegan and vegetarian society websites and position statements. Subject index terms included ‘plant-based’, ‘plant-based diet’, ‘plant-centric’, ‘plant-centred’ and ‘definition’ and the final search builder was ((plant-based OR plant-based diet OR plant-centred OR plant-centric) AND (definition)). For data on nutrient intake from PB diets and/or compliance with recommendations, an electronic search was also conducted in PubMed and Web of Science. Subject index terms included ‘plant-based’, ‘plant-based diet’, ‘vegan’, ‘vegetarian’, ‘pescatarian’, ‘semi-vegetarian’, ‘flexitarian’, ‘portfolio diet’, ‘Mediterranean’, ‘DASH’, ‘healthy US-style diet’, ‘planetary health diet’, ‘Nordic diet’ and ‘nutrient’, ‘nutrient intake’, ‘diet quality’ and ‘adults’ and the final search builder was ((plant-based OR plant-based diet OR vegan OR vegetarian OR pescatarian OR semi-vegetarian OR flexitarian OR portfolio diet OR Mediterranean OR DASH OR healthy US-style diet OR planetary health diet OR Nordic diet) AND (nutrient OR nutrient intake OR diet quality) AND (adults)).
Plant-based definitions
Table 1 outlines the PB diet definitions identified in this review. Traditionally, PB diets referred to vegetarian diets, which include fruits, vegetables, grains, nuts, seeds, beans and pulses but exclude animal-derived foods in different amounts(34). A lacto-vegetarian diet excludes meat, fish and eggs but includes dairy, an ovo-vegetarian diet excludes meat, fish and dairy, but includes eggs and a lacto-ovo vegetarian diet, generally excludes meat and fish but includes dairy and eggs(Reference Dinu, Abbate and Gensini5,Reference Satija and Hu13,Reference Rocha, Laster and Parag35–Reference Newby, Tucker and Wolk49) . Veganism refers to a philosophy or way of life rooted in animal welfare, which seeks to exclude the use of animals for food, clothing and other purposes(50). In dietary terms, the vegan diet is the most extreme form of a vegetarian diet and excludes all foods and beverages wholly or partly derived from animals(Reference Rocha, Laster and Parag35,Reference Melina, Craig and Levin48,50) . Iterations of a vegan diet are defined in the literature, such as a whole-food vegan diet (excludes processed foods), a whole-food low-fat vegan diet (excludes processed and high fat plant foods) and a raw food vegan diet (excludes all cooked food)(Reference Rocha, Laster and Parag35,Reference Melina, Craig and Levin48,Reference Tuso, Stoll and Li51–Reference Trapp and Levin61) .
Table 1. Definitions of plant-based diets
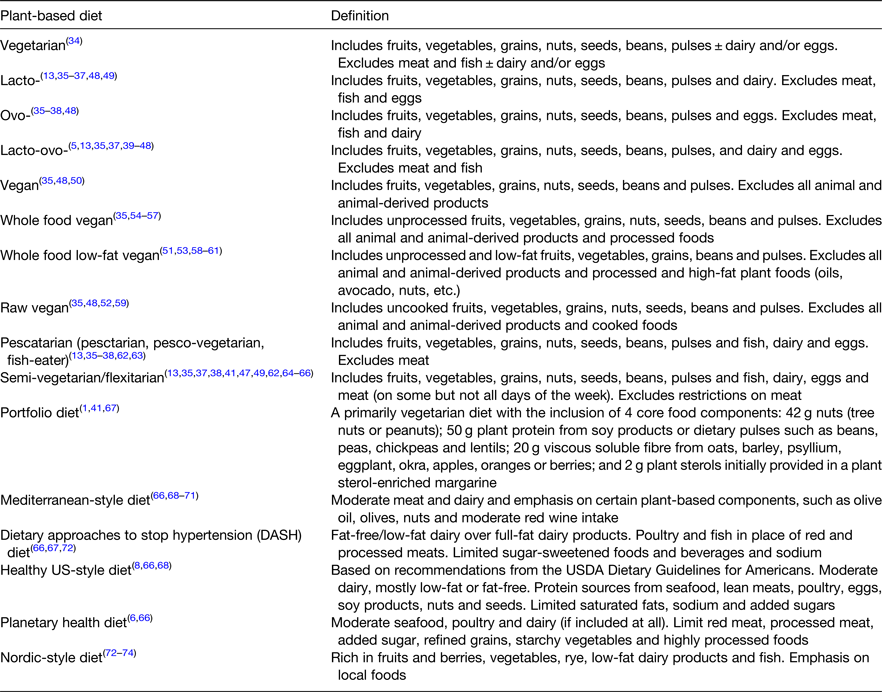
Other variations of vegetarian diets include the pescatarian diet, which is similar to a lacto-ovo vegetarian diet but additionally includes fish(Reference Satija and Hu13,Reference Rocha, Laster and Parag35–Reference Yokoyama, Levin and Barnard38,Reference Turner-McGrievy, Davidson and Wingard62,Reference Wozniak, Larpin and de Mestral63) . The flexitarian or semi-vegetarian diet is described as a primarily vegetarian diet but allows some animal food consumption, however, the amount and type of animal foods varies, from a specified amount of animal food per month to exclusion of red meat only but inclusion of poultry, fish and other animal foods(Reference Satija and Hu13,Reference Rocha, Laster and Parag35,Reference Corrin and Papadopoulos37,Reference Yokoyama, Levin and Barnard38,Reference Ferdowsian and Barnard41,Reference Clarys, Deliens and Huybrechts47,Reference Newby, Tucker and Wolk49,Reference Turner-McGrievy, Davidson and Wingard62,Reference Derbyshire64–Reference Hemler and Hu66) .
Other primarily PB diets, associated with good health and sustainability, are now included within the PB literature. These diets are also high in fruits, vegetables, grains, nuts, seeds, beans and pulses and encourage moderate (or no) intake of animal-derived foods but emphasise certain PB components. The portfolio diet, originally developed to incorporate cholesterol-lowering foods into one diet, is a primarily vegetarian diet, but with specific proportions of four core PB components, i.e. 42 g of nuts, 50 g plant protein, 20 g viscous soluble fibre and 2 g plant sterols(Reference Chiavaroli, Nishi and Khan1,Reference Ferdowsian and Barnard41,Reference Chiavaroli, Viguiliouk and Nishi67) . The Mediterranean-style diet places an emphasis on olive oil, olives, nuts and moderate red wine consumption(Reference Hemler and Hu66,Reference Hemler and Hu68–Reference Trichopoulou, Martínez-González and Tong71) . The dietary approaches to stop hypertension (DASH) diet emphasises fat-free/low-fat dairy over full fat, and limits sodium and added sugar, which is quite similar to the healthy US-style diet(8,Reference Hemler and Hu66–Reference Hemler and Hu68,Reference Eichelmann, Schwingshackl and Fedirko72) . Both the DASH and healthy US-style diet additionally promote protein sources other than red and processed meats, such as seafood, lean meats, poultry, eggs, soy products, nuts and seeds. The planetary health diet places an emphasis on limiting highly processed foods and the Nordic-style diet emphasises low-fat dairy, fish, fruits, berries, rye and local foods(Reference Willett, Rockström and Loken6,Reference Hemler and Hu66,Reference Eichelmann, Schwingshackl and Fedirko72–Reference Kanerva, Rissanen and Knekt74) .
As not all PB diets conform to one diet type, plant-based dietary indexes (PDI) have recently been developed to measure adherence to a PB dietary pattern within an omnivorous population(Reference Martínez-González, Sánchez-Tainta and Corella75). PDI are a type of dietary quality index which positively weight PB foods and negatively weight animal foods. They offer an alternative to defining PB diets in terms of complete exclusion of some or all animal foods. While not all PB foods are necessarily ‘healthy’, some studies have differentiated between what the authors describe as ‘healthy’ and ‘unhealthy’ PB dietary patterns by positively weighting healthy PB foods (e.g. wholegrain cereal products) and negatively weighting low-quality PB foods (e.g. refined cereal products); however, what is included and excluded in the healthy PDI and unhealthy PDI varies between studies(Reference Satija, Bhupathiraju and Rimm23,Reference Kim, Caulfield and Rebholz24,Reference Chen, Zuurmond and van der Schaft76) .
While PDI can help to make associations between adherence to a PB diet and health outcomes, they rarely provide information on nutrient contributions to the diet from the various components and so little is known about the impact of PB diets on nutritional quality. Therefore, this review also aimed to examine the nutritional role of PB diets in adults specifically investigating the intake of energy, macro- and micronutrients and compliance with current dietary recommendations when consuming a PB diet compared to an omnivorous diet.
The nutritional role of plant-based diets
Eleven observational and intervention studies comparing PB diets (raw vegan, vegan, lacto-vegetarian, lacto- and/or ovo vegetarian, pescatarian, semi-vegetarian and Mediterranean) with omnivorous diets (meat-eater or baseline diets) met the eligibility criteria for this review and are described in Table 2. Hereafter, all variations of PB diets within these studies will be referred to collectively as PB diets and all comparative/baseline diets will be described as omnivorous. Tables 3–5 present the energy, macronutrients, dietary fibre and micronutrient intake data from these studies. All values are reported as mean intakes with the exception of one study, which reported medians, as outlined in the respective tables. Where energy in MJ and percentage energy (%E) of nutrients were not provided, this was calculated using standardised conversion factors for easier comparison between studies(77). Micronutrient intakes are reported from the food component of the diet only (i.e. intakes from nutritional supplements are not included). Where retinol was presented as international units (IU), this was converted using standardised conversions for easier comparison between studies(78).
Table 2. Characteristics of studies comparing nutrient intakes from plant-based diets with omnivorous diets
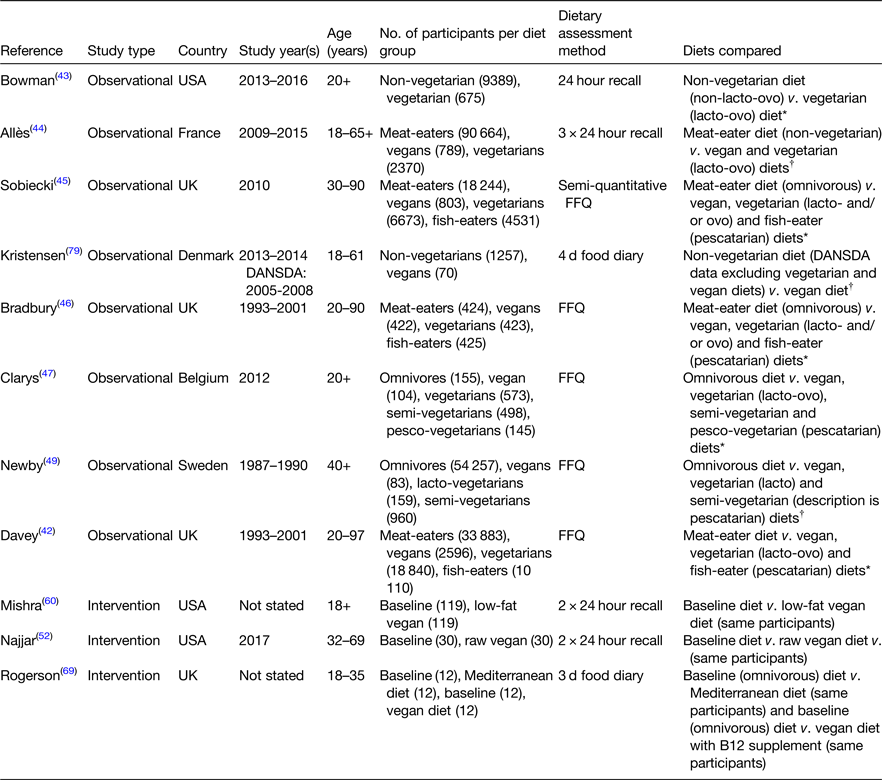
DANSDA, The Danish National Survey of Dietary Habits and Physical Activity.
* Classified into consumer groups by the researcher based on participant self-reported intakes.
† Self-reported consumers.
Table 3. Mean daily energy and macronutrient intakes from plant-based diets compared to omnivorous diets
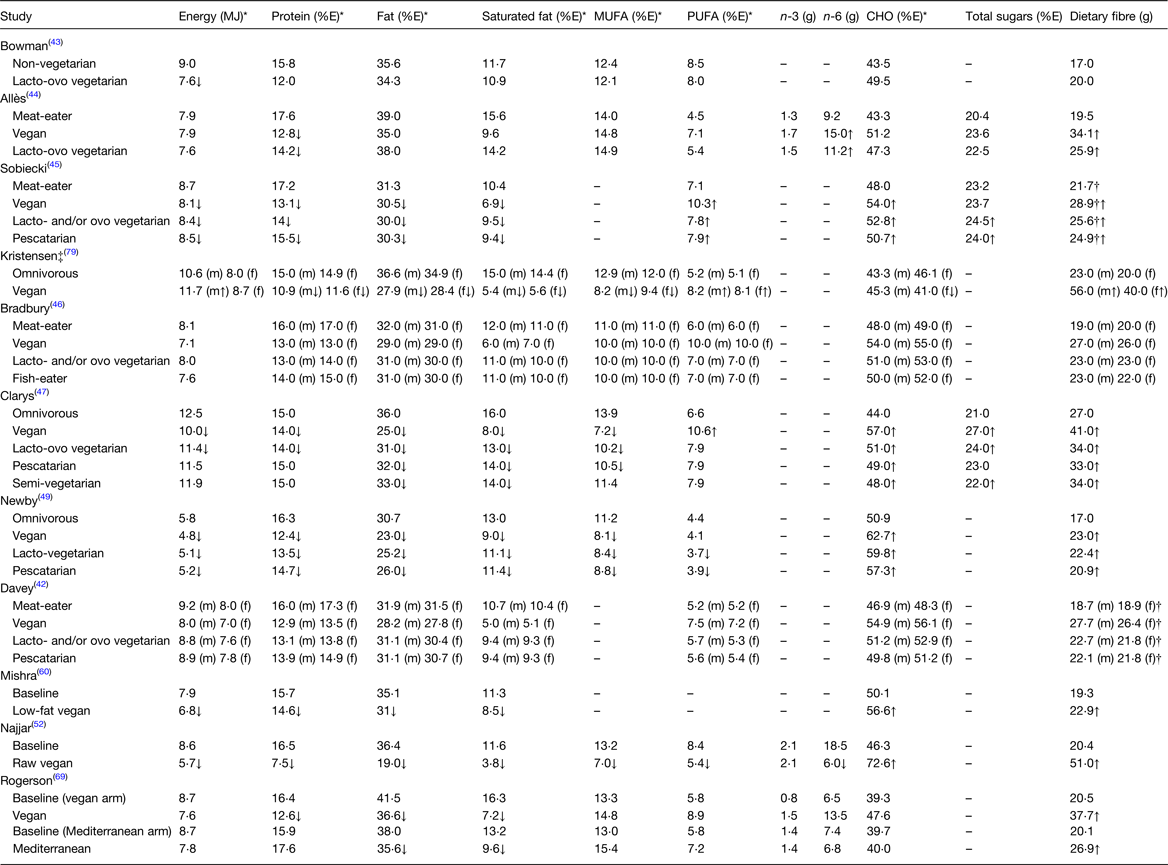
CHO, carbohydrate; – indicates no data available; m, males; f, females.
All values are reported as mean intakes, except for where ‡ (median) is present; *where energy in MJ and percentage energy (%E) of nutrients were not provided, this was calculated using standardised conversion factors(77).
†NSP reported.
↑↓Indicates significantly higher or lower in plant-based diet. Arrows within () indicate a significant difference within males or females only.
Table 4. Mean daily vitamin intakes in plant-based diets compared to omnivorous diets
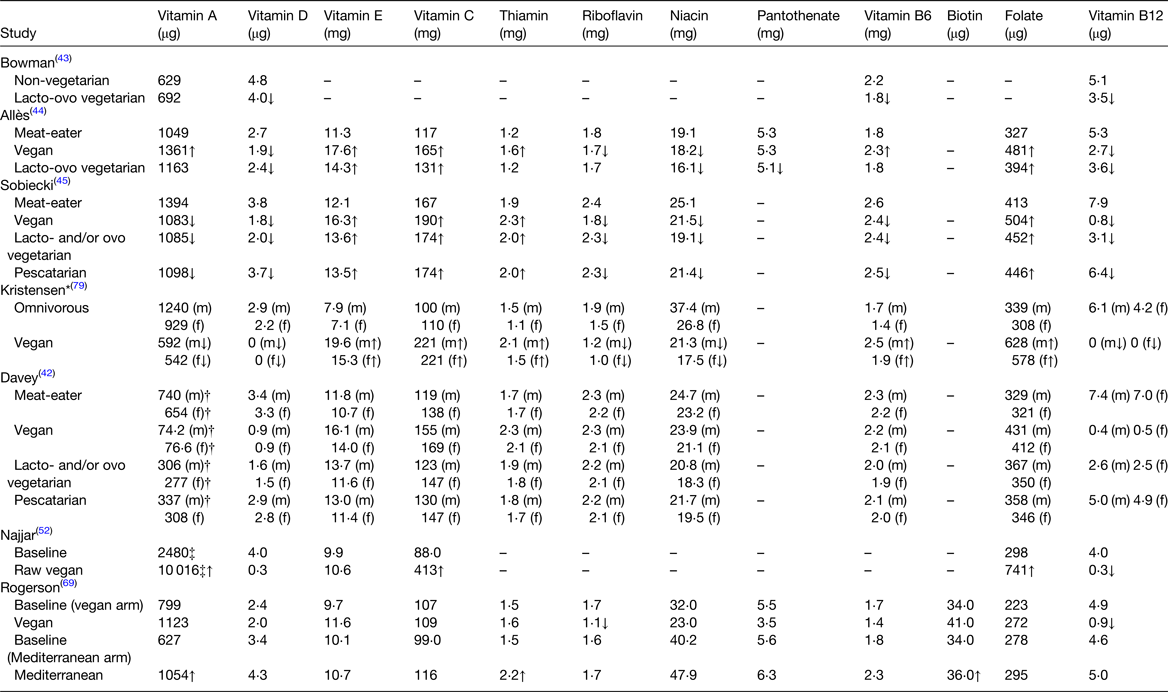
All values are reported as mean intakes, except for where * (median) is present ↑↓ indicates significantly higher or lower in plant-based diet. Arrows within () indicate a significant difference within males or females only.
†Retinol; ‡converted to μg from IU using standardised conversions(78).
Table 5. Mean daily mineral intakes in plant-based diets compared to omnivorous diets
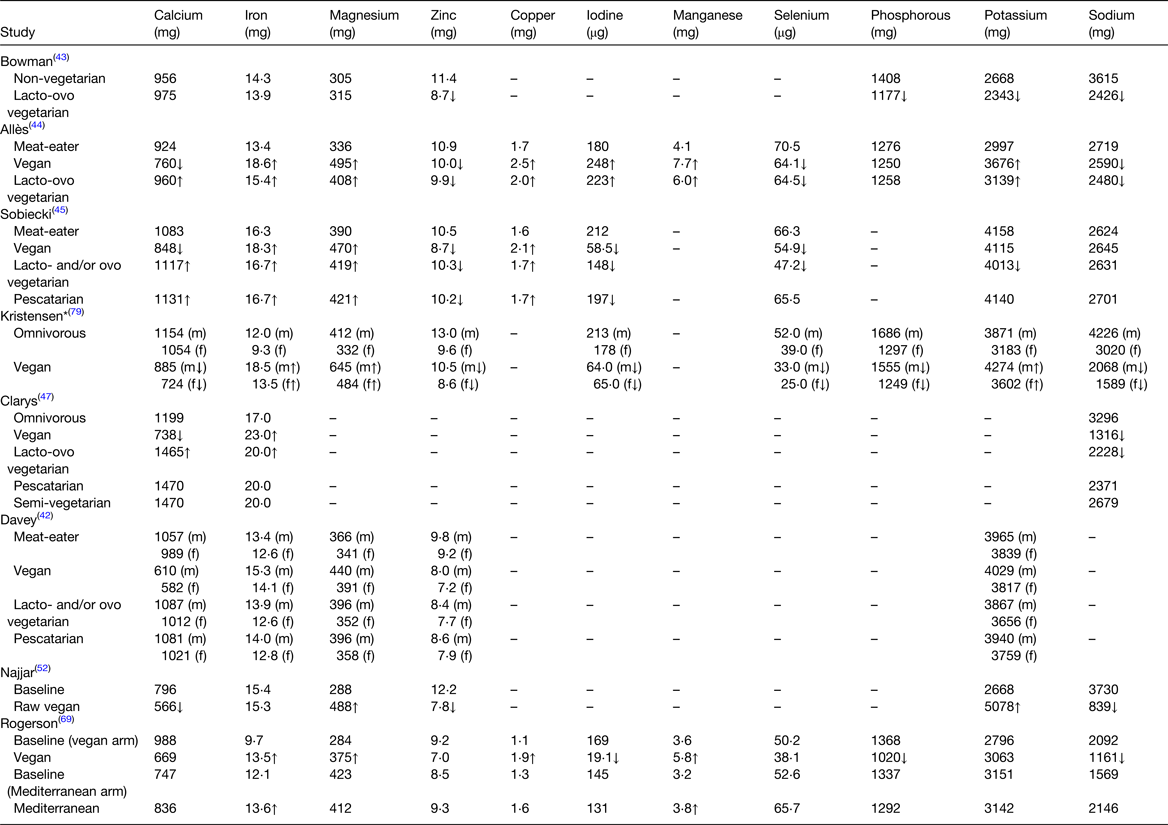
All values are reported as mean intakes, except for where * (median) is present ↑↓ indicates significantly higher or lower in plant-based diet. Arrows within () indicate a significant difference within males or females only.
Energy and macronutrients
Data from both observational and intervention studies showed that the intake of energy from PB diets (regardless of type) (5–12 MJ) was lower or similar than that of omnivorous diets (6–13 MJ)(Reference Davey, Spencer and Appleby42–Reference Clarys, Deliens and Huybrechts47,Reference Newby, Tucker and Wolk49,Reference Najjar, Moore and Montgomery52,Reference Mishra, Xu and Agarwal60,Reference Rogerson, Macas and Milner69,Reference Kristensen, Madsen and Hansen79) . While this may be expected due to the lower energy density of many staple plant foods, such as fruits, vegetables, legumes and wholegrains, compared to animal-derived products, it may also be partly explained by the conscious lifestyle choice of many who consume PB diets(Reference Orlich, Singh and Sabaté80–Reference De Backer and Hudders82). With regard to protein intake, this review found that despite protein intake being lower or similar from PB diets (8–18 % of total energy intake (%E)) compared to omnivorous diets (15–18 %E)(Reference Davey, Spencer and Appleby42–Reference Clarys, Deliens and Huybrechts47,Reference Newby, Tucker and Wolk49,Reference Najjar, Moore and Montgomery52,Reference Mishra, Xu and Agarwal60,Reference Rogerson, Macas and Milner69,Reference Kristensen, Madsen and Hansen79) , protein intake from both PB and omnivorous diets met generally accepted population guidelines(Reference Sobiecki, Appleby and Bradbury45,Reference Kristensen, Madsen and Hansen79) . In contrast, at an individual level, a study by Allès et al.(Reference Allès, Baudry and Mejean44) found that 27 % of vegans and 15 % of vegetarians had intakes below the acceptable distribution intake range for total protein compared to 4 % of omnivores and Sobiecki et al.(Reference Sobiecki, Appleby and Bradbury45) reported that 8–16 % of vegans and 6–10 % of vegetarians had inadequate intakes for protein compared to 1–3 % of meat-eaters. Since many PB diets lack the main sources of high biological value protein that are found in animal-derived products, protein intake from PB diets should be carefully considered to ensure that not only an adequate amount of protein is consumed, but that the variety of protein sources provides a full complement of essential amino acids(Reference Mariotti and Gardner83).
With regard to total fat intake, PB diets (19–38 %E) provided a lower or similar amount of fat compared to omnivorous diets (31–42 %E)(Reference Davey, Spencer and Appleby42–Reference Clarys, Deliens and Huybrechts47,Reference Newby, Tucker and Wolk49,Reference Najjar, Moore and Montgomery52,Reference Mishra, Xu and Agarwal60,Reference Rogerson, Macas and Milner69,Reference Kristensen, Madsen and Hansen79) with studies generally showing that intake of total fat from both the PB and omnivorous diets was in line with widely accepted population recommendations for fat intake(Reference Sobiecki, Appleby and Bradbury45,Reference Kristensen, Madsen and Hansen79) . Given that some of the key sources of saturated fat (i.e. animal products) are restricted or eliminated from PB diets, it is not unexpected that intake of saturated fat from PB diets (4–14 %E) was lower or similar compared to omnivorous diets (10–16 %)(Reference Davey, Spencer and Appleby42–Reference Clarys, Deliens and Huybrechts47,Reference Newby, Tucker and Wolk49,Reference Najjar, Moore and Montgomery52,Reference Mishra, Xu and Agarwal60,Reference Rogerson, Macas and Milner69,Reference Kristensen, Madsen and Hansen79) . A study by Sobiecki et al.(Reference Sobiecki, Appleby and Bradbury45) investigating compliance to nutrient recommendations found that mean saturated fat intake from PB diets (7–9⋅5 %E) met dietary recommendations from the UK Department of Health (DOH) of <10 %E, while intake from omnivorous diets exceeded recommendations, however only by 0⋅4 %E. However, it is important to acknowledge that intakes of saturated fat in most Western populations is considerably >10 %E and so the wider applications of this study should be interpreted with caution(Reference Rippin, Hutchinson and Jewell84).
Studies in this review found that intake of MUFA was lower or similar from PB diets (7–15 %E) compared to omnivorous diets (11–14 %E)(Reference Bowman43,Reference Allès, Baudry and Mejean44,Reference Bradbury, Crowe and Appleby46,Reference Clarys, Deliens and Huybrechts47,Reference Newby, Tucker and Wolk49,Reference Najjar, Moore and Montgomery52,Reference Rogerson, Macas and Milner69,Reference Kristensen, Madsen and Hansen79) . Regarding PUFA intakes, most studies reported a similar or higher intake of PUFA from PB diets (5–11 %E) compared to omnivorous diets (5–9 %E)(Reference Davey, Spencer and Appleby42–Reference Clarys, Deliens and Huybrechts47,Reference Rogerson, Macas and Milner69,Reference Kristensen, Madsen and Hansen79) , however two studies reported a lower intake of PUFA from PB diets (4–5 %E) compared to omnivorous diets (4–8 %E)(Reference Newby, Tucker and Wolk49,Reference Najjar, Moore and Montgomery52) . Regardless, studies investigating compliance to nutrient recommendations found that PUFA intake from both PB and omnivorous diets met recommendations from the UK DOH and the Nordic Nutrition Recommendations (NNR)(Reference Sobiecki, Appleby and Bradbury45,Reference Kristensen, Madsen and Hansen79) . Intake of n-3 and n-6 was similar or higher from the PB diets (n-3: 1–2 g, n-6: 7–15 g), compared to omnivorous diets (n-3: <1–2 g, n-6: 7–9 g)(Reference Allès, Baudry and Mejean44,Reference Rogerson, Macas and Milner69) with the exception of Najjar et al.(Reference Najjar, Moore and Montgomery52) who reported a lower intake of n-6 from a raw vegan diet (6 g), compared to an omnivorous diet (19 g) which may be explained by the exclusion of oils and PB fats from this diet. It should be noted that while the body can convert α-linolenic acid from PB foods, such as nuts and seeds to n-3, research suggests that the process is inefficient and that n-3 from animal sources (i.e. oily fish) is more bioavailable(Reference Swanson, Block and Mousa12,Reference Lane, Derbyshire and Li85,Reference Saunders, Davis and Garg86) .
This review found that carbohydrate intake was similar or higher from PB diets (40–73 %E) compared to omnivorous diets (39–51 %E)(Reference Davey, Spencer and Appleby42–Reference Clarys, Deliens and Huybrechts47,Reference Newby, Tucker and Wolk49,Reference Najjar, Moore and Montgomery52,Reference Mishra, Xu and Agarwal60,Reference Rogerson, Macas and Milner69,Reference Kristensen, Madsen and Hansen79) with studies showing that carbohydrate intake from PB diets met recommendations while carbohydrate intake in omnivorous diets was below recommendations from the UK Scientific Advisory Committee on Nutrition and the NNR(Reference Sobiecki, Appleby and Bradbury45,Reference Kristensen, Madsen and Hansen79) . Furthermore, at an individual level, a study by Allès et al.(Reference Allès, Baudry and Mejean44) showed that 36 % of meat-eaters had intakes below the acceptable distribution range for carbohydrates, compared to 16 % of vegans and 23 % of vegetarians. With regard to dietary sugars, those consuming PB diets had a similar or higher intake (22–27 %E) of total sugar compared to omnivorous diets (20–23 %E)(Reference Allès, Baudry and Mejean44,Reference Sobiecki, Appleby and Bradbury45,Reference Clarys, Deliens and Huybrechts47) . No study reported free sugar intake and only Kristensen et al.(Reference Kristensen, Madsen and Hansen79) provided an estimate of added sugar intake, where intake of added sugars in the PB diet was lower (3–4 %E) than the omnivorous diet (8 %E) (data not shown). However, the omnivorous diet in this study may not be representative of a typical adult diet in the Western world where intakes of added sugars are typically about 10 %E(Reference Walton, Bell and Re87). Intake of dietary fibre was similar or higher from PB diets (20–56 g) compared to omnivorous diets (17–27 g)(Reference Davey, Spencer and Appleby42–Reference Clarys, Deliens and Huybrechts47,Reference Newby, Tucker and Wolk49,Reference Najjar, Moore and Montgomery52,Reference Mishra, Xu and Agarwal60,Reference Rogerson, Macas and Milner69,Reference Kristensen, Madsen and Hansen79) with studies showing that dietary fibre intake from PB diets (NSP 25–29 g, dietary fibre 40–56 g) met recommendations from the UK Scientific Advisory Committee on Nutrition and NNR, while intake from omnivorous diets (NSP 22 g, dietary fibre 20–23 g) did not(Reference Sobiecki, Appleby and Bradbury45,Reference Kristensen, Madsen and Hansen79) . These findings may be expected due to the high dietary fibre content of PB foods such as fruits, vegetables, legumes and wholegrains, compared to animal-derived products.
Micronutrients
Intake of vitamin A from PB diets (692–10 016 μg) was generally higher than omnivorous diets (627–2480 μg), however two studies reported lower intakes from the PB diet (542–1098 μg) compared to the omnivorous diet (929–1394 μg)(Reference Bowman43–Reference Sobiecki, Appleby and Bradbury45,Reference Najjar, Moore and Montgomery52,Reference Rogerson, Macas and Milner69,Reference Kristensen, Madsen and Hansen79) . One study reported that retinol intakes from the PB diet were lower than the omnivorous diet which is not surprising given that animal-derived products are key sources of retinol(Reference Davey, Spencer and Appleby42). Regardless, the prevalence of inadequate intakes of vitamin A is low with just 1–8 % of those consuming PB diets and 1–3 % of omnivores having inadequate intakes of vitamin A(Reference Sobiecki, Appleby and Bradbury45).
Vitamin D intake was lower or similar from PB diets (0–4μg) compared to omnivorous diets (2–5 μg)(Reference Davey, Spencer and Appleby42–Reference Sobiecki, Appleby and Bradbury45,Reference Najjar, Moore and Montgomery52,Reference Rogerson, Macas and Milner69,Reference Kristensen, Madsen and Hansen79) , which is not an unexpected finding, given that, natural dietary sources of vitamin D are limited to animal-derived products (with the exception of mushrooms grown under UV light). Kristensen et al.(Reference Kristensen, Madsen and Hansen79) found that vitamin D intakes from the PB diet and the omnivorous diet were below national recommendations (NNR) which is not surprising given that low intakes of vitamin D are reported in populations globally(Reference Cashman and Kiely88,Reference Kiely and Black89) . Even in non-vegetarians, supplementation is often recommended to ensure adequate intakes particularly in winter months(Reference Amrein, Scherkl and Hoffmann90,Reference Cashman91) . Furthermore, foods fortified with vitamin D, e.g. ready-to-eat breakfast cereals or PB alternative foods, may make a useful contribution to vitamin D(Reference Alcorta, Porta and Tárrega19,Reference Kiely and Black89,Reference Buttriss and Lanham-New92) .
Intake of vitamin E was similar or higher from PB diets (11–20 mg) compared to omnivorous diets (7–12 mg)(Reference Davey, Spencer and Appleby42,Reference Allès, Baudry and Mejean44,Reference Sobiecki, Appleby and Bradbury45,Reference Najjar, Moore and Montgomery52,Reference Rogerson, Macas and Milner69,Reference Kristensen, Madsen and Hansen79) , partially explained by the presence of vitamin E in vegetable oils, nuts and green vegetables. Studies showed that intake of vitamin E from both PB diets and omnivorous diets did not meet recommendations from the US Institute of Medicine and the NNR; however, one study found that vitamin E intake from the PB diet met population recommendations (NNR)(Reference Sobiecki, Appleby and Bradbury45,Reference Kristensen, Madsen and Hansen79) .
Intake of vitamin C was similar or higher from PB diets (109–413 mg) compared to omnivorous diets (88–167 mg)(Reference Davey, Spencer and Appleby42,Reference Allès, Baudry and Mejean44,Reference Sobiecki, Appleby and Bradbury45,Reference Najjar, Moore and Montgomery52,Reference Rogerson, Macas and Milner69,Reference Kristensen, Madsen and Hansen79) , but vitamin C intake from both PB and omnivorous diets met population recommendations nonetheless (UK DOH and NNR)(Reference Sobiecki, Appleby and Bradbury45,Reference Kristensen, Madsen and Hansen79) .
Intakes of thiamin, folate and biotin were similar or higher from PB diets compared to omnivorous diets(Reference Davey, Spencer and Appleby42–Reference Sobiecki, Appleby and Bradbury45,Reference Najjar, Moore and Montgomery52,Reference Rogerson, Macas and Milner69,Reference Kristensen, Madsen and Hansen79) . Studies investigating compliance with recommendations found that intakes of thiamin and folate in both PB and omnivorous diets were in line with recommendations from the UK DOH for general population intake (however, for folate this is dependent on the dietary reference value used as recommendations vary between countries)(Reference Sobiecki, Appleby and Bradbury45,Reference Kristensen, Madsen and Hansen79) . Vitamin B6 intake was lower or similar from PB diets (1⋅4–2⋅3 mg) in most studies, compared to omnivorous diets (1⋅7–2⋅6 mg)(Reference Davey, Spencer and Appleby42–Reference Sobiecki, Appleby and Bradbury45,Reference Rogerson, Macas and Milner69,Reference Kristensen, Madsen and Hansen79) , which is expected as meat and fish are good sources of vitamin B6. However, vitamin B6 intake from the vegan diet according to Allès et al.(Reference Allès, Baudry and Mejean44) and Kristensen et al.(Reference Kristensen, Madsen and Hansen79) was similar or higher (1⋅8–2⋅5 mg) compared to omnivorous diets (1⋅4–1⋅8 mg). Regardless, intake of vitamin B6 from both PB and omnivorous diets met population recommendations from the UK DOH and the NNR(Reference Sobiecki, Appleby and Bradbury45,Reference Kristensen, Madsen and Hansen79) . Intakes of other B vitamins, such as riboflavin, niacin and pantothenate, were lower or similar from PB diets compared to omnivorous diets(Reference Davey, Spencer and Appleby42–Reference Sobiecki, Appleby and Bradbury45,Reference Najjar, Moore and Montgomery52,Reference Rogerson, Macas and Milner69,Reference Kristensen, Madsen and Hansen79) . Studies investigating compliance with recommendations found that intakes of riboflavin and niacin from both PB and omnivorous diets met national population recommendations (UK DOH and NNR)(Reference Sobiecki, Appleby and Bradbury45,Reference Kristensen, Madsen and Hansen79) . Vitamin B12 intake was lower or similar from PB diets (0–6 μg) compared to omnivorous diets (4–8 μg)(Reference Davey, Spencer and Appleby42–Reference Sobiecki, Appleby and Bradbury45,Reference Najjar, Moore and Montgomery52,Reference Rogerson, Macas and Milner69,Reference Kristensen, Madsen and Hansen79) , which is expected as vitamin B12 is naturally found in animal-derived products, including meat, fish eggs and dairy and not usually a constituent of PB foods(93). Vitamin B12 intake from diets which included some animal-derived foods, including lacto- and/or ovo vegetarian and pescatarian diets, as well as omnivorous diets met population recommendations; however, intake of vitamin B12 from the vegan diet did not meet population recommendations (UK DOH and NNR)(Reference Sobiecki, Appleby and Bradbury45,Reference Kristensen, Madsen and Hansen79) . As vegan foods do not naturally contain vitamin B12, the consumption of dietary supplements and/or fortified foods is required to maintain an adequate supply of vitamin B12 in the diet of vegans or those who significantly limit their intake of animal-derived foods(Reference Rizzo, Laganà and Rapisarda94).
Generally, calcium intake from PB diets varied depending on the level of exclusion of dairy products(Reference Davey, Spencer and Appleby42–Reference Sobiecki, Appleby and Bradbury45,Reference Clarys, Deliens and Huybrechts47,Reference Najjar, Moore and Montgomery52,Reference Rogerson, Macas and Milner69,Reference Kristensen, Madsen and Hansen79) . For example, intake of calcium from vegan diets was lower or similar (566–885 mg) compared to omnivorous diets (747–1199 mg)(Reference Davey, Spencer and Appleby42,Reference Allès, Baudry and Mejean44,Reference Sobiecki, Appleby and Bradbury45,Reference Clarys, Deliens and Huybrechts47,Reference Najjar, Moore and Montgomery52,Reference Rogerson, Macas and Milner69,Reference Kristensen, Madsen and Hansen79) , while intake of calcium from other PB diets which include dairy (i.e. vegetarian, pescatarian, semi-vegetarian and Mediterranean) was similar or higher (960–1470 mg) compared to omnivorous diets(Reference Davey, Spencer and Appleby42–Reference Sobiecki, Appleby and Bradbury45,Reference Clarys, Deliens and Huybrechts47,Reference Rogerson, Macas and Milner69) . Kristensen et al.(Reference Kristensen, Madsen and Hansen79) showed that intake of calcium from both the PB and omnivorous diet generally met population recommendations (NNR). However, Sobiecki et al.(Reference Sobiecki, Appleby and Bradbury45) found that the prevalence of inadequate intake of calcium was notable in meat eaters (26–39 %) but higher in vegans (52–64 %).
Intake of iron from PB diets was similar or higher (13–23 mg) compared to omnivorous diets (9–17 mg)(Reference Davey, Spencer and Appleby42–Reference Sobiecki, Appleby and Bradbury45,Reference Clarys, Deliens and Huybrechts47,Reference Najjar, Moore and Montgomery52,Reference Rogerson, Macas and Milner69,Reference Kristensen, Madsen and Hansen79) , which may be unexpected; however, a recent review has suggested that higher iron intakes from PB diets may be due to consumption of green leafy vegetables, beans, nuts and seeds which are highly consumed in PB diets(Reference Bakaloudi, Halloran and Rippin25). While studies considered in this review found that iron intake from both PB and omnivorous diets met recommendations from the UK DOH and the NNR, it is important to acknowledge the bioavailability of haem iron compared to non-haem iron. The US Institute of Medicine recommends an iron intake 1⋅8 times higher for vegetarians than that of omnivores due to the lower bioavailability of non-haem iron arising from PB foods compared with the haem iron from animal-derived sources(Reference Sobiecki, Appleby and Bradbury45,Reference Trumbo, Yates and Schlicker95) . While lower ferritin levels have been reported in those consuming PB diets compared to omnivorous diets, a recent systematic review found that there is no difference in the prevalence of iron deficiency between those following a PB diet and those following an omnivorous diet(Reference Bakaloudi, Halloran and Rippin25).
Zinc intake was lower or similar from PB diets (7–11 mg) compared to omnivorous diets (9–13 mg)(Reference Davey, Spencer and Appleby42–Reference Sobiecki, Appleby and Bradbury45,Reference Najjar, Moore and Montgomery52,Reference Rogerson, Macas and Milner69,Reference Kristensen, Madsen and Hansen79) . Kristensen et al.(Reference Kristensen, Madsen and Hansen79) showed that both PB and omnivorous diets met population recommendations (NNR). While Sobiecki et al.(Reference Sobiecki, Appleby and Bradbury45) found that 4–27 % of vegetarians and vegans had inadequate intake of zinc compared to 2–8 % of meat-eaters; however when adjusted for bioavailability, 30–55 % of vegetarians and 56–74 % vegans had inadequate intake of zinc. Those consuming PB diets may have up to 50 % higher requirements of zinc due to lower bioavailability of zinc-rich plant foods, which contain phytate, a zinc inhibitor, compared to animal-derived sources(Reference Allen, Carriquiry and Murphy11,Reference Bakaloudi, Halloran and Rippin25,Reference Trumbo, Yates and Schlicker95) .
Intake of iodine was generally lower or similar from PB diets (19–197 μg) compared to omnivorous diets (145–213 μg), which is as expected given key sources of iodine include fish, seafood, eggs and milk(Reference Sobiecki, Appleby and Bradbury45,Reference Rogerson, Macas and Milner69,Reference Kristensen, Madsen and Hansen79) . One exception was a study by Allès et al.(Reference Allès, Baudry and Mejean44), who reported higher iodine intakes in PB diets. A high consumption of PB drinks was observed among vegans (419 g/d), which (if fortified with iodine) may explain this higher iodine intake(Reference Allès, Baudry and Mejean44). However, studies comparing intakes to recommendations from the US Institute of Medicine and NNR found that intakes from omnivorous diets generally met recommendations for iodine while intakes from PB diets did not, with approximately 30 % of vegetarians and 93–94 % of vegans estimated to have inadequate intakes of iodine(Reference Sobiecki, Appleby and Bradbury45,Reference Kristensen, Madsen and Hansen79) .
Intake of selenium was lower or similar from PB diets (25–66 μg) compared to omnivorous diets (39–71 μg)(Reference Allès, Baudry and Mejean44,Reference Sobiecki, Appleby and Bradbury45,Reference Rogerson, Macas and Milner69,Reference Kristensen, Madsen and Hansen79) . While animal-derived foods are a good source of selenium, the content of selenium in plant foods depends on the content of the soil in which it is grown and therefore varies significantly but is generally lower(Reference Trumbo, Yates and Schlicker95). However, studies which compared intakes to recommendations from the US Institute of Medicine and NNR found that intakes from both the PB and omnivorous diets did meet recommendations(Reference Sobiecki, Appleby and Bradbury45,Reference Kristensen, Madsen and Hansen79) .
Those consuming PB diets had similar or higher intakes of magnesium compared to omnivorous diets and studies showed that magnesium intake from both diets met population recommendations (UK DOH and NNR)(Reference Davey, Spencer and Appleby42–Reference Sobiecki, Appleby and Bradbury45,Reference Najjar, Moore and Montgomery52,Reference Rogerson, Macas and Milner69,Reference Kristensen, Madsen and Hansen79) . Few studies provided data on intakes of copper, manganese and phosphorous; however, where available, intakes of copper and manganese were similar or higher and intake of phosphorous was lower or similar from PB diets compared to omnivorous diets(Reference Bowman43–Reference Sobiecki, Appleby and Bradbury45,Reference Najjar, Moore and Montgomery52,Reference Rogerson, Macas and Milner69,Reference Kristensen, Madsen and Hansen79) .
Potassium intake was generally similar or higher from PB diets (3063–5078 mg) compared to omnivorous diets (2668–3965 mg)(Reference Davey, Spencer and Appleby42–Reference Sobiecki, Appleby and Bradbury45,Reference Najjar, Moore and Montgomery52,Reference Rogerson, Macas and Milner69,Reference Kristensen, Madsen and Hansen79) , with the exception of two studies which showed a lower intake of potassium in vegetarian diets compared to omnivorous diets(Reference Bowman43,Reference Sobiecki, Appleby and Bradbury45) . Nonetheless, studies found that potassium intake in both the PB and omnivorous diets met population recommendations (World Health Organisation and NNR)(Reference Sobiecki, Appleby and Bradbury45,Reference Kristensen, Madsen and Hansen79) .
Sodium intake was lower or similar from PB diets (839–2701 mg) compared to omnivorous diets (1569–4226 mg); however, intake of sodium in both the PB and omnivorous diets exceeded population recommendations (UK DOH and NNR)(Reference Sobiecki, Appleby and Bradbury45,Reference Kristensen, Madsen and Hansen79) . This is not unexpected as it is widely reported that sodium intake worldwide is well in excess of recommendations(Reference Brown, Tzoulaki and Candeias96).
Conclusions
A global shift towards a more PB diet has been recommended for both health and environmental sustainability. This review aimed to summarise the definitions of PB diets globally and to investigate the nutritional role of PB diets in adults. This review found that there is a wide range of PB definitions in the literature including the traditional vegetarian diets, which exclude animal-derived foods in different amounts including lacto-vegetarian, ovo-vegetarian, lacto-ovo vegetarian, pescatarian and vegan. Furthermore, definitions have expanded to include semi-vegetarian/flexitarian diets which allow some animal-derived food consumption. Other diets (e.g. portfolio, Mediterranean-style, DASH, healthy US-style, planetary health and Nordic-style diets) are generally high in fruit, vegetables, legumes, wholegrains, nuts and seeds, and place further emphasis on certain PB components, such as olive oil, olives, nuts and moderate red wine intake or specific proportions of PB components and encourage moderate (or no) intake of animal-derived foods. PDI which positively weight PB foods and negatively weight animal foods have also been developed to measure adherence to a PB dietary pattern in an omnivorous diet, however, what is defined within each PDI varies.
Notwithstanding the variations in PB diet definitions, data from observational and intervention studies have shown that those consuming a PB diet have lower or similar intakes of energy, protein, total fat, saturated fat, MUFA and added sugar and higher or similar intakes of carbohydrate, PUFA (including n-3 and n-6), total sugars and dietary fibre than those consuming an omnivorous diet.
Those consuming a PB diet had lower or similar intakes of vitamin D, riboflavin, niacin, pantothenate, vitamin B6, vitamin B12, zinc, iodine, selenium, phosphorous and sodium and higher or similar intakes of vitamins A, E, C, thiamin, folate, biotin, iron, magnesium, copper, manganese and potassium than those consuming an omnivorous diet. Findings for calcium varied depending on the level of exclusion of dairy products with intakes from vegan diets being lower than other PB and omnivorous diets.
Overall, this review has highlighted that those consuming a PB diet are more likely to meet recommended intakes for carbohydrate, dietary fibre and vitamin E and are less likely to meet recommendations for protein, vitamin B12 and iodine compared to omnivores. Regardless of consumer type, both PB consumers and omnivores were noted to have low intakes of vitamin D and calcium and high intakes of sodium compared to recommendations.
While intakes of protein, n-3, iron and zinc were generally sufficient from the PB diet, it is important to acknowledge the lower bioavailability of these nutrients from PB foods compared to animal-derived products. As dietary patterns shift towards a more PB diet there is a need for further studies to investigate the role of PB diets for nutritional adequacy and status in populations currently accustomed to consuming a primarily omnivorous diet.
Acknowledgements
The authors would like to thank the Irish section of the Nutrition Society for inviting the present review paper as part of the postgraduate review competition.
Financial Support
This work was supported by funding from the Irish Department of Agriculture Food and the Marine.
Conflict of Interest
None.
Authorship
G. K., L. K. and J. W. contributed to the scope of this review. G. K. contributed to the data extraction and wrote the first draft. All authors contributed to the writing of the final manuscript. All authors critically reviewed the manuscript and approved the final version submitted for publication.








