Introduction
Guillain–Barré syndrome (GBS) is the most common cause of acute flaccid paralysis worldwide. GBS is essentially a clinical diagnosis; however, cerebrospinal fluid (CSF) analysis is recommended to exclude other diseases and lessen the possibility of misdiagnosis.Reference Sejvar, Kohl and Gidudu1 A common CSF finding in GBS is albuminocytological dissociation, defined as elevated protein levels (>45 mg/dl) and ≤5 cells.Reference Sejvar, Kohl and Gidudu1,Reference Fokke, van den Berg, Drenthen, Walgaard, van Doorn and Jacobs2 Half of GBS present elevated CSF protein levels within 1 week since symptom onset and 80% within 2 weeks.Reference Brettschneider, Petzold, Süssmuth and Tumani3
GBS is exclusively a disease of the peripheral nervous system.4 Pathophysiologically, there is inflammation of the nerve root, causing venular congestion of small root veins and plasma proteins leak from the nerve root blood vessels into the subarachnoid space.Reference Brettschneider, Petzold, Süssmuth and Tumani3 When nerve roots are compromised, proximal limb weakness and radicular pain occur.Reference Brettschneider, Petzold, Süssmuth and Tumani3,Reference Berciano, Sedano and Pelayo-Negro5
Rapid progression of symptoms in GBS causes patients to seek early medical attention (≤7 days). This subset of patients has increased risk of invasive mechanical ventilation (IMV), which is also a risk factor for prolonged hospital stay, and consequently poor outcomes at 3 and 6 months.Reference Walgaard, Lingsma and Ruts6
Early Guillain–Barré syndrome is considered in patients in which weakness occurs within 7 days of symptom onset.Reference Berciano, Orizaola and Gallardo7 In this subset of patients, neurophysiological findings have been described in previous articles. Various CSF biomarkers have been proposed to assess prognosis and response to therapy, including tau, antigangliosides, hypocretin-1, neurofilament, eleveated protein levels and basic myelin protein.Reference Illes and Blaabjerg8 However, the clinical and prognostic implication of albuminocytological dissociation has not been described.
Materials and Methods
An ambispective cohort study of patients with GBS defined by Asbury criteria during the period from January 1, 2017, to June 30, 2021, in a single reference neurological center in Mexico was conducted. Patient’s data from the years 2017 and 2018 was collected retrospectively and data from the years 2019–2021 prospectively. Selection included patients >18 years, arrived at our institution within 7 days since symptom onset, had CSF analysis within 7 days since symptom onset and had nerve conduction studies performed. Albuminocytological dissociation was considered in patients having elevated protein levels (>45 mg/dl) and ≤5 cells in CSF analysis.Reference Doets, Verboon and van den Berg9 We excluded patients with fever, immunosuppression, seizures, or clinical suspicion of a central nervous system infection, as it may alter CSF analysis.
Baseline characteristics were obtained to describe our population. We considered: age, gender, history of infection, assessment of muscle strength through the Medical Research Council (MRC) scale and the GBS disability scale at the time of diagnosis,Reference Hughes, Newsom-Davis, Perkin and Pierce10 cranial nerve involvement, IMV, and length of hospital stay. We also considered cardiovascular autonomic dysfunction as changes in heart rate (bradycardia and/or tachycardia) or blood pressure (hypotension and/or hypertension) not explained by any other medical condition (example: infection or secondary to medications) on admission or during hospitalization at the discretion of the treating physician.
We obtained the following information from nerve conduction studies: compound muscle action potential (CMAP) (mV), distal latency (ms) and nerve conduction velocities (m/s) of the median, ulnar, fibular, and tibial motor nerves, and sensitive action potential (SNAP) (µV) of the median and sural sensory nerves. Uncini’s criteria were applied to classify patients into electrophysiological variants; we also considered any variant with conduction block: the presence of pCMAP/dCMAP amplitude ratio <0.7 (excluding tibial nerve) in at least one of the motor nerves explored in any electrophysiological variant (acute inflammatory demyelinating polyneuropathy (AIDP) or axonal).Reference Uncini and Kuwabara11
Good outcome was considered if the patient was able to walk unaided (Guillain-Barré disability score [GDS] ≤2 points) at 3-month follow-up.
Statistical Analysis
Quantitative variables were described as mean (SD) or median (IQR) depending on their distribution. Categorical variables are described in frequencies and percentages. To search for differences between groups, we used x 2 and Fisher’s exact test for categorical variables. Student’s t-test was considered to compare means, and Mann-Whitney U test to compare medians. A p value <0.05 was considered statistically significant. Through multivariable binary logistic regression, risk factors associated with early cerebrospinal fluid albuminocytological dissociation were sought. The results were expressed as odds ratio (OR) with 95% confidence intervals. To validate the multivariable model, the Hosmer-Lemshow goodness-of-fit test was used and the performance of the model was evaluated through area under the curve.
Results
We included 240 patients who fulfilled Asbury criteria for GBS. One hundred fourteen patients had a lumbar puncture performed within 7 days within symptom onset. Twenty patients were further excluded because they were diagnosed with a central nervous system infection, leaving 94 patients for analysis.
Baseline Characteristics of Our Population
Mean age was 45.94 ± 17.1 years and 67% were male. Thirty-eight percent had a history of diarrhea and median time from symptom onset to admission was 5 days (IQR 3–6). Mean GDS at admission was 4 (IQR 3–4), MRC score at admission 32.4 ± 15.7 points, and 34% required IMV. Fifty-four percent had cranial nerve involvement, and 28.7% had autonomic dysfunction. The median time from symptom onset to CSF analysis was 5 days (IQR 3–6), and median protein levels were 36 mg/dl (27–54). Figure 1 depicts the presence or absence of albuminocytological dissociation grouped by days since symptom onset. The most frequent electrophysiological variant was acute motor axonal neuropathy (AMAN) (39.4%) followed by AIDP (38.3%). The rest of the baseline characteristics are summarized in Table 1.
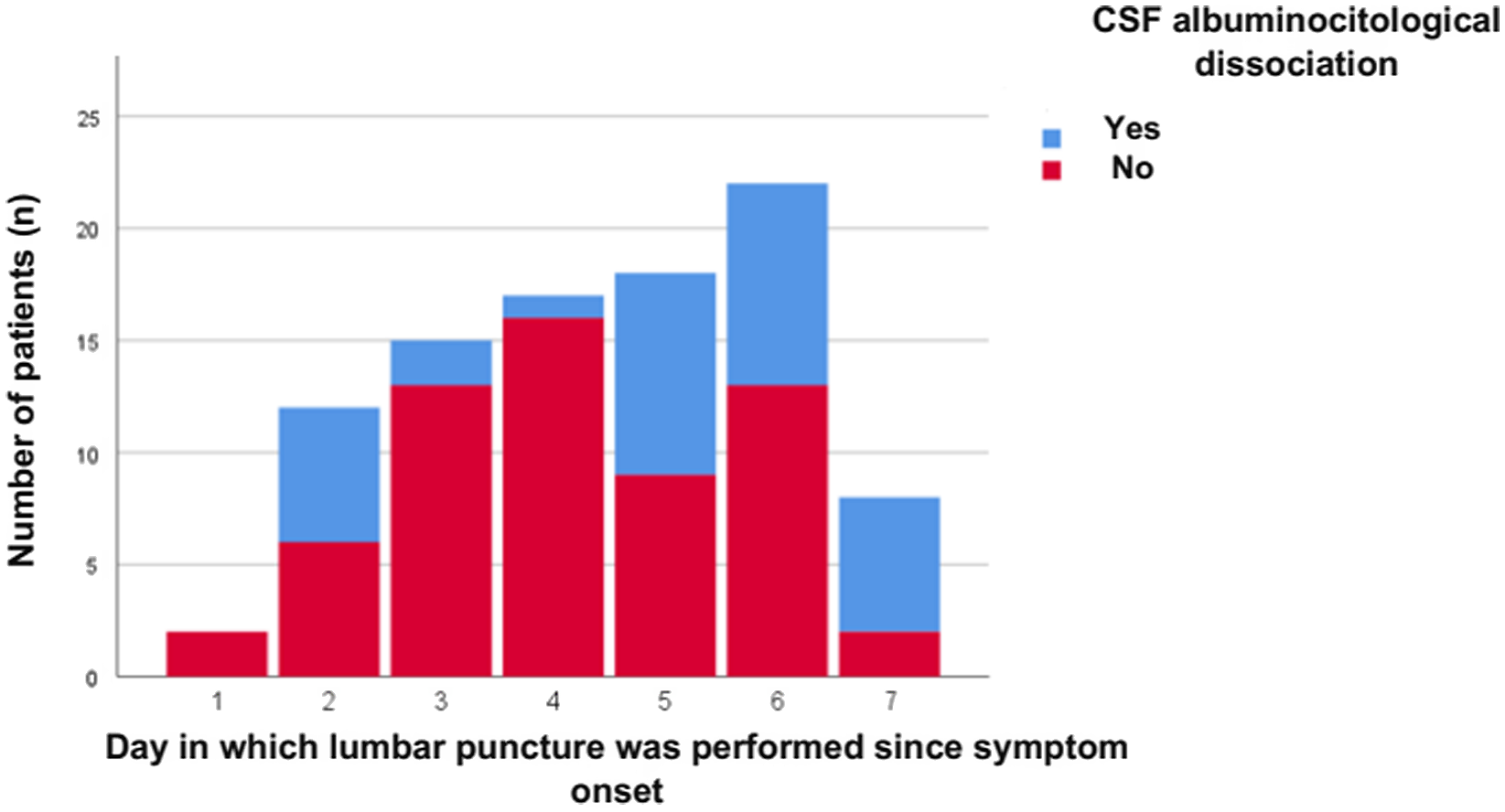
Figure 1: Number of patients with vs without albuminocytological dissociation according to the day cerebrospinal fluid was obtained.
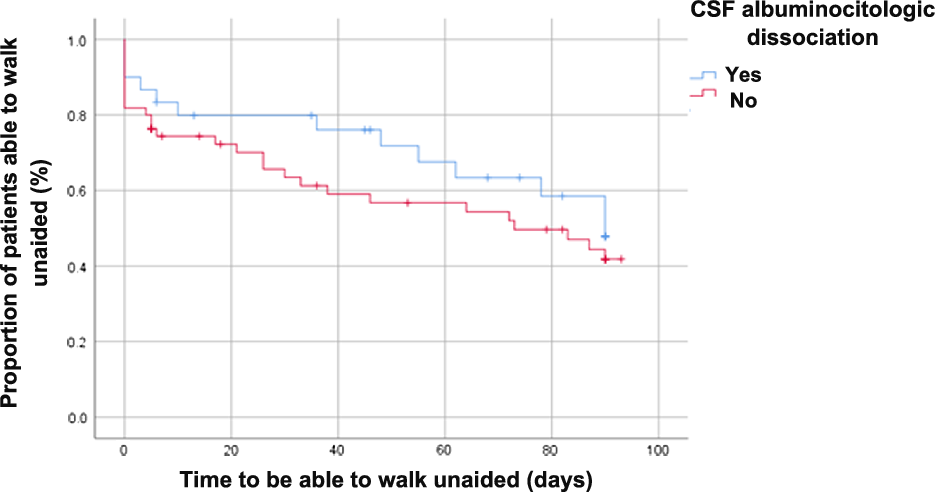
Figure 2: Proportion of patients able to walk unaided with vs without early albuminocytological dissociation.
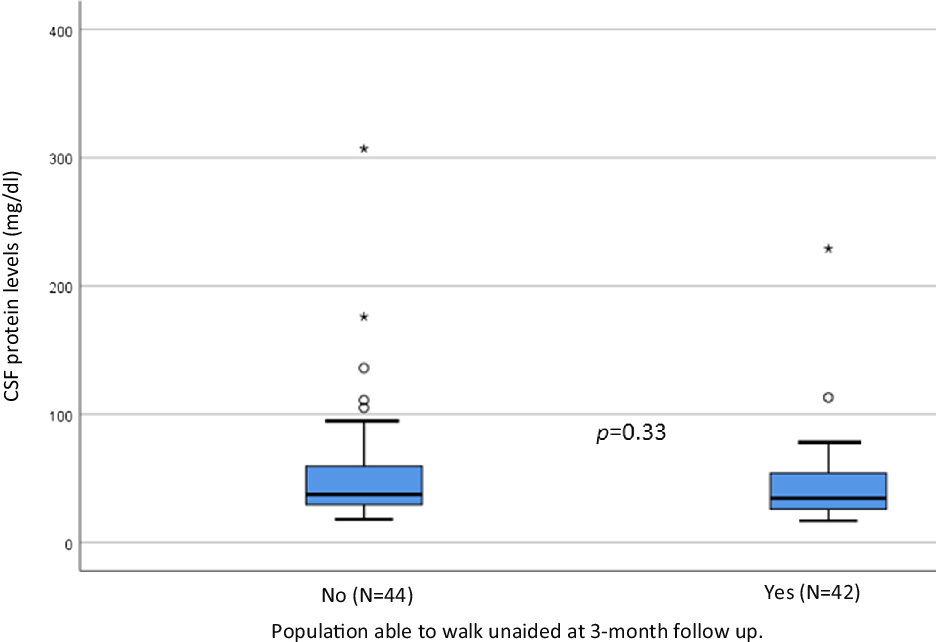
Figure 3: CSF protein levels at admission in patients with and without recovery of independent walking at three months of follow-up.
Table 1: Baseline characteristics of our patients
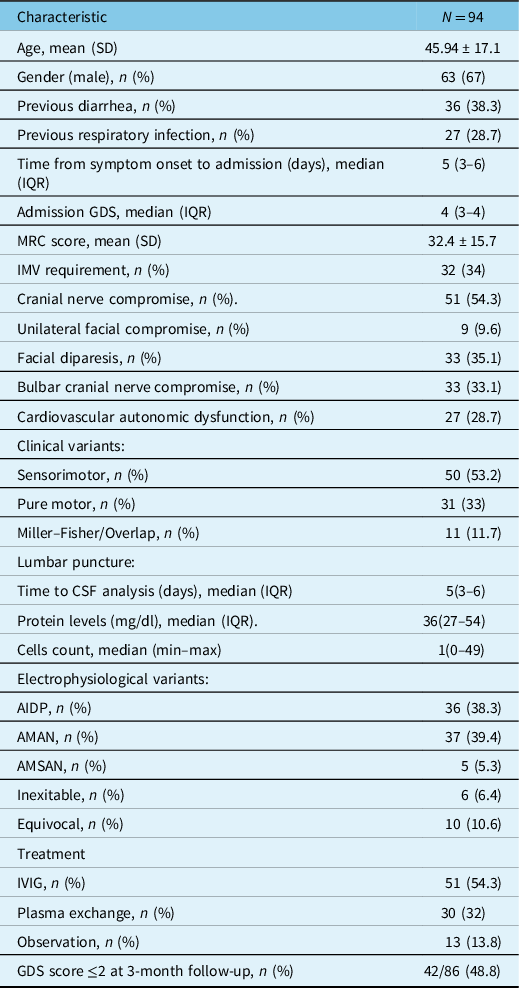
SD: standard deviation, IQR: interquartile range, MRC: medical research council, IMV: invasive mechanical ventilation, GDS: Guillain-Barré disability score, AIDP: acute inflammatory demyelinating polyneuropathy, AMAN: acute motor axonal neuropathy, AMSAN: acute motor sensory axonal neuropathy.
Comparative Analysis
When comparing the group with versus without albuminocytological dissociation only the time since symptom onset to diagnosis was significant: (median) 5 (3.5–6) days vs 4 (3–5.5) days, p = 0.02 (Table 2). Regarding electrophysiological characteristics, we found significant differences in the frequency of the different electrophysiological variants: AIDP [60.6% vs 26.2%, p = 0.002], AMAN [21.2% vs 49.1%, p = 0.009] and acute motor sensory axonal neuropathy (AMSAN) [12.1% vs 1.6%, p = 0.05]. In addition, we observed significant differences in the frequency of any variant (AIDP or axonal) with the presence of at least one complete nerve conduction block (54.5% vs 31.1%, p = 0.043). The SNAPs of the median and sural nerves were lower in the group with early dissociation. In addition, 18 (19.7%) of our population presented absence of sural nerve recording: 11 patients with AIDP, 5 with AMSAN and 2 patients with the Inexcitable variant. This finding was observed more frequently in the early albuminocytological dissociation group (36.3% vs 9.8%, p = 0.005). There were no significant differences in the distal CMAPs of the motor nerves (median, ulnar, fibular and tibial) between groups.
Analyzing clinical and paraclinical factors related to presenting early albuminocytological dissociation, interestingly we observed that the presence of conduction block in any variant (OR 3.21, CI95% 1.12–9.16, p = 0.002), the absence of sural nerve registry (OR 5.69, CI95% 1.48–21.83, p = 0.011) and the greater number of days from the onset of symptoms upon admission (OR 1.57, CI95% 1.12–2.21, p = 0.009) are independent risk factors. Model performance through ROC curves is 0.81, CI 95% (0.72–0.90), p = < 0.001 (Table 3).
Table 2: Comparison between patients with versus without albuminocytological dissociation

SD: standard deviation, IQR: interquartile range, MRC: medical research council, IMV: invasive mechanical ventilation, GDS: Guillain-Barré disability score, AIDP: acute inflammatory demyelinating polyneuropathy, AMAN: acute motor axonal neuropathy, AMSAN: acute motor sensory axonal neuropathy, CMAPs: compound muscle action potential, SNAPs: sensory nerve action potential.
Follow-up and Prognosis
We did not observe significant differences in recovery of independent walking in short term between both groups (with vs without albuminocytological dissociation) in the comparative frequencies (Table 2) and Kaplan–Meyer analysis (Figure 2). We did not observe significant differences in protein levels between the population with vs. without recovery of independent walking at three months of follow-up (Figure 3).
Discussion
Albuminocytological dissociation is a common finding in GBS and directly related to nerve root inflammation. It has also been associated with increased deposition of antibodies, complements, and products of active myelin breakdown. It is present in half patients within 1 week of symptom onset and 80% within 2 weeks.Reference Wakerley and Yuki12 It is considered a supportive criterion for Asbury GBS criteria, as well as definer to a greater level of certainty in Brighton group criteria.Reference Fokke, van den Berg, Drenthen, Walgaard, van Doorn and Jacobs2,Reference Asbury and Cornblath13
Albuminocytological dissociation is classically defined in GBS as protein levels >0.45mg/L in CSF with normal cells.Reference Fokke, van den Berg, Drenthen, Walgaard, van Doorn and Jacobs2,Reference Doets, Verboon and van den Berg9 Recently, several authors consider that the upper limit range (URL) of total protein levels in CSF should be adjusted to the patient's age to increase diagnostic sensitivity.Reference Bourque, Brooks, McCudden, Warman-Chardon and Breiner14 A systemic review considers that protein upper limit level in CSF for patients older than 50 years should be >0.60 mg/L.Reference Breiner, Moher, Brooks, Cheng, Hegen, Deisenhammer, McCudden and Bourque15 A limitation of our study is that all patients were classified according to the classic definition of albuminocytological dissociation. A study by Sahin et al concluded that elevated CSF protein levels were associated with poor 6-month outcome.Reference Sahin, Cinar and Karsidag16 Other study concluded that albuminocytological dissociation does not correlate with clinical severity in GBS.Reference Saba, Hossieny and Arnold17 Nonetheless, this last study was underpowered, and elevated CSF protein levels seemed to be potentially associated with the need for mechanical ventilation, but studies with a greater number of patients are required. To date, there is no clear evidence regarding patients with early dissociation (<7 days). We did not find any difference in motor recovery in patients with early dissociation vs. those with normal CSF.
Requirement for IMV occurs in 30% of cases in GBS. Patients who present to medical services early (≤7 days of evolution) tend to be severe, due to a higher frequency of involvement of lower cranial nerves and lower MRC scores, which imply a risk of requiring mechanical ventilation.Reference López-Hernández, Colunga-Lozano, Galnares-Olalde and Vargas-Cañas18 In our population, 34% of patients required mechanical ventilation. Interestingly, we did not observe clinical differences in the frequency of mechanical ventilation, lower cranial nerve involvement, and MRC score, between patients with early albuminocytological dissociation and those without it. In patients in which lumbar puncture is performed within 7 days, there was no clear association between protein elevation and poor outcome.
We found in our patients that elevated protein levels were more common in AIDP than in axonal variants in early CSF analysis. We hypothesize that the demyelinating variant might have also increased production of antibodies, early root inflammation, and early active myelin breakdown.Reference Sahin, Cinar and Karsidag16 In our population with GBS with early admission, the most frequent electrophysiological variant was AMAN 39.4%. Although it is controversial in the literature if albuminocytological dissociation is more frequent in some variant than in others, we clearly observed in this population the most frequent association is AIDP (60.6%).
Only half of patients with proximal weakness or radicular pain, which are related to root compromise had elevated CSF protein levels. This explains that root inflammation is not the only mechanism to produce CSF protein elevation, as mentioned before, or that root inflammation should be severely compromised to increase protein levels.Reference Berciano, Orizaola and Gallardo7
A marker of clinical severity in GBS is the presence of autonomic dysfunction, which occurs in 20–32% of cases, regardless of the electrophysiological variant (axonal or demyelinating).Reference Zaeem, Siddiqi and Zochodne19,Reference Michel-Chávez, Chiquete, Gulías-Herrero, Carrillo-Pérez, Olivas-Martínez, Macías-Gallardo, Aceves-Buendía, Ruiz-Ruiz, Bliskunova, Portillo-Valle, Cobilt-Catana, Ortiz-Quezada, Durán-Coyote, Rodríguez-Perea, Aguilar-Salas, Cantú-Brito, García-Ramos and Estañol20 The main are heart rate or blood pressure variability. Autonomic dysfunction generally occurs in severe clinical forms of GBS (GDS ≥ 3).Reference Zaeem, Siddiqi and Zochodne19 Anatomically the sympathetic autonomic fibers leave the spinal cord towards the paravertebral ganglion chains, close to the nerve roots. However, patients with early albuminocytological dissociation did not present a higher frequency of autonomic dysfunction.
The attack on the peripheral nerve by inflammatory cells or through complement is directed against molecules found at different levels, such as gangliosides at the nodal or paranodal level.Reference Fehmi, Scherer and Willison21 The inflammatory attack can affect some nerves more, translating clinically into some clinical variants (sensory motor, pure motor, pharyngocervicobrachial, paraparetic, etc).4 Likewise, some nerves may be more electrophysiologically affected. In this study, we did not observe differences in axonal damage through the distal CMAPs of both motor nerves in the upper and lower extremities.
As previously mentioned, early dissociation in GBS is not related to any clinical characteristic or unfavorable outcome in our population, as in other studies.Reference Saba, Hossieny and Arnold17 However, little is known about electrophysiological characteristics in early dissociation. When analyzing factors related to early dissociation in our population, we observed that no electrophysiological variant was statistically significant. Interestingly, we observed that the presence of a conduction block in any variant represents an OR 3.2 risk factor. This finding could be explained by the fact that the presence of conduction blocks represents an active inflammation through antibodies directed towards the proximal portions of the nerves.Reference Sahin, Cinar and Karsidag16 We already know that one of the typical electrophysiological characteristics in GBS is the preservation of the sural nerve, however, 11% of patients have an affected sural nerve (absence of electrophysiological recording of the sural nerve).Reference Bromberg and Albers22 In our multivariate model, we observed that sural absence is a risk factor. The involvement of the sural nerve (in this case, absence of electrophysiological recording of the sural nerve) occurs more frequently in the AIDP variant, especially in severe clinical presentation.Reference Bromberg and Albers22 Another point is that sural nerve compromise is more frequent at older ages due to previous damage to the nerve over the years. In our population, the population with early dissociation is slightly older, although this result is not statistically significant.Reference Umapathi, Koh, Cheng, Goh and Lim23
Conclusion
Albuminocytological dissociation is a common finding in patients with GBS, especially in those within 2 weeks of symptom onset. Early dissociation is not associated with any severe clinical characteristic or unfavorable outcome. It is more common to see in AIDP rather than axonal variants.
Table 3: Uni- and multivariate analysis
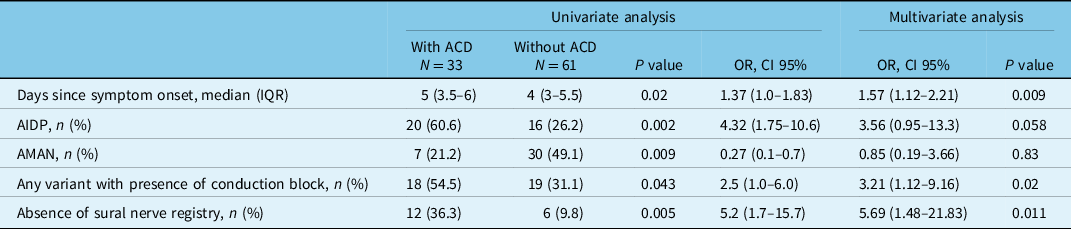
General model: Chi-square 28.94, GL 5, p = 0.001.
Hosmer-Lemshow: Chi-square 4.30, GL 8, p = 0.82.
Model accuracy AUC 0.81 CI 95% (0.72–0.90), p = <0.001.
ACD: albuminocytological dissociation, CI: confidence interval, OR: odds ratio, IQR: interquartile range, AIDP: acute inflammatory demyelinating polyneuropathy, AMAN: acute motor axonal neuropathy. NCS: nerve conduction studies.
Conflicts of Interest
VCES, GOJA, LVF, GGM, GA, and LHJC declare no conflicts of interest.
Author Roles
VCES: writing, reviewing, and editing.
GOJA: writing, reviewing, and editing.
LVF: writing, reviewing, design, and editing.
GGM: writing and reviewing.
LHJC: writing, reviewing, design, and editing.








