Disease-related malnutrition is a common complication in sick and hospitalized patients with deleterious effects on morbidity and mortality(Reference Pirlich, Schutz and Norman1). Malnourished patients have an impaired muscle function, poorer functionality and experience a lower quality of life(Reference Norman, Schutz, Kemps, Lubke, Lochs and Pirlich2, Reference Norman, Kirchner, Lochs and Pirlich3).
Several studies have shown that malnourished patients exhibit lower body cell mass assessed either by bioelectrical impedance analysis (BIA) or more sophisticated methods(Reference Norman, Kirchner, Lochs and Pirlich3, Reference Barbosa-Silva, Barros, Post, Waitzberg and Heymsfield4).
Assessing body composition in hospitalized patients is, however, not always easy, since sophisticated methods are time consuming, expensive and not suitable for routine clinical use. The non-invasive BIA is a simpler method which can easily be employed as a bed-side method in hospital. BIA is considered a reliable tool for body compartment calculation in healthy individuals who have no fluid imbalance, no body shape abnormalities and are within a certain BMI range (16–34 kg/m2). Furthermore, appropriate (i.e. age-, sex- and population-specific) equations for the calculation of body compartments must be applied(Reference Barbosa-Silva and Barros5).
However, these necessary assumptions for the calculation of body compartments using the impedance parameters obtained by BIA (e.g. homogenous composition, fixed cross-sectional area and consistent distribution of current density) are frequently not applicable in sick and hospitalized patients and this is mainly due to disturbed hydration as in nephrotic syndrome, renal failure, cardiac insufficiency, obesity or liver cirrhosis(Reference Kyle, Piccoli and Pichard6–Reference Coppini, Waitzberg and Campos9), or modifications in distribution of extra- and intracellular water.
Bioelectrical impedance vector analysis (BIVA), on the other hand, uses the plot of the impedance parameters resistance (R) and reactance (Xc) normalized per height as a bivariate vector in the R Xc graph(Reference Piccoli, Rossi, Pillon and Bucciante10). BIVA thus provides information about hydration status and body cell mass and integrity since reactance is the capacitative effect produced by the tissue interfaces and cell membranes, whereas bioelectric resistance is the pure opposition of a biological conductor to the flow of an alternating electric current. It has gained attention since it has been shown to be clinically useful in monitoring hydration status in renal patients undergoing chronic haemodialysis(Reference Piccoli7), patients with liver cirrhosis(Reference Guglielmi, Mastronuzzi, Pietrini, Panarese, Panella and Francavilla11), critically ill patients(Reference Piccoli, Pittoni, Facco, Favaro and Pillon12) and obese patients with stable and changing weight(Reference Piccoli, Brunani, Savia, Pillon, Favaro, Berselli and Cavagnini13).
In the present observational study, we wanted to investigate how benign disease-related malnutrition assessed by the Subjective Global Assessment (SGA), as well as nutritional status by BMI, are reflected in the R Xc graph.
Subjects and methods
Patients (n 242) who were hospitalized at our Department of Gastroenterology, Hepatology and Endocrinology were recruited for the study. Patients were considered for inclusion if they were older than 18 years and were suffering from benign gastrointestinal disease.
A priori defined exclusion criteria were malignant disease and life expectancy less than three months. Severe hyperhydration, estimated by clinical assessment and by ultrasound in patients with liver cirrhosis, as well as implanted defibrillators were considered further exclusion criteria for the BIA.
All measurements were made within 48 h of admission to hospital. All patients gave written informed consent and the Ethics Committee of the Charite Universitätsmedizin approved the study.
Subjective Global Assessment
The SGA was carried out using the protocol developed by Detsky et al. (Reference Detsky, McLaughlin, Baker, Johnston, Whittaker, Mendelson and Jeejeebhoy14). It relies on the patient's history regarding weight loss, dietary intake, gastrointestinal symptoms, functional capacity and physical signs of malnutrition (loss of subcutaneous fat or muscle mass, oedema, ascites). Patients were classified as well nourished (A), moderately or suspected of being malnourished (B) or severely malnourished (C).
Whole body impedance measurements
BIA was performed using a Nutriguard M (Data Input GmbH, Darmstadt, Germany) applying alternating electric currents of 800 μA at 50 kHz and R and Xc were measured. The phase angle reflects the contributions between R and Xc (i.e. the arc tangent of the ratio of reactance to resistance transformed to degrees).
Patients were measured in the morning after an overnight fast, in the supine position with arms and legs abducted from the body. Source and sensor electrodes (Ag/AgCl, Bianostic Classic® Electrodes; Data Input) were placed on the dorsum of both hand and foot of the dominant side of the body.
The CV of repeated measurements of R and Xc at 50 kHz was assessed in five patients: CV was < 1·5 % for R and < 2·6 % for Xc.
R Xc graph
BIVA uses the plot of direct measurements of the vector components R and Xc. According to the R Xc graph, R and Xc normalized for body height are plotted as a bivariate random vector (Xc/H v. R/H) on the R–Xc plane. The vector distribution is described by its associated 95 % CI (confidence ellipse in the R–Xc plane). The shortening or lengthening of the vector indicates hydration status in the form of oedema or dehydration, respectively, whereas a migration sideways indicates increase or decrease in dielectric mass (cell membranes and tissue interfaces) of soft tissues(Reference Piccoli, Rossi, Pillon and Bucciante10).
Anthropometric measurements
Weight and height were documented and used to calculate BMI (weight (kg)/height (m)2). Mid upper arm circumference was determined and triceps skinfold was measured with a Holtain caliper (Crymych, UK) on the non-dominant arm. Arm muscle area and arm fat area were calculated applying the formula by Gurney & Jelliffe(Reference Gurney and Jelliffe15).
Muscle function
Handgrip strength was measured in the non-dominant hand with a Jamar® dynamometer. The patients performed the test while sitting comfortably with shoulder adducted and neutrally rotated, the elbow flexed to 90°, forearm and wrist in neutral position. The patients were instructed to perform a maximal isometric contraction. The test was repeated twice within 30 s and the highest value of the three measurements was recorded.
Laboratory parameter
All laboratory parameters (C-reactive protein, albumin) were determined according to standard methods.
Statistics
Statistical analysis was carried out using the software package SPSS© version 13 (SPSS Inc., Chicago, IL, USA).
All data are presented as median and interquartile range. Multiple comparison was performed by Kruskal–Wallis test. Following a significant test result, comparisons between the SGA classes or BMI classes were performed using the Mann–Whitney U test. Spearman's correlation was calculated to assess the relationship between variables.
Statistically significant differences between the mean vectors of the SGA or BMI classes were assessed with the Hotelling's T 2 test for vector analysis. Mahalanobis distance D, a generalized measure of distance between the groups defined by two correlated variables, was also calculated. Vector analysis was performed with BIVA software(Reference Piccoli and Pastori16).
An acceptable level of statistical significance was established a priori at P < 0·05.
Results
In total 242 patients (121 female) were recruited for the study. All patients suffered from benign gastrointestinal disease, mostly liver disease (n 82), chronic inflammatory bowel disease (n 82), biliary (n 16), gastritis/oesophagitis (n 22), pancreatic disease (n 13), diverticulosis/diverticulitis (n 6) and other (n 21). Ethnic origin of all patients was Caucasian.
Disease-related malnutrition
Ninety-eight patients were considered well nourished (40 %), ninety-four were classified moderately malnourished (39 %) and fifty patients were classified severely malnourished (21 %) according to the SGA.
Age was not significantly different between well-nourished, moderately and severely malnourished patients and sex distribution was only different between well-nourished and severely malnourished with a higher percentage of men in the SGA class C as shown in Table 1. BMI, arm muscle area and arm fat area decreased significantly with SGA class and albumin and C-reactive protein was significantly different only between well-nourished and moderately as well as severely malnourished. Hand grip strength was significantly lower in severely malnourished than moderately malnourished or well-nourished patients.
Table 1 Demographic, nutritional and laboratory characteristics of the study population according to the Subjective Global Assessment (SGA)
(Median values and interquartile ranges)

AFA, arm fat area; AMA, arm muscle area; CRP, C-reactive protein; R/H: resistance standardized per height; Xc/H, reactance standardized per height.
Median values were significantly different from those of the SGA B group:
**P < 0·01,
***P < 0·001.
Median values were significantly different from those of the SGA C group:
†† P < 0·01,
††† P < 0·001.
Median values were significantly different from those of the SGA A group:
‡ P < 0·05,
‡‡ P < 0·01,
‡‡‡ P < 0·001.
Impedance parameters
Phase angle decreased significantly with SGA class (see Fig. 1). We also obtained a discreet correlation between percentage weight change in the preceding 6 months and the phase angle (r 0·221, P = 0·001), and with R/H at 50 kHz (r − 0·203, P = 0·002).
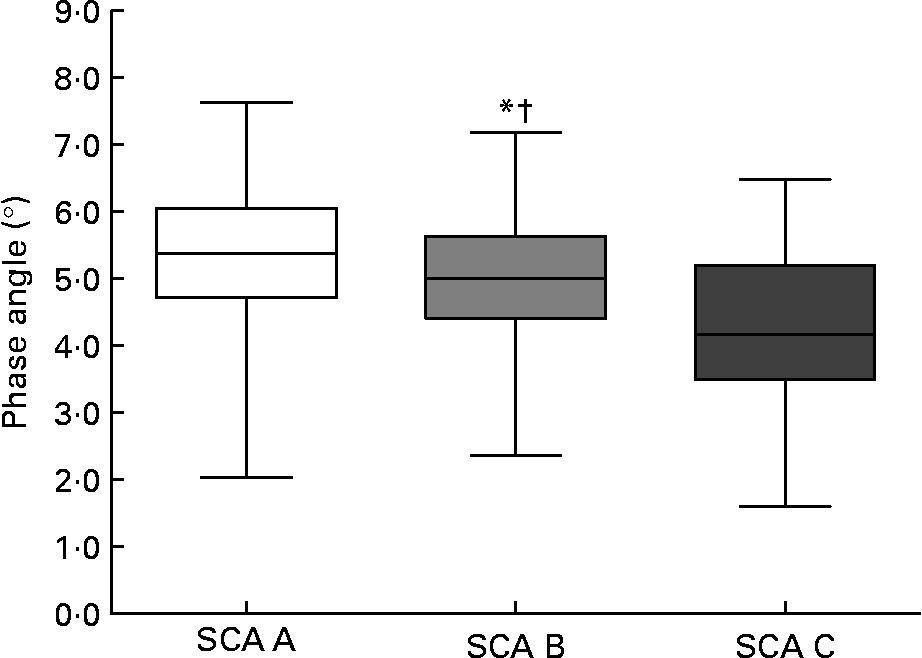
Fig. 1 Phase angle decreases significantly with Subjective Global Assessment (SGA). The box plots display the minimum, the maximum and the 25th, 50th and 75th percentiles. Values were significantly different from those of the SGA A group: *P = 0·033. Values were significantly different from those of the SGA C group: †P < 0·0001.
A significant vector displacement was observed between moderately and severely malnourished patients due to a reduced Xc component with preserved R, whereas there was no significant difference between the mean vector position of well-nourished and moderately malnourished patients (see Fig. 2(A)).

Fig. 2 (A), Significant vector migration (![]() ) between well-nourished (Subjective Global Assessment (SGA) A) v. malnourished patients (SGA B and C) due to decreased reactance standardized per height (Xc/H) component with preserved resistance standardized per height (R/H). A v. B: T 2 4·9, P = 0·09, D 0·32; B v. C: T 2 19·3, P < 0·0001, D 0·77. (B), Vector migration (
) between well-nourished (Subjective Global Assessment (SGA) A) v. malnourished patients (SGA B and C) due to decreased reactance standardized per height (Xc/H) component with preserved resistance standardized per height (R/H). A v. B: T 2 4·9, P = 0·09, D 0·32; B v. C: T 2 19·3, P < 0·0001, D 0·77. (B), Vector migration (![]() ) of BMI classes (I: < 18·5 kg/m2; II: 18·5–24·9 kg/m2; III: 25–29·9 kg/m2; IV: ≥ 30·0 kg/m2) in the opposite direction. I v. II: T 2 33·4, P = 0·0001, D 1·04; II v. III: T 2 5·8, P = 0·0575, D 0·42; III v. IV: T 2 9·4, P = 0·0132, D 0·77. See Subjects and methods section for details and explanation of confidence ellipses and vector migration. D, Mahalanobis distance; T 2, Hotelling's test.
) of BMI classes (I: < 18·5 kg/m2; II: 18·5–24·9 kg/m2; III: 25–29·9 kg/m2; IV: ≥ 30·0 kg/m2) in the opposite direction. I v. II: T 2 33·4, P = 0·0001, D 1·04; II v. III: T 2 5·8, P = 0·0575, D 0·42; III v. IV: T 2 9·4, P = 0·0132, D 0·77. See Subjects and methods section for details and explanation of confidence ellipses and vector migration. D, Mahalanobis distance; T 2, Hotelling's test.
BMI stratification
When stratifying the study population in BMI classes (class I: < 18·5; class II: 18·5–24·9; class III: 25·0–29·9; class IV: ≥ 30·0 kg/m2), we obtained significant vector displacement between class I and II, as well as between class III and class IV, whereas the vector migration between class II and III missed significance (see Fig. 2(B)). Phase angle was only significantly lower in class I than class II as well as III. As anticipated, R/H decreased significantly with increasing BMI, whereas Xc/H was only different between BMI class III and IV (see Table 2).
Table 2 Demographic, nutritional and laboratory characteristics of the study population according to BMI
(Median values and interquartile ranges)

AFA, arm fat area; AMA, arm muscle area; CRP, C-reactive protein; R/H: resistance standardized per height; Xc/H, reactance standardized per height.
Median values were significantly different from those of the BMI class I group:
*P < 0·05,
***P < 0·001.
Median values were significantly different from those of the BMI class II group:
† P < 0·05,
††† P < 0·001.
Median values were significantly different from those of the BMI class III group:
‡ P < 0·05,
‡‡ P < 0·01.
Anthropometric measurements such as arm muscle area and arm fat area decreased with decreasing BMI, age was only different between BMI class II and III/IV and sex distribution remained equal. Hand grip strength was significantly different between BMI class I and classes II–IV.
Subgroup analysis
We also aimed to study the vector migration caused by the SGA within the BMI classes, but only class II (18·5–24·9 kg/m2) yielded sufficiently high patient numbers in the SGA classes (SGA A: n 46; SGA B: n 62; SGA C: n 24) for comparative analysis. Here again, the SGA produced a distinguished vector migration, which was statistically significant between SGA A and B v. C. (see Fig. 3(A)) and revealed drastically altered Xc component with virtually unchanged resistance.

Fig. 3 (A), Significant vector migration between severely malnourished (Subjective Global Assessment (SGA) C) v. well- and moderately malnourished patients (SGA A and B) due to decreased reactance standardized per height (Xc/H) component with preserved resistance standardized per height (R/H) within BMI class II (18·5–24·9 kg/m2). A v. B: T 2 1·2, P = 0·554, D 0·21; B v. C: T 2 8·7, P < 0·017, D 0·71. (B), Significantly displaced confidence ellipses and mean vectors of malnutrition (SGA C) v. underweight (BMI class I: < 18·5 kg/m2). BMI I v. SGA C: T 2 7·8, P = 0·025, D 0·59. See Subjects and methods section for details and explanation of confidence ellipses and vector migration. D, Mahalanobis distance; T 2, Hotelling's test.
We also compared underweight by BMI (BMI class I < 18·5 kg/m2) with malnutrition as assessed by the SGA (SGA C) and observed significant different vector and confidence ellipse positions (see Fig. 3(B)).
Discussion
In the present study we investigated how benign disease-related malnutrition determined by the SGA is reflected in the R Xc graph. We observed a significant vector migration dictated by the SGA, with a decreased Xc component implying abnormal tissue structure and integrity in malnutrition. These altered electric tissue properties were, however, not seen in patients with underweight according to BMI.
Sophisticated body composition in the routine clinical setting is difficult. BIA which is considered an easy non-invasive and inexpensive bed-side method is a reliable tool for body compartment calculation in healthy individuals, but equations for the calculation of body compartments must be validated for various disease states(Reference Kyle, Piccoli and Pichard6). The BIVA providing information on cell mass and integrity as well as hydration status has gained attention over recent years as it is independent from both body weight measurement or equation inherent errors(Reference Piccoli, Rossi, Pillon and Bucciante10).
In the present study population, the mean vector of the severely malnourished patients was significantly displaced when compared to both well-nourished and moderately malnourished patients. This vector migration was due to a significantly reduced Xc component with a preserved R component indicating impaired tissue structure in severe malnutrition. The Xc/H and R/H components of well-nourished and moderately malnourished patients were comparable.
There are several known factors influencing vector position and migration such as BMI, age, ethnic origin and sex. Increasing BMI is associated with both decreasing R and Xc components producing very typical vector patterns(Reference Bosy-Westphal, Danielzik, Dorhofer, Later, Wiese and Muller17).
Age and sex distribution was comparable between the SGA groups, but BMI decreased significantly with SGA class. It could therefore be expected that the vector migration would be entirely dictated/affected by the BMI thus indicating that the decreased Xc component in malnutrition is simply due to the lower body mass. When stratifying the study population according to BMI (class I: < 18·5; class II: 18·5–24·9; class III: 25·0–29·9; class IV: ≥ 30·0 kg/m2), however, and studying the different vector patterns of the SGA classes and the BMI classes, it is apparent that vector displacement in severe malnutrition is not induced by the lower BMI. The mean vector of the BMI migrated in the opposite direction to the mean vector of the SGA when going from high BMI ( ≥ 30 kg/m2) to low BMI ( < 18·5 kg/m2).
The vector migration of the BMI groups is consistent with the vector migration shown in the retrospective analysis of the large NHANES databank by Piccoli et al. (Reference Piccoli, Pillon and Dumler18) as well as the data by Bosy-Westphal et al. of 214 294 healthy adults(Reference Bosy-Westphal, Danielzik, Dorhofer, Later, Wiese and Muller17) with both R/H and Xc/H components actually decreasing with increasing BMI.
The pattern of the impedance vector distribution according to SGA classes, on the other hand, revealed virtually unaltered R/H with significantly decreasing Xc/H in the severely malnourished (SGA C), despite simultaneously decreased BMI.
Moreover, we obtained a similar vector migration dictated by the SGA in the subgroup analysis of patients with similar BMI (18·5–24·9 kg/m2) which clearly indicates that the vector migration of the SGA is independent from BMI.
Also, comparing underweight patients with BMI < 18·5 kg/m2 and patients who were classified severely malnourished according to the SGA (median BMI 19·4 kg/m2), it is evident that these groups exhibit statistically different R/H and Xc/H values despite comparable BMI and phase angle.
The present results imply that malnutrition defined by the SGA is associated with abnormal tissue structure as well as loss of body mass and that these altered electric tissue properties are not seen in underweight according to BMI.
Disease-related malnutrition assessed by the SGA is mainly characterized by ongoing weight loss and is thus a marker of catabolism. It has repeatedly been shown that more than 10 % weight loss in the preceding 6 months is invariably associated with clinically obvious impairment of organ function, and is predictive of postoperative complications(Reference Hill19, Reference Windsor and Hill20). The SGA does not consider current weight or BMI, which allows identification of malnourished patients despite a high or inconspicuous BMI.
Taken together, the present study, which is the first investigation of benign disease-related malnutrition in the BIVA, demonstrated significant disturbances in electric tissue properties in malnutrition as assessed by the SGA which are not seen in underweight according to BMI. The present results, moreover, add to the body of evidence that the BMI alone is not a suitable tool to identify disease-related malnutrition.
Conclusion
Clinical malnutrition as assessed by the SGA is associated with a distinctive bioelectrical vector migration which is strikingly different from the migration caused by the BMI. SGA vector migration is distinguishably marked by decreased Xc implying that abnormal tissue structure, and not reduced body mass only, occurs in disease-related malnutrition. These alterations are not seen in underweight according to BMI which is reflected by an increased R. BIVA appears to be an attractive tool to identify disease-related malnutrition and to monitor nutritional intervention.
Acknowledgements
This project was supported by a grant from the Charite Universitätsmedizin Berlin, research project no. 05/25 369. K. N. and M. P. designed the study; C. S. and A. K. collected the data; K. N. analysed the data; L. V and H. L. provided significant advice; K. N., C. S., L. V. and M. P. wrote the manuscript. The authors have no conflicts of interest.







