INTRODUCTION
Mumps is usually labelled a mild disease and although it was previously a common childhood disease its true significance has probably been underestimated. The most characteristic feature of mumps is parotitis, a swelling of salivary glands, but other more severe and unusual symptoms can occur in mumps infection, including meningitis, encephalitis, orchitis in post-pubertal men and pancreatitis [1]. Mumps was the leading cause of sensorineural deafness in the pre-vaccination era [Reference Peltola2]. Several complications are known to occur at higher rates in adults than in children with mumps disease, including pancreatitis and orchitis [1].
The development of a mumps vaccine originates from research conducted by Johnson & Goodpasture in the 1930s [Reference Johnson and Goodpasture3] with the first inactivated vaccine used in the USA from 1950 [1]. Live attenuated mumps virus vaccines were first used during the 1960s in the Soviet Union and USA. The USA started using the Jeryl Lynn vaccine strain whereas the Soviet Union developed a strain called Leningrad-3. Both these strains have been used subsequently in Europe, in addition to others such as the Rubini, Urabe, RIT and Leningrad-Zagreb (L-Z) strains [Reference Galazka, Robertson and Kraigher4]. Currently, mumps vaccine is used in all European countries, as a component of the trivalent measles-mumps-rubella (MMR) vaccine [5].
The widespread use of mumps vaccines has decreased the incidence of the disease substantially [Reference Galazka, Robertson and Kraigher4]. Although the USA and some European countries initially succeeded in controlling mumps by vaccination with two doses at high coverage [Reference Peltola2, Reference Bart, Orenstein and Hinman6], outbreaks have since occurred after a long period of very low incidence in some countries, but not in others [Reference Peltola7, Reference Vandermeulen, Leroux-Roels and Hoppenbrouwers8]. The immunogenicity and effectiveness of the mumps component of MMR vaccine has been variable. Immunogenicity studies have shown that the seroconversion rate after mumps vaccination varies from 80% to 98% according to vaccine strain [Reference Galazka, Robertson and Kraigher4]. In recent outbreaks the effectiveness of mumps vaccination has varied depending on the number of vaccine doses given and the mumps vaccine virus strain used [Reference Castilla9–Reference Gupta, Best and MacMahon12]. Mumps vaccine effectiveness has been estimated from 63% to 97% for the Jeryl Lynn, Urabe and L-Z strains, but as low as 6% for the Rubini strain [Reference Galazka, Robertson and Kraigher4].
During recent years, outbreaks have occurred in teenage populations, many of whom had previously received mumps vaccine, in the USA [Reference Dayan and Rubin11, Reference Dayan13], Canada, Australia [Reference Ferson and Konecny14], and in several European countries [Reference Vandermeulen, Leroux-Roels and Hoppenbrouwers8, Reference Gupta, Best and MacMahon12, Reference Brockhoff15, Reference Kaaijk16]. Some studies have suggested this is due to waning immunity [Reference Schwarz17–Reference Davidkin20]. However, a comparison of the results of seroprevalence and immunity studies of mumps undertaken in different countries has been difficult because of different methods used in testing serum antibody levels. Furthermore, no international reference serum for mumps antibodies is available [Reference Mauldin21]. Finally, there are still questions regarding the level of antibody that provides protection and the rate of decrease of antibody levels with time after vaccination or natural infection [Reference Vandermeulen, Leroux-Roels and Hoppenbrouwers8, Reference Vyse22].
The European Sero-Epidemiology Network (ESEN) was established to coordinate and harmonize the collection of serological data. The ESEN2 project was a continuation of the original ESEN project extending to new partner countries and infections. One article published in connection to the ESEN project [Reference Nardone23] dealt with mumps seroprevalence data from six countries collected between 1994 and 1998. It stated that current MMR immunization programmes will need to be strengthened in a number of countries to be able to control mumps in Western Europe, and that serosurveillance of mumps is an important component of evaluating disease control. In the current study, we relate seroprevalence, epidemiological and vaccination data collected from 18 ESEN countries to their subsequent risk of mumps outbreaks in order to inform vaccination strategies.
METHODS
Study population
The study used data collected in 18 countries participating in ESEN2. These are Belgium, Bulgaria, Cyprus, Czech Republic, Finland, Hungary, Ireland, Israel, Latvia, Lithuania, Luxembourg, Malta, Romania, Slovakia, Slovenia, Spain, Sweden, and the UK. The data consists of three main parts: epidemiological data concerning the national vaccine programme (historical vaccine uptake, vaccine schedule and vaccine strain used); disease incidence data collected through national surveillance; and seroprevalence data, i.e. mumps IgG antibody levels in age- and sex-stratified serum collections from the general population of each participating country.
Epidemiological data collection
Initial epidemiological data collection was undertaken in 2002 with electronic questionnaires sent to all participating countries. Data were collected on vaccination programme, vaccination campaigns, vaccination strains used, target groups, vaccination schedule and annual coverage of MMR1 and MMR2. Data was also collected on historical annual mumps incidence (total, by age group, by vaccination status), and outbreaks.
In 2009, the survey was updated with data for the period 2002–2008 and the older data were complemented with more detailed information. This information was collected through a second questionnaire that was sent to participating ESEN2 countries in March 2009. The questionnaires were pre-filled with available information by the study team and the participating countries were asked to update the questionnaires with available information.
Serum collection
Eighteen European countries took part, testing for mumps IgG antibody in sera population banks collected between 1996 and 2004 (Table 1). The sera were obtained either by population-based random sampling (6/18 countries), by residual sera collected from routine laboratory testing (11/18), or a combination of these two methods (1/18). The approach of using residual sera has been used previously and been shown to provide a good representation of infectious disease antibody prevalence [Reference Kelly24, Reference Osborne25]. Ethical approval was sought for serum collections according to national guidance. Sera were collected from all age groups, were equally distributed between males and females and were geographically representative of each country (Table 1). Project guidelines recommended that 100 samples be tested in each 1-year age band of persons aged <20 years and 200 samples for each 5-year age band of persons aged 20 to ⩾60 years. Project targets were achieved only in Romania, Slovakia, Spain and the UK. The rest of the countries had slightly fewer samples from each age group, but the number of samples was distributed proportionally by age group in all countries. Three countries deviated from the sampling plan with Spain only collecting samples for ages 2–39 years, Luxembourg only from persons aged ⩾4 years, and Sweden only from certain age groups (Table 1).
Table 1. Type and number of samples, and year of collection for the seroprevalence survey

* Sera collected from 2, 5, 8, 10, 14, 17, 20–34 and ⩾65 years age groups.
Assay standardization
The Robert Koch Institute, Berlin, Germany acted as the reference centre for the mumps standardization as described by Tischer et al. [Reference Tischer26]. The reference centre prepared a panel of 151 sera evenly spread throughout the range from low negatives to high positives using the enzyme immunoassay (EIA) Enzygnost® (Dade Behring Marburg GmbH, Germany). This panel was distributed to participating laboratories where they were tested twice with the quantitative EIA of their choice. Once before main serum bank testing to evaluate test performance against the reference centre and a second time during testing of the main serum bank [Reference Kafatos, Andrews and Nardone27, Reference Andrews28].
Initially, the panel results of the second test of the national centre were regressed against the reference centre's results, thus obtaining standardization equations. These country-specific standardization equations were used to convert the local quantitative results into standardized reference titres.
Each participating laboratory's serum bank was tested with the same validated assay used for testing the reference panel. Eight of the 18 countries used Dade Behring mumps IgG EIA like the reference laboratory, two used in-house EIAs and the remaining eight used EIA IgG assays from six different manufacturers [Reference Tischer26]. The reference laboratory's assay cut-off (which was 230 arbitrary units) was used to categorize the standardized measurements into negative and positive. The equivocal (or low positive) results were merged with the positives in a similar way to previous ESEN studies [Reference Andrews28].
In four of the 18 countries (Finland, Israel, Spain, Sweden), the national serum banks had been tested over a year before the distribution of the reference panel and an alternative method was used, designated back-standardization. This involved selecting a panel of 150 samples from each national main bank and subsequently testing these panels by the reference laboratory. The resulting standardization equations were used to standardize the main bank results in the same way as described above [Reference Tischer26].
Data analysis
The data collected through the epidemiological questionnaire were compared with the seroprevalence data to see how they related to the vaccine uptake data, type of vaccines used, and mumps incidence by age group and birth cohort.
The ESEN2 countries were grouped into four categories depending on their mean reported vaccination coverage for the first dose of MMR (MMR1) in the decade between 1999 and 2008: (i) very high average coverage countries (>95%), (ii) high average coverage countries (>90–95%), (iii), intermediate coverage countries (80–90%), and (iv) countries with no mumps vaccination programme (Tables 2–4).
Odds ratios (ORs) and 95% confidence intervals (CIs) were calculated for all explanatory factors.
Univariable analysis using logistic regression was performed to investigate the relationship between occurrence of mumps outbreaks (mumps incidence >1/100 000 population per year between 2004 and 2008) and a number of explanatory factors. ORs with their corresponding 95% CIs were estimated and the associations were tested using Pearson's χ2 and Fisher's exact tests. The explanatory factors examined were geometric mean titres (GMTs) of mumps antibodies (actual GMTs), age of reported cases (<5, 5–9, 10–14, 15–19, ⩾20 years), seroprevalence (⩽80% or >80%), MMR1 coverage (MMR1 uptake in % in the year of peak mumps incidence), number of years between doses (<4, 4–8, >8 years) and type of vaccine used (Rubini strain ever used or not).
In the multivariable logistic regression model only variables that could influence whether or not a country would reach the WHO mumps control target of <1 case/100 000 population (i.e. age, MMR1 coverage, number of years between doses and type of vaccine used) were included. The multivariable model was based on 75 observations (the number of non-missing data across all the variables included in the model).
Microsoft Excel (Microsoft, USA) was used for obtaining equations for standardizing the serological results, while Stata v. 11.2 (StataCorp, USA) was used for the univariable and multivariable analysis.
RESULTS
All 18 ESEN2 countries who had tested their serum banks for mumps IgG responded to the questionnaire.
Finland was the first ESEN2 country to introduce mumps vaccination, for all males enrolling in compulsory army service from 1960. Bulgaria, Sweden and Slovenia introduced mumps-containing vaccines in the 1970s in childhood programmes whereas 13 countries introduced mumps vaccine between 1980 and 1991 for children. Romania, in 2004, was the last participating country to introduce a mumps-containing vaccine (after the serosurvey was undertaken) of the participating countries. Currently (2011), all the 18 ESEN2 countries include two doses of MMR vaccine in their vaccination programme (Table 2), although with wide variations in both the age at which the vaccine doses are administered (MMR1: 12–18 months; MMR2: 21 months to 13 years) and the reported uptake (average MMR1 coverage 1999–2008: 80·7–99·9%). The vaccination programmes are voluntary in all the ESEN2 countries except Czech Republic.
Table 2. Vaccination coverage in ESEN2 countries and the vaccine strains used

* Based on only two measurements (1996 and 2007).
† Earlier data on strain missing.
Several different mumps vaccination strains have been used in the ESEN2 countries. Most countries have used more than one mumps vaccination strain since they implemented vaccination (Table 2). The most commonly used strains are Urabe and Jeryl Lynn. Spain also used the Rubini strain from 1992 to 1999 and Malta used the Rubini strain for a catch-up campaign for persons aged 13–15 years in 1994. Some of the former Eastern European countries have used the Leningrad-3 strain, the L-Z strain and the Sofia-6 strain.
Age-specific mumps antibody seropositivity rates and antibody titres varied greatly between the 18 countries. The countries that experienced mumps outbreaks (defined as >1 mumps case/100 000 population; n = 10) had slightly, but significantly (P = 0·013), lower geometric mean antibody titres (2·64 vs. 2·85) in all age groups compared to the countries that did not have outbreaks (n = 7).
The data have been interpreted according to the four groupings of countries by vaccination coverage, as follows.
No vaccination
Romania is the only country that did not include mumps vaccination in its immunization schedule at the time of the serosurvey (conducted in 2002 in Romania). Although Romania did introduce MMR1 in 2004 and MMR2 in 2005, no data on vaccination coverage were available at the time of data collection (Fig 1). Due to the lack of routine mumps vaccination at the time of the serosurvey, the data from Romania illustrate what incidence rates and mumps antibody seroprevalence were like in the pre-vaccination era. The age-specific mumps incidence was very high in children, with a peak of 1400/100 000 in the 5–9 years age group. Very few cases of mumps were reported in people aged >20 years. Outbreaks occurred every 2–5 years. As shown in Figure 1, the age-specific seroprevalence increases slowly and steadily with age: 50% having acquired antibodies by age 7 years and >85% by age 20 years.

Fig. 1 [colour online]. Mumps incidence in Romania in 2002 compared to mumps antibody seroprevalence. Mumps vaccination was started in 2004.
Intermediate vaccination coverage countries
Belgium, Cyprus, Ireland, Malta and the UK all started mumps vaccination programmes in the late 1980s and have had two-dose programmes since 1995. Reported mean MMR1 vaccination coverage was 80–90% between 1999 and 2008. All countries in the intermediate vaccination coverage group have similar MMR vaccination schedules and in all these countries the first cohorts who received MMR were aged 15–19 years at the time of the ESEN serosurvey. The vaccination coverage data, where known, show lower uptake in 15–19 years cohorts than in younger age cohorts. All these countries, except Belgium, have seen mumps outbreaks since 2004 with peak incidence rates in the 15–19 years age groups (Table 3). Belgium also experienced an outbreak after the data collection was completed (spring 2011). We have used the UK data as an example of this group (Fig. 2). The UK experienced a large outbreak of mumps in 2005. During this outbreak the peak incidence was almost 700/100 000 in the 15–19 years age group (born 1986–1990) with the 20–24 years age group (born 1981–85) accounting for the second highest incidence. The latter group was the first to be offered MMR as part of the childhood vaccination schedule with the start of the mumps vaccination programme. By the time of the serosurvey (undertaken in 2000), this group should have been offered a second dose of vaccine. Reported MMR1 coverage was then on average only 69% in this age group and the MMR2 coverage unknown in the UK. Cyprus and Ireland also had outbreaks mainly in the 15–19 years age group (born 1985–1989) at around the same time as the UK.
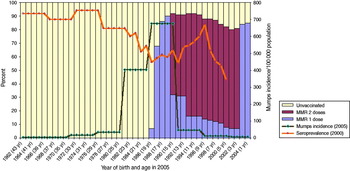
Fig. 2 [colour online]. Mumps incidence in the UK in 2005 by age group compared to mumps vaccination status and mumps antibody seroprevalence (MMR2 was introduced in 1995 but coverage measurements started in 1999).
Table 3. Mumps incidence in the ESEN2 countries in 2004–2008, including outbreaks and outbreak strain genotypes
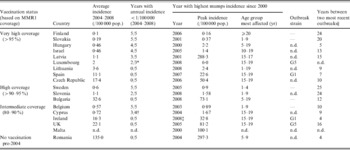
n.d., No data.
* Data only available for 3 years.
† Data only available for 4 years.
‡ Outbreak ongoing since 2004–2008 highest incidence.
§ Or years since last outbreak in case no outbreaks were seen in the last 10 years.
The seropositivity rates for mumps antibodies are highly variable ranging from 53·1% (UK) to 87·3% (Ireland) in the 15–19 years age group (Table 4).
Table 4. Prevalence of mumps antibodies as a proportion of the tested samples in the different ESEN2 countries

* Age 2–4 years
† Age 4 years only.
‡ Age groups for Sweden: 2, 5, 8–11 , 14–15 , 17–18 , 20–34 , ⩾65 years.
High vaccination coverage countries
Sweden, Slovenia and Bulgaria started childhood vaccination programmes with mumps-containing vaccines in 1971–1979 and two-dose programmes from 1982 to 2002. Reported mean MMR1 vaccination coverage of these programmes was 90–95% between 1999 and 2008. Although Bulgaria experienced an outbreak of mumps in 2008 after an inter-epidemic period of 12 years, Sweden and Slovenia have had low mumps incidence rates for more than 20 years, although Slovenia has had difficulties keeping the incidence <1/100 000 population (Table 3). Whereas Sweden has only vaccinated using the Jeryl Lynn strain, Slovenia first used Leningrad-3 and L-Z strains before changing to Jeryl Lynn. Bulgaria used the Sofia-6, Leningrad-3 and Urabe strains before Jeryl Lynn was introduced.
We selected Bulgaria to illustrate this group (Fig. 3). The persons most affected by the 2008 mumps outbreak in Bulgaria were aged 5–19 years (born 1989–2003), with incidence rates of almost 280/100 000. Persons aged <15 years had reported vaccination coverage of MMR1 of around 90% but older persons had lower reported vaccination coverage. As MMR was introduced in 1993 for those aged 13 months, persons aged 17 years in 2008 were the first age group to have been vaccinated against mumps as children and those aged 18–19 years were therefore unvaccinated.
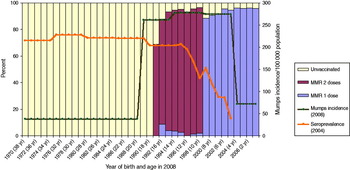
Fig. 3 [colour online]. Mumps incidence in Bulgaria in 2008 by age group compared to mumps vaccination status and mumps antibody seroprevalence.
The mumps antibody seroprevalence data differ greatly between the three countries in the ‘high vaccination coverage’ group with Slovenia showing higher seropositivity rates than the other two. Bulgaria has the lowest seroprevalence rates of <40% in children aged 1–4 years and a peak seroprevalence of 75·1% in persons aged 15–19 years. This stands in contrast to the reported two-dose vaccination coverage of almost 95% in Bulgaria. Seroprevalence increases from 71·3% in 2-year-olds in Sweden to 76·6% in persons aged 20–34 years, whereas Slovenia already has seroprevalence rates of >95% in the 5–9 years age group (Table 4).
Very high vaccination coverage countries
Nine of the participating countries reported average MMR1 vaccination coverage of more than 95% between 1999 and 2008. These were Czech Republic, Finland, Hungary, Israel, Latvia, Lithuania, Luxembourg, Slovakia and Spain.
Hungary and Slovakia (Figs 4, 5) are typical examples of the high vaccination coverage group with seroprevalence levels >80% in the population aged >2 years.

Fig. 4 [colour online]. Mumps incidence in Hungary in 2007 by age group compared to mumps vaccination status and mumps antibody seroprevalence.

Fig. 5 [colour online]. Mumps incidence in Slovakia in 2006 by age group compared to mumps vaccination status and mumps antibody seroprevalence.
In the ‘very high vaccination coverage’ countries, mumps incidence has remained very low in most countries, with average incidence rates in Finland, Hungary, Israel and Slovakia of <1/100 000 in the last 5 years (Table 3). Apart from Israel, these four countries have mumps antibody seroprevalence rates >80% from the age of 5 years. The mumps cases that do occur are very few and do not follow a specific age or vaccination coverage pattern. Slovakia has seroprevalence rates >90% from the age of 10 years and has not had a mumps outbreak for 20 years. Similar to most other countries in this group, Slovakia gives the first MMR dose at age 14 months and the second dose at age 10 years.
Latvia, Lithuania and Luxembourg had slightly higher mumps incidence rates than the above-mentioned countries (>1/100 000 population), and Luxembourg reported an outbreak of mumps in young military recruits in 2008 [Reference Mossong29].
The Czech Republic and Spain stand out in this group with average mumps incidence rates >10/100 000 between 2004 and 2008 and both experienced outbreaks (Table 3). The Czech Republic experienced a large outbreak in 2006 (Fig. 6), as did Spain in 2007 (Fig. 7). The outbreak in the Czech Republic had a peak incidence of almost 300/100 000 in the 15–19 years age group. This group was highly vaccinated with two doses of MMR (Jeryl Lynn and Urabe strains). Of particular note, is that the routine mumps programme is given at an unusually short interval with the first dose at age 15 months and second dose at age 21–25 months (since the start of the programme in 1987). The Spanish outbreak also had its peak incidence in the 15–19 years age group, with a maximum incidence of almost 85/100 000. This group had received two doses of MMR (Rubini and Jeryl Lynn strains) at ages 12–14 months and 3–6 years with an average vaccination coverage just below 90% for MMR1, and unknown coverage for MMR2.

Fig. 6 [colour online]. Mumps incidence in the Czech Republic in 2006 by age group compared to mumps vaccination status and mumps antibody seroprevalence.

Fig. 7 [colour online]. Mumps incidence in Spain in 2007 by age group compared to mumps vaccination status and mumps antibody seroprevalence (MMR2 was introduced in 1996 for persons aged 11–13 years, but was given to children aged 3-6 years from 2000).
Factors influencing outbreaks
In the univariable analysis, there were significantly lower odds of having an outbreak in countries with higher GMTs of antibody (OR 0·09, 95% CI 0·01–0·71). There were also lower odds of having an outbreak in countries with seroprevalence >80% overall (OR 0·12, 95% CI 0·03–0·47). All countries with <95% reported MMR1 coverage reported an outbreak, whereas for coverage ⩾95%, about a third did not (P < 0·001). Finally, the odds for having an outbreak was lower in countries with an interval of >4 years between doses (P = 0·033). No significant difference was found between age groups (P = 0·25) or the type of vaccine used (P = 1·00) (Table 5).
Table 5. Results of the univariable and multivariable analyses of factors (listed as variables) that could influence mumps outbreaks
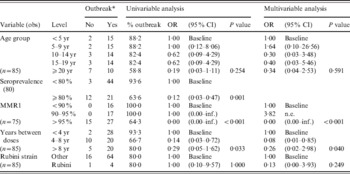
OR, Odds ratio; CI, confidence interval; n.e., not possible to estimate.
* Defined by incidence with 1/100 000 cut-off.
The MMR1 vaccine coverage of ⩾95% remained significant in the multivariable model (P < 0·001). Moreover, an interval between 4 and 8 years between doses was associated with lower odds of having an outbreak (OR 0·08, 95% CI 0·01–0·85) (Table 5).
DISCUSSION
Despite long-standing mumps vaccination programmes with high coverage in Europe, a majority of ESEN2 countries have in recent years experienced mumps outbreaks or failed to reach the <1 case/100 000 population WHO control target. A small number of countries maintained excellent control. Our findings suggest several explanatory factors for the observed increase in incidence.
In almost all countries with mumps outbreaks, the peak incidence was seen in the 15–19 years age group. This group is generally the first to receive MMR, i.e. the one that falls between those who acquired natural immunity to mumps and those that have high vaccination coverage. However, their seroprevalence rates are not lower than in other age groups (except in the UK), and the GMT of mumps IgG is also high. Although none of the populations show an apparent waning of antibodies detected by EIA, there may have been a decline in protection with time since mumps vaccination. This has been shown as age-specific decreases in vaccine effectiveness [Reference Cohen10], increased risk of developing mumps with time after vaccination [Reference Cortese30, Reference Vandermeulen31] and higher attack rates with time since vaccination [Reference Hersh32–Reference Schaffzin34]. There are also numerous studies showing decreased antibody levels against mumps with time since vaccination [Reference Dayan and Rubin11]. Studies of contact patterns indicate that this age group has a higher number of close contacts than adults and young children which could also help explain the high mumps incidence [Reference Mossong35].
Age-specific mumps antibody titres were lower in mumps outbreak countries but no correlation was seen between those cohorts most affected by mumps outbreaks (aged 15–19 years) and the seroprevalence data. The seroprevalence data could therefore not be used alone to predict outbreaks. It has been shown previously that mumps antibody seroprevalence is difficult to relate to risk of mumps infection [Reference Hanna-Wakim36]. Current data do not give conclusive evidence what level of mumps antibodies should be considered protective, but it does seem that high levels of circulating mumps antibodies are important in protection against outbreaks [Reference Ilonen37, Reference Hyoty38]. On the other hand, cell-mediated immune responses seem to play an important role in protection against mumps, also in the absence of measurable antibodies [Reference Hanna-Wakim36, Reference Ilonen37].
The differences in antibody levels between the populations studied may be induced by several factors, among them the uptake of mumps vaccine, the different immunogenicity of mumps vaccines or differences in schedules (e.g. spacing between doses) and previous exposure to mumps infection. Even two doses of mumps vaccine will not achieve a 100% seroconversion so a group of susceptible individuals will always remain in the population [Reference Dayan and Rubin11]. In the data from Bulgaria, however, there was a marked discrepancy between the reported high vaccination coverage and the observed low seroprevalence, similar to that seen in studies of rubella [Reference Nardone39] and measles [Reference Andrews40]. This could be due to denominator figures for vaccination coverage calculation not including relatively large, unregistered, under-vaccinated population groups, such as travellers or Roma populations [Reference Masseria, Mladovsky and Hernandez-Quevedo41]. Although our analysis could not show a significant association between having used the Rubini strain and experiencing a mumps outbreak, this strain has previously been shown to achieve a vaccine effectiveness of only 0–33% in outbreaks [Reference Dayan and Rubin11] and 0–12·4% in non-outbreak studies [Reference Toscani42–Reference Chamot44]. This is likely to be an important explanatory factor for outbreaks observed in older cohorts in Spain and Malta.
High reported coverage of MMR1 was associated with reduced risk of mumps outbreaks. This is as expected, and reviews of mumps vaccine effectiveness in outbreaks have shown that the major explanatory factor in most outbreaks appeared to be incomplete vaccination coverage [Reference Dayan and Rubin11]. However, studies indicate that one dose of mumps-containing vaccine does reduce the risk of complications after mumps infection [Reference Hahne45]. Although the effectiveness of two doses of mumps vaccine is higher than that of one dose [Reference Dayan and Rubin11], mumps outbreaks have also occurred in populations with high two-dose coverage. For example, in a series of mumps outbreaks that occurred in the USA in 2006 some investigations showed that >99% of the patients had been vaccinated twice [Reference Cortese30]. A recent publication on the same outbreak found that the cases had lower pre-outbreak mumps antibody levels than non-cases [Reference Cortese46]. However, studies of antibody responses after vaccination with the Jeryl Lynn strain, and other strains, have shown that primary vaccine failure in recipients of two doses appears an unlikely cause of mumps outbreaks in vaccinated persons [Reference Davidkin, Valle and Julkunen47, Reference Kuno-Sakai, Ozaki and Kimura48]. Secondary vaccine failure (waning protection) may provide the explanation in combination with close contact (as in a higher educational establishment like university) [Reference Brockhoff15]. This might explain why these older age groups are mainly affected. It will be important to continue surveillance to observe whether this phenomenon is related to waning immunity and thus likely to be an on-going issue, or rather is related to a partially vaccinated cohort in those countries who introduced the mumps programme at lower coverage with one dose, and will thus be a one-off phenomenon. Investigation of outbreaks also provides an opportunity to disentangle this phenomenon.
An interesting finding in this study was the association between a long time interval between the first and second MMR doses and decreased risk of outbreaks. This was most noticeable in the case of the Czech Republic and Slovakia where the vaccination programmes were identical, including the strains used and uptake reported, except for the time interval between the two MMR doses. The Czech Republic, with 6–10 months between the two doses, experienced outbreaks whereas Slovakia, with 9 years between doses, did not. This could be interpreted as giving the second dose to older children, as well as a long interval between doses, increases the protection against mumps. WHO recommends at least a 1-month interval between the first and second doses [1] but our results show that a longer interval is associated with reduced risk of subsequent mumps outbreaks. Administering the second MMR dose before the age of 2 years has been suggested to induce shorter protection against mumps [Reference Davidkin49]. Our findings suggest an interval of at least 4 years between two vaccination doses is optimal. It should be noted that mumps vaccination strategies are dependent on the two other infections that the MMR vaccine protects against (measles and rubella). The age at the second dose is often decided based to a large extent on protection against measles.
There are some limitations to our study. Although the seroprevalence data collection was standardized for the ESEN studies, the data collected on mumps vaccine coverage and mumps incidence rates were from regular national surveillance systems of individual countries. The data could have been collected in different ways with different case definitions, and figures are therefore not necessarily completely comparable across countries. However, these are the national figures provided by the study countries and the best data available. Not all countries collected the pre-specified number of samples from all age groups from 1 to ⩾60 years, but we have checked that data are not skewed. Vaccination schedules have been changed over time; especially regarding the strains used, and this has not been controlled for in the data analysis. The Rubini strain was only used in two of the 18 ESEN2 countries, and the sample size was therefore too small to show a significant association with mumps outbreaks. However, data from other studies do indicate that the vaccine effectiveness from this strain is lower than for the other mumps vaccine strains. Although the approach of using residual sera in data collection has been used previously and has been shown to give a good representation of common childhood infections in the population, this method might lead to certain risk groups, e.g. difficult to reach groups, not being represented in the samples, but those under-vaccinated due to medical reasons might be overrepresented.
CONCLUSIONS
Epidemiological and serological data were synthesized in an attempt to explain why outbreaks occur in highly vaccinated populations. Preventing outbreaks and controlling mumps most likely requires several elements, including high-coverage two-dose vaccination programmes with a highly immunogenic mumps vaccine of the same strain given with 4–8 years between doses. These findings should be taken into consideration for the planning of mumps vaccination programmes.
ACKNOWLEDGEMENTS
This work was partially funded by a grant from the European Commission (contract number QLK2-CT-2000-00542). We thank all the members of the ESEN2 country teams who contributed to this work, including Professor Liz Miller at the Health Protection Agency Centre for Infection (UK), Emilia Lupulescu from the Viral Reference Laboratory, Cantacuzino Institute (Romania), Tiia Lepp at the Swedish Institute for Infectious Disease Control, Dr Maria Victoria Martinez de Aragón (Centro Nacional de Epidemiología, Instituto de Salud Carlos III, Madrid), Natalija Zamjatina and Larisa Savrasova at the Virology Unit, Laboratory of the State Agency of Infection Centre (Latvia), Yair Aboudy (Central Virology Laboratory, Ministry of Health, Israel).
DECLARATION OF INTEREST
None.














