Polycystic ovary syndrome (PCOS) is defined as an endocrine disease resulting from a hormonal imbalance that affects 5–18 % of women of reproductive age, making it an important public health problem in view of the co-morbidities and prevalence currently presented(Reference Kelley, Brown and Diehl1). The syndrome is characterised by symptoms such as menstrual irregularity, anovulatory infertility, clinical and biochemical hyperandrogenism, as well as other metabolic manifestations, which affect from 30 to 70 % of women with PCOS(Reference Chittenden, Fullerton and Maheshwari2–Reference Altieri, Cavazza and Pasqui4).
The hormonal imbalance that occurs with PCOS can influence the reproductive, metabolic and psychological health of patients. Clinical manifestations may include precocious puberty, acne, alopecia, seborrhoea, irregular menstrual cycles, hirsutism, infertility and complications in pregnancy(Reference Vanky, Kjøtrød and Salvesen5). Anxiety, depression and non-acceptance of body image are frequent psychological co-morbidities(Reference Blay, Aguiar and Passos6). Metabolic impairment includes the primary effect of insulin resistance (IR) on muscle and adipose tissue, with compensatory hyperinsulinaemia, associated with intrinsic β-cell dysfunction, type 2 diabetes mellitus and gestational diabetes, hyperlipidaemia, increased risk of CVD, obesity, sleep apnoea, non-alcoholic fatty liver disease and the metabolic syndrome (MetS)(Reference Teede, Deeks and Moran7).
In 2018, a guideline for the assessment and management of PCOS was published by the American Society for Reproductive Medicine/European Society of Human Reproduction and Embryology, which endorsed the Rotterdam criteria for the diagnosis of PCOS in adult women who were not in menopause(8). Among these criteria, PCOS can be diagnosed when at least two of the three proposed criteria are present, categorised as follows: (1) androgen status – clinical or biochemical hyperandrogenism; (2) menstrual history – oligo- or anovulation or (3) ovarian appearance – polycystic morphology on ultrasound(8).
The PCOS state of low-grade inflammation is similar to that of other non-communicable diseases, such as obesity, type 2 diabetes mellitus and CVD(Reference Barrea, Marzullo and Muscogiuri9). The role of inflammation in PCOS has been the subject of several studies, and associations have been found between increased levels of inflammation markers (high-sensitivity C-reactive protein (hs-CRP), ferritin, TNF and IL-6 and IL-18) and oxidative stress (OS) markers (malondialdehyde (MDA), total antioxidant capacity – TAC, nitric oxide (NO) and GSH) with PCOS(Reference Sathyapalan and Atkin10–Reference Sóter, Ferreira and Sales12).
In fact, a case–control study(Reference Sóter, Ferreira and Sales12) that aimed to evaluate the relationship between polymorphisms in genes encoding inflammation-associated cytokines and the metabolic profiles of Brazilian women with PCOS (n 97) v. a control group (n 99) observed that fasting glucose levels varied according to IL-6 genotype, while the hirsutism score, 2-h glucose tolerance test, total cholesterol and TAG levels varied according to the IL-10 genotype. Serum lipid levels were also related to interferon-γ and transforming growth factor-β genotypes, suggesting that cytokine gene polymorphisms may promote abnormal metabolic features in PCOS(Reference Sóter, Ferreira and Sales12).
Another case–control study(Reference Enechukwu, Onuegbu and Olisekodiaka13) aimed to determine the relationship between OS markers and lipid profiles in patients with PCOS. This study included fifty PCOS patients and fifty healthy controls and revealed that serum MDA levels were significantly higher in PCOS patients than in controls and that TAC was significantly lower in the PCOS group(Reference Enechukwu, Onuegbu and Olisekodiaka13). It is known that TAC is also reduced in many diseases such as hypertension, type 2 diabetes mellitus, obesity and the MetS(Reference Minihane, Vinoy and Russell14).
Among the environmental factors, numerous nutrients are known to modulate the inflammatory response and contribute to the protection and treatment of non-communicable diseases, such as MUFA and PUFA(Reference Minihane, Vinoy and Russell14–Reference Nasef, Mehta and Ferguson16). The long-chain n-3 fatty acids, namely α-linolenic acid (ALA), EPA and DHA, are commonly considered ‘essential’ fatty acids, since they are not synthesised in the human body and are mostly obtained from the diet(Reference Russo17). EPA and DHA are found naturally in marine sources, including cold water fish, shellfish and seaweed. ALA can be converted to DHA or EPA after ingestion and is found in seeds such as chia, flaxseed and pumpkin seeds, as well as in vegetable and oilseeds like nut oils. In addition, it is also present in small amounts in other vegetable sources, such as spinach and kale(Reference Simopoulos18).
Evidence suggests that n-3 fatty acid supplementation increases insulin sensitivity and plasma adiponectin levels, reduces hyperinsulinaemia, plasma TAG and liver fat and attenuates inflammation and the OS response in adults(Reference Cussons, Watts and Mori19,Reference Carpentier, Portois and Malaisse20) . However, the role of n-3 fatty acid supplementation in controlling OS and chronic low-grade inflammation in women with PCOS is still uncertain. Therefore, the present study aimed to systematically review and meta-analyse randomised controlled trials (RCT) investigating the influence of n-3 fatty acid supplementation on inflammatory and OS markers in patients with PCOS.
Methods
The conduct and design of this systematic review and meta-analysis followed the predetermined protocol according to the Cochrane Handbook’s recommendations(Reference Higgins, Thomas and Chandler21). Results were reported in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses statement(Reference Liberati, Altman and Tetzlaff22). The protocol of the current study was published on the International Prospective Register of Systematic Reviews (PROSPERO) (CRD42019129199).
Search strategy
The PICO acronym search question was composed of: P (participants) = Women with Polycystic Ovary Syndrome; I (intervention) = n-3 fatty acid supplementation; C (control) = Placebo and O (outcomes) = Inflammatory and OS markers levels. A literature review was performed by searching the electronic databases Medline/PubMed (Medical Literature Analysis and Retrieve System Online), Cochrane Central Register of Controlled Trials (CENTRAL), Scopus and Lilacs (Latin American and Caribbean Health Sciences) until November 2019 to identify RCT, which reported the effect of n-3 fatty acid supplementation on inflammatory and OS markers in PCOS patients over 18 years of age. The initial search included the Medical Subject Headings terms ‘Polycystic Ovary Syndrome’ and ‘Fatty Acids, Omega-3’. It also included the entry terms associated with a high-sensitivity strategy for the search of RCT developed by The Cochrane Collaboration(Reference Higgins, Thomas and Chandler21). Online Supplementary material 1 describes the search strategy used on the PubMed database.
The same terms were used to search for clinical studies in the National Institutes of Health (www.clinicaltrials.gov), the Brazilian Registry of Clinical Trials (www.ensaiosclinicos.gov.br) and the Turning Research into Practice (www.tripdatabase.com) databases. All potentially eligible studies were considered for review, regardless of the language and date of publication. A manual search was also implemented in the reference lists of relevant reviews(Reference Amini, Tehranian and Movahedin23–Reference Pundir, Charles and Sabatini27).
Inclusion and exclusion criteria
We included only RCT that analysed the effect of n-3 fatty acid supplementation on inflammatory and OS markers in women with PCOS. The outcome was considered as changes in the concentration or activity of these markers from baseline until the end of the study. Studies that did not report the outcomes of interest, non-randomised studies and those that included children, adolescents (under 18 years of age) or pregnant women were excluded. Studies that did not present as endpoints inflammatory or OS markers were also excluded.
Study selection and data extraction
Initially, the studies retrieved from the databases were input into a single electronic library, and duplicates were excluded using the EndNote® software. Two reviewers (J. A. G. T. and M. T. A.) independently analysed the titles and abstracts of the articles retrieved from the literature search, reviewed the full text of the published articles and extracted the data using a standardised data extraction tool. Any disagreements between the reviewers regarding the study data were resolved by a third investigator (K. B. G. or V. E. A.).
The extracted data included the number of participants, study design, trial duration and patients’ demographic and anthropometric characteristics (age and BMI). n-3 Fatty acid supplementation data from the intervention and control groups were collected. Informative data about inflammatory and OS markers collected at baseline and the end of the study were extracted. Percentage changes in biomarker concentrations were calculated for the studies that presented baseline values.
Assessment of bias across studies and quality of evidence
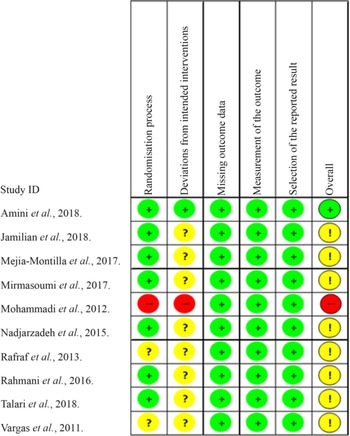
The risk of bias of the studies and the quality of evidence were assessed independently by two reviewers (J. A. G. T. and M. T. A.) following the Cochrane guidelines(Reference Higgins, Savović, Page, Higgins, Thomas and Chandler28), and a third reviewer (K. B. G. or V. E. A.) resolved any disparity. The Cochrane Risk of Bias Tool for Randomized Trials – Rob 2.0 was applied to assess the risk of bias in individual studies according to the recommendations of the Cochrane Collaboration(Reference Higgins, Savović, Page, Higgins, Thomas and Chandler28,Reference Sterne, Savović and Page29) . The Rob 2.0 is structured into five domains: (1) bias arising from the randomisation process; (2) bias due to deviations from intended interventions; (3) bias due to missing outcome data; (4) bias in measurement of the outcome and (5) bias in selection of the reported result. The response options for the signalling questions are: (1) yes; (2) probably yes; (3) probably no; (4) no and (5) no information(Reference Sterne, Savović and Page29).
The Grading of Recommendations, Assessment, Development and Evaluation approach(Reference Balshem, Helfand and Schünemann30) was used to assess the quality of the evidence for each outcome: hs-CRP, adiponectin, visfatin, NO, GSH, MDA and TAC. This approach assesses the strength of the evidence quality by including factors that can decrease quality (e.g. methodological quality, directness of evidence, heterogeneity, precision of effect estimates and risk of publication bias) or increase it (e.g. large magnitude of effect, reduction or spurious effect due to plausible confounding factors, dose–response gradient). Each evaluated factor was rated as high, moderate, low or very low(Reference Higgins, Savović, Page, Higgins, Thomas and Chandler28,Reference Balshem, Helfand and Schünemann30) .
Statistical analyses
Differences between the mean values and standard deviations at baseline and at the end of the study were used to report the changes in inflammatory and OS marker concentrations(Reference Follmann, Elliott and Suh31). Heterogeneity between studies was assessed by Cochran’s Q test, and P ≤ 0·10 was considered statistically significant. The I 2 test was also performed to evaluate the magnitude of heterogeneity, which was considered high if I 2 ≥ 50·0 %. The pooled estimates of the weighted mean differences (WMD) for NO, GSH, TAC and MDA and the estimates of the standard mean differences (SMD) for hs-CRP, adiponectin and visfatin between n-3 fatty acid supplementation and control groups were calculated using the random effects model(Reference DerSimonian and Laird32), since significant heterogeneity among studies was identified in preliminary models. This approach also provided a more conservative assessment of the average effect size. Subsequently, sensitivity (subgroup) analyses were conducted by including variables to determine how much of the between-study difference could be explained by these variables. Publication bias was not accessed through funnel plot asymmetry, since according to the Cochrane Handbook(Reference Higgins, Thomas and Chandler21), it should be used when there are at least ten studies included in the meta-analysis, and all outcomes evaluated in the present meta-analysis had, at maximum, seven studies included. The statistical analyses were performed using Review Manager 5® software. Significant values were considered as P < 0·05 with the 95 % CI.
Results
Study characteristics
Fig. 1 shows the flow chart of the study selection process. The electronic database search identified 323 studies. After analysis of the titles, 210 abstracts were maintained and, subsequently, 178 were excluded after abstract reading. In this stage, the Kappa coefficient of agreement between the two investigators was 0·933. Eighteen RCT were selected for full-text reading after analysis of the abstracts; eight studies were excluded for not fulfilling the inclusion criteria, and thus, ten(Reference Amini, Bahmani and Foroozanfard33–Reference Vargas, Almario and Buchan42) randomised clinical trials were included in this systematic review with meta-analysis.
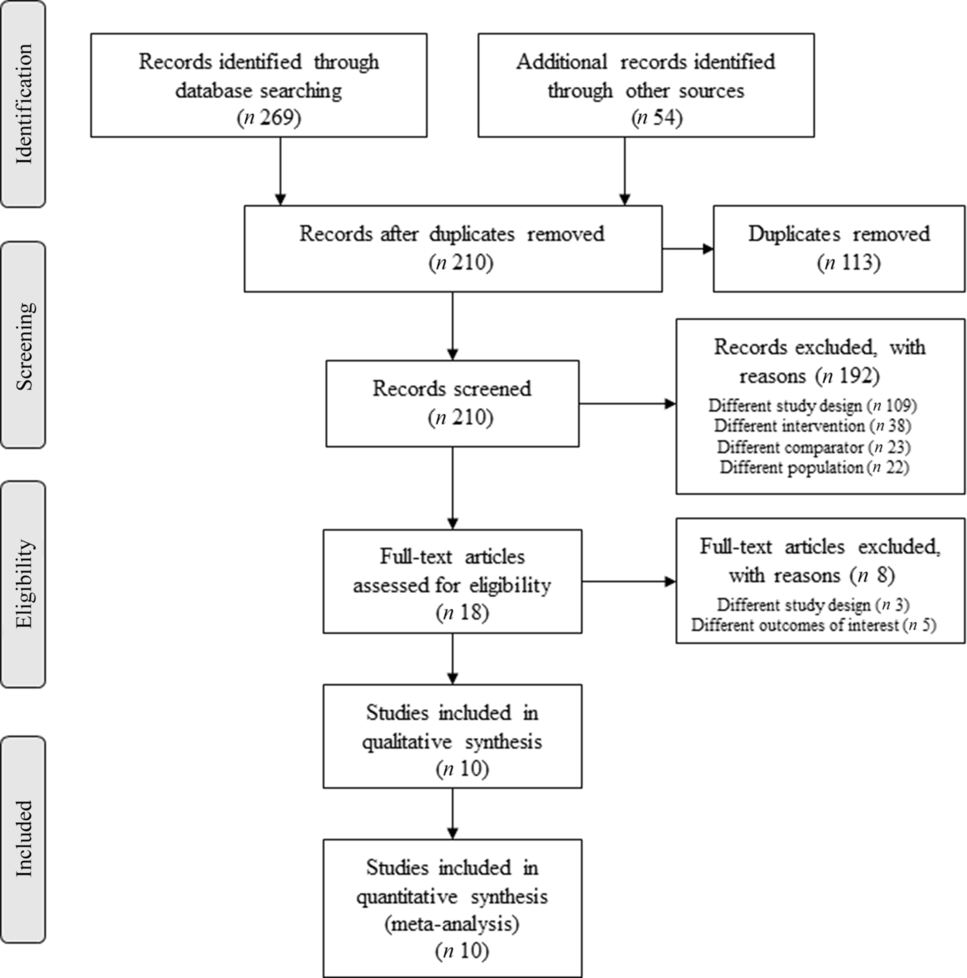
Fig. 1. Flow chart of the literature search and the study selection process.
One study(Reference Vargas, Almario and Buchan42) was included as two independent reports because the findings were described by different interventions of interest (flaxseed and fish oils). The total sample size of all studies comprised 381 patients diagnosed with PCOS (nine studies(Reference Amini, Bahmani and Foroozanfard33–Reference Talari, Poladchang and Hamidian41) diagnosed by the Rotterdam criteria and one study(Reference Vargas, Almario and Buchan42) by the National Institutes of Health criteria) with a mean age of 27·05 years and 384 controls with a mean age of 27·10 years. All the included studies were parallel RCT that comprised 6–12 weeks of follow-up. Subgroup analyses based on sources of n-3 fatty acids (fish oil v. flaxseed and n-3 fatty acids v. n-3 fatty acids plus vitamins D and E) were performed to check the sources of heterogeneity between the studies. However, no significant differences were found between subgroups after the tests.
The included studies were grouped according to the assessed outcome: (1) inflammatory(Reference Amini, Bahmani and Foroozanfard33–Reference Rafraf, Mohammadi and Asghari-Jafarabadi39,Reference Talari, Poladchang and Hamidian41,Reference Vargas, Almario and Buchan42) and (2) OS(Reference Amini, Bahmani and Foroozanfard33,Reference Jamilian, Samimib and Mirhosseinic34,Reference Mirmasoumi, Fazilati and Foroozanfard36,Reference Rahmani, Samimi and Ebrahimi40,Reference Talari, Poladchang and Hamidian41) marker changes, when supplemented with just n-3 fatty acids(Reference DerSimonian and Laird32,Reference Mejia-Montilla, Reyna-Villasmil and Domínguez-Brito35–Reference Rafraf, Mohammadi and Asghari-Jafarabadi39,Reference Vargas, Almario and Buchan42) or co-supplemented with vitamin D(Reference Jamilian, Samimib and Mirhosseinic34) or E(Reference Rahmani, Samimi and Ebrahimi40,Reference Talari, Poladchang and Hamidian41) in patients with PCOS. Table 1 presents the characteristics of the studies included. Online Supplementary material 2 shows the changes in biomarkers of the included studies. Online Supplementary material 3 presents the laboratory characteristics of the participants before and after n-3 fatty acid intervention.
Table 1. Characteristics of the studies investigating inflammation and oxidative stress markers concentrations from n-3 fatty acid supplementation
(Mean values and standard deviations)
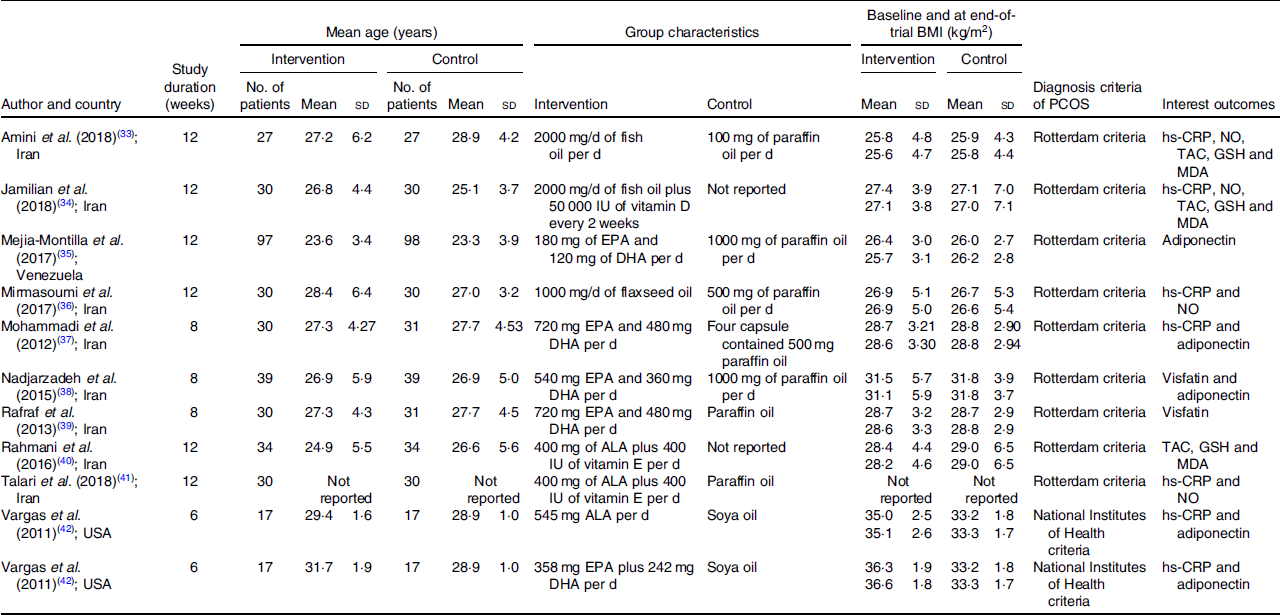
PCOS, polycystic ovary syndrome; hs-CRP, high-sensitivity C-reactive protein; NO, nitric oxide; TAC, total antioxidant capacity; MDA, malondialdehyde; IU, international units; ALA, α-linolenic acid.
Inflammatory markers – high-sensitivity C-reactive protein
Of the ten selected studies, six(Reference Amini, Bahmani and Foroozanfard33,Reference Jamilian, Samimib and Mirhosseinic34,Reference Mirmasoumi, Fazilati and Foroozanfard36,Reference Mohammadi, Rafraf and Farzadi37,Reference Talari, Poladchang and Hamidian41,Reference Vargas, Almario and Buchan42) investigated the effects of n-3 fatty acid supplementation on the circulating concentrations of hs-CRP compared with a placebo group. The mean follow-up time of the studies was 9 weeks (6–12 weeks), and they included thirty-four to sixty participants (mean age 28 years). The mean BMI was 29·57 kg/m2, and one study(Reference Vargas, Almario and Buchan42) used National Institutes of Health criteria to diagnose PCOS. Two studies(Reference Amini, Bahmani and Foroozanfard33,Reference Vargas, Almario and Buchan42) had fish oil as the intervention, three(Reference Mirmasoumi, Fazilati and Foroozanfard36,Reference Mohammadi, Rafraf and Farzadi37,Reference Vargas, Almario and Buchan42) with flaxseed oil and two with n-3 fatty acid co-supplementation with D(Reference Jamilian, Samimib and Mirhosseinic34) and E(Reference Talari, Poladchang and Hamidian41) vitamins. One study(Reference Jamilian, Samimib and Mirhosseinic34) did not report the placebo used, four studies(Reference Amini, Bahmani and Foroozanfard33,Reference Mirmasoumi, Fazilati and Foroozanfard36,Reference Mohammadi, Rafraf and Farzadi37,Reference Talari, Poladchang and Hamidian41) used paraffin oil and one(Reference Vargas, Almario and Buchan42) soya oil as the placebo.
Overall, n-3 fatty acid intervention decreased significantly hs-CRP concentrations when compared with the control group (SMD –0·29 (95 % CI –0·56, –0·02) mg/l; I 2 = 38 %, P for heterogeneity: 0·14; Fig. 2(a)).
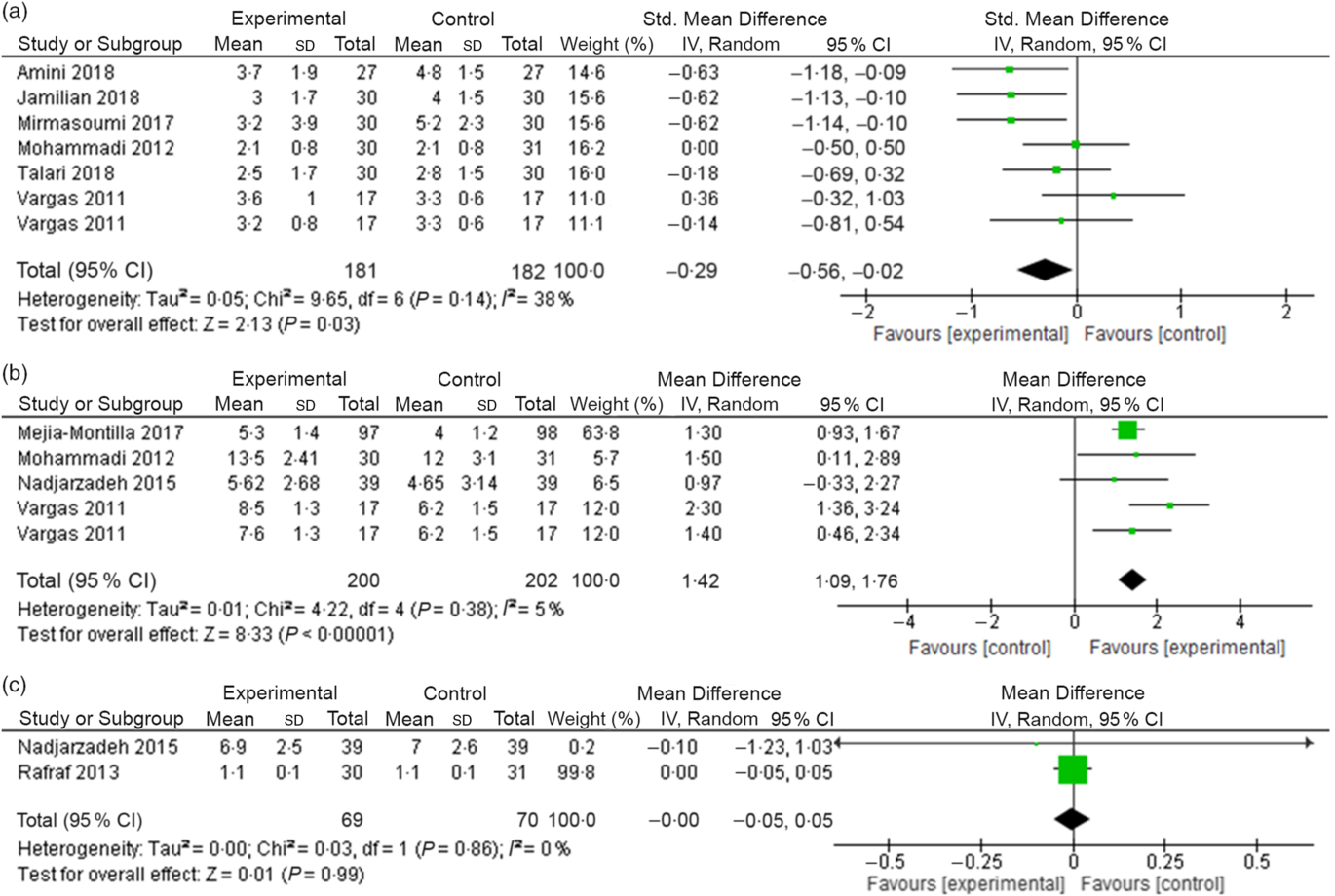
Fig. 2. (a) Change in high-sensitivity C-reactive protein (mg/l) concentrations due to n-3 fatty acid supplementation. (b) Change in adiponectin (ng/ml) concentrations due to n-3 fatty acid supplementation. (c) Change in visfatin (ng/ml) concentrations due to n-3 fatty acid supplementation.
Inflammatory markers – adiponectin
A total of four studies(Reference Mejia-Montilla, Reyna-Villasmil and Domínguez-Brito35,Reference Mohammadi, Rafraf and Farzadi37,Reference Nadjarzadeh, Dehghani-Firouzabadi and Daneshbodi38,Reference Vargas, Almario and Buchan42) were included in meta-analysis that evaluated the effects of n-3 fatty acid supplementation on the circulating concentrations of adiponectin compared with a placebo group. The studies included 34–195 participants (mean age 27 years), and one study(Reference Vargas, Almario and Buchan42) used National Institutes of Health criteria to diagnose PCOS. The mean follow-up time of the studies was 8 weeks (6–12 weeks), and the mean BMI was 27·76 kg/m2. Four studies(Reference Mejia-Montilla, Reyna-Villasmil and Domínguez-Brito35,Reference Mohammadi, Rafraf and Farzadi37,Reference Nadjarzadeh, Dehghani-Firouzabadi and Daneshbodi38,Reference Vargas, Almario and Buchan42) had fish oil as the intervention and one(Reference Vargas, Almario and Buchan42) used flaxseed oil as n-3 fatty acid supplementation.
The pooled data from four studies showed a significant effect of n-3 fatty acid supplementation on increasing adiponectin concentrations (weighted mean difference 1·42 (95 % CI 1·09, 1·76) ng/ml; I 2 = 5 %, P for heterogeneity: 0·38; Fig. 2(b)).
Inflammatory markers – visfatin
The meta-analysis that evaluated the concentrations of visfatin, under n-3 fatty acid supplementation when compared with a placebo group, included two studies(Reference Nadjarzadeh, Dehghani-Firouzabadi and Daneshbodi38,Reference Rafraf, Mohammadi and Asghari-Jafarabadi39) . Both studies used the Rotterdam criteria for PCOS diagnosis, and the intervention was composed of 180 mg of EPA and 120 mg of DHA. The mean follow-up time was 8 weeks. In addition, the mean age and BMI were 27·2 and 30·12 kg/m2, respectively.
In the meta-analysis, we did not observe a decrease of visfatin concentrations in the n-3 fatty acid supplementation group when compared with the control group. (weighted mean difference –0·00 (95 % CI –0·05, 0·05) ng/ml; I 2 = 0 %, P for heterogeneity: 0·86; Fig. 2(c)).
Oxidative stress markers – nitric oxide
Four studies(Reference Amini, Bahmani and Foroozanfard33,Reference Jamilian, Samimib and Mirhosseinic34,Reference Mirmasoumi, Fazilati and Foroozanfard36,Reference Talari, Poladchang and Hamidian41) were included in the meta-analysis that evaluated serum levels of NO in the n-3 fatty acid group compared with a placebo. The mean follow-up time of the studies was 12 weeks, and they included 54–68 participants (mean age 27 years). The mean BMI was 26·57 kg/m2, and all studies used the Rotterdam criteria for PCOS diagnosis. Two studies(Reference Mirmasoumi, Fazilati and Foroozanfard36,Reference Talari, Poladchang and Hamidian41) used flaxseed oil in the intervention group, and the Talari et al. study co-supplemented the group with vitamin E(Reference Talari, Poladchang and Hamidian41). Another two studies used fish oil as n-3 fatty acid supplementation, and Jamilian et al. (Reference Jamilian, Samimib and Mirhosseinic34) co-supplemented with vitamin D. Three studies(Reference Amini, Bahmani and Foroozanfard33,Reference Mirmasoumi, Fazilati and Foroozanfard36,Reference Talari, Poladchang and Hamidian41) used paraffin oil as the placebo.
n-3 fatty acid supplementation did not decrease serum levels of NO in the intervention group, when compared with the placebo group (SMD –0·01 (95 % CI –0·69, 0·67) (mmol/l); I 2 = 85 %, P for heterogeneity: 0·0002; Fig. 3(a)).
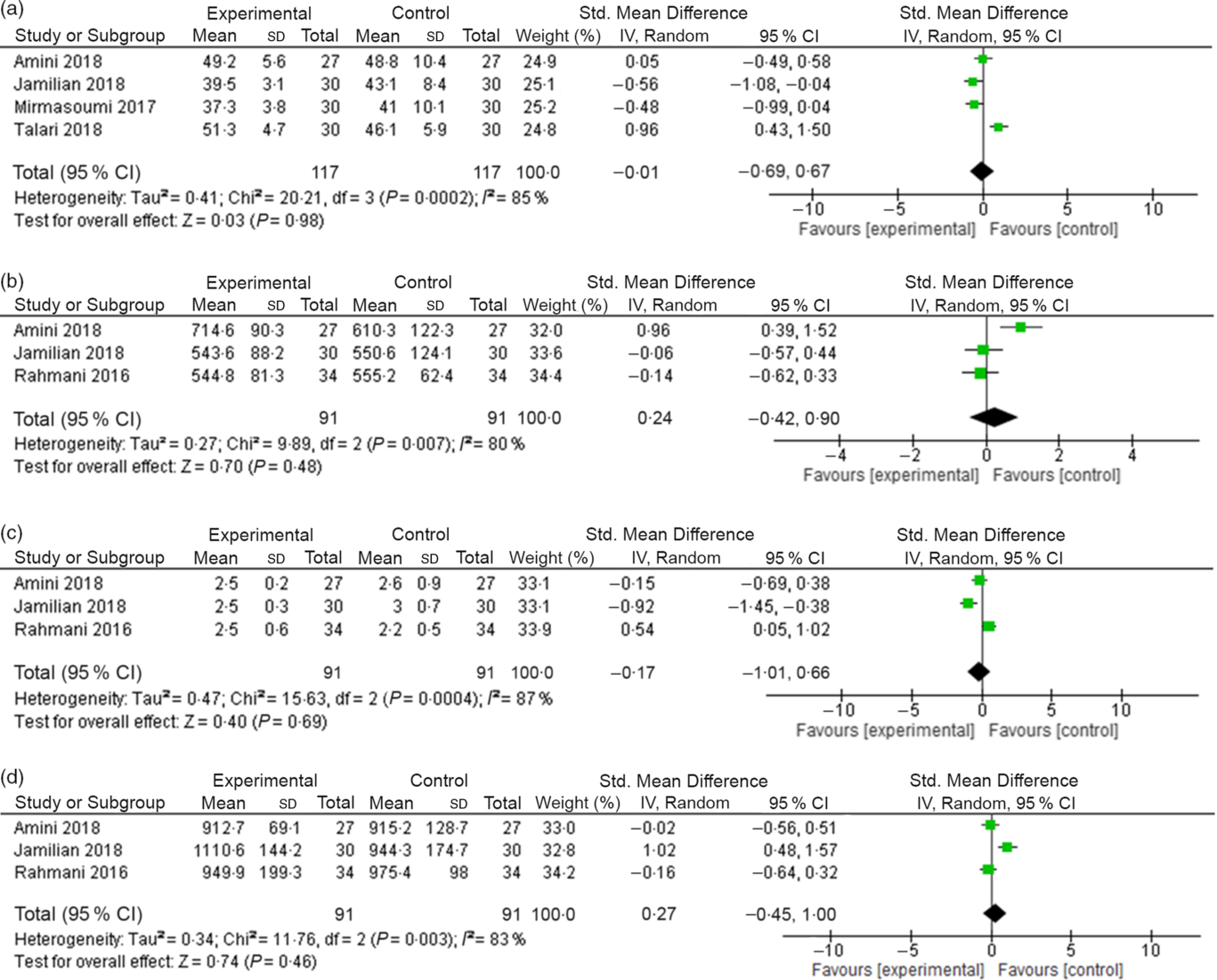
Fig. 3. (a) Change in nitric oxide (NO) (mmol/l) levels due to n-3 fatty acid supplementation. (b) Change in GSH (mmol/l) levels due to n-3 fatty acid supplementation. (c) Change in malondialdehyde (mmol/l) levels due to n-3 fatty acid supplementation. (d) Change in total antioxidant capacity (mmol/l) levels due to n-3 fatty acid supplementation.
Oxidative stress markers – GSH, malondialdehyde and total antioxidant capacity
For meta-analysis of these OS markers, three studies(Reference Amini, Bahmani and Foroozanfard33,Reference Jamilian, Samimib and Mirhosseinic34,Reference Rahmani, Samimi and Ebrahimi40) were included that evaluated the influence of n-3 fatty acid supplementation on serum levels of GSH, MDA and TAC when compared with the placebo group. The mean follow-up of the studies was 12 weeks, and they included 54–68 participants (mean age 27 years). The mean BMI was equal to 27·19 kg/m2, and all studies used Rotterdam criteria for PCOS diagnosis. Two studies used fish oil as n-3 fatty acid supplementation, and the study by Jamilian et al. (Reference Jamilian, Samimib and Mirhosseinic34) co-supplemented with vitamin D. Another study(Reference Rahmani, Samimi and Ebrahimi40) included used flaxseed oil plus vitamin E as the intervention. One study(Reference Amini, Bahmani and Foroozanfard33) reported using paraffin oil as the placebo.
We did not observe a significant decrease in serum levels of GSH, MDA or TAC in the group n-3 fatty acids when compared with the placebo group (GSH – SMD 0·24 (95 % CI –0·42, 0·90) (mmol/l); I 2 = 80 %, P for heterogeneity: 0·007; Fig. 3(b); MDA – SMD –0·17 (95 % CI –1·01, 0·66) (mmol/l); I 2 = 87 %, P for heterogeneity: 0·0004; Fig. 3(c) and TAC – SMD 0·27 (95 % CI –0·45, 1·00) (mmol/l); I 2 = 83 %, P for heterogeneity: 0·003; Fig. 3(d)).
Risk of bias and quality of the body of evidence
The risk of bias in the included studies is summarised in Fig. 4. In domain 1, regarding the process of randomisation, one study presented a high risk of bias because it did not report a randomisation process on methods. Domain 2, the risk of bias due to deviations from the intended interventions, was classified as low because no studies presented a possible negative effect of assignment to intervention. Domain 3, regarding the risk of bias due to missing outcome data, was classified as low risk related to the availability of data from 95 % of the participants in all included studies. In the risk of measuring the result – domain 4, we did not identify a likely directional bias, that is, measuring results that are not suitable for the outcomes that the authors planned to evaluate – directed to one of the interventions. For domain 5 – risk of selection of the reported result, we evaluated this domain as low risk because no individual study presented any evidence of selective reporting bias of results. Overall, eight(Reference Jamilian, Samimib and Mirhosseinic34–Reference Mirmasoumi, Fazilati and Foroozanfard36,Reference Nadjarzadeh, Dehghani-Firouzabadi and Daneshbodi38–Reference Vargas, Almario and Buchan42) studies were evaluated as some concerns related to the intervention, one study(Reference Amini, Bahmani and Foroozanfard33) was evaluated as low risk and another study(Reference Mohammadi, Rafraf and Farzadi37) as high risk, associated with evaluation of domains 1 and 2.
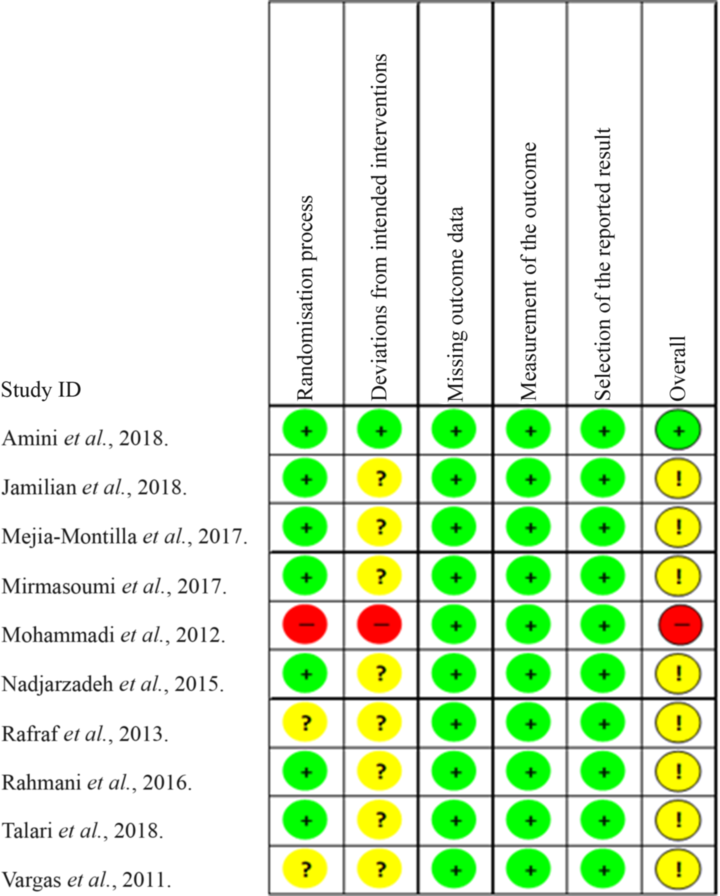
Fig. 4. Cochrane Collaboration bias risk graph. ![]() , Low risk;
, Low risk; ![]() , some concerns;
, some concerns; ![]() , high risk.
, high risk.
The quality of the body of evidence for each outcome of the current systematic review is described in online Supplementary material 4. The directness of evidence was classified as high considering the precision of the main effect estimates of interest. The results were highly heterogeneous, and no dose–response effect could be established. For one of the outcomes evaluated (inflammation biomarker changes), a clinically relevant effect of great magnitude was demonstrated. In summary, the quality of the body of evidence of this systematic review was classified as moderate.
Discussion
The present systematic review with meta-analysis of RCT analysed the role of n-3 fatty acid supplementation in PCOS patients considering plasma concentrations of inflammatory and OS markers. Intervention studies that compared n-3 fatty acid supplementation with a placebo were associated with no difference in visfatin levels nor in OS markers. However, it was observed that n-3 fatty acid supplementation significantly increased the circulating concentrations of adiponectin and decreased hs-CRP levels in PCOS patients when compared with placebo.
PCOS results in a pro-inflammatory state, and the development of metabolic dysfunction is supported by chronic low-grade inflammation. This state may be associated with the accumulation of visceral fat, glucose intolerance, IR and dyslipidaemia, all of which are presented in patients diagnosed with PCOS(Reference Ebejer and Calleja-Agius43). We observed in the present systematic review that n-3 fatty acid supplementation can reduce the levels of hs-CRP, an inflammatory maker known to be increased in women with PCOS(Reference Ebejer and Calleja-Agius43), which can predict a cardiovascular event(Reference González, Rote and Minium44). hs-CRP is an acute-phase protein produced by the liver following stimulation by IL-6, the endocrine cytokine originating from adipocytes(Reference Moshage, Roelofs and van Pelt45,Reference Ouchi, Kihara and Funahashi46) .
The mononuclear cells of women with PCOS are in an activated state, as evidenced by increased plasma hs-CRP, which may play a role in obesity(Reference González, Rote and Minium44). A meta-analysis of thirty-one articles (n 3648 women) that aimed to evaluate the status of serum inflammatory markers in women with PCOS showed that circulating hs-CRP was 96 % higher in women with PCOS compared with controls (95 % CI 71 %, 122 %, P < 0·0001) and it was associated with a high prevalence of obesity in the women with PCOS in the studies analysed(Reference Escobar-Morreale, Luque-Ramírez and González47). n-3 Fatty-acid-derived mediators have been implicated in the resolution of inflammation, with a significant protective effect by inhibition of NF-κB activity, which rises in many inflammatory diseases(Reference Kang and Weylandt48). In fact, our results corroborate the findings of a systematic review and meta-analysis that aimed to evaluate the effect of n-3 fatty acid supplementation on serum levels of inflammatory biomarkers in patients on haemodialysis. This study showed a significant decrease in serum levels of hs-CRP in the n-3 fatty acid supplementation group when compared with placebo (SMD –2·09; 95 % CI –3·62, –0·56, P < 0·05)(Reference Dezfouli, Moeinzadeh and Taheri49).
The state of low-grade inflammation is the result of the accumulation of visceral fat because this tissue is capable of producing cytokines, chemokines and other adipokines, such as adiponectin, which act, directly or indirectly, as mediators of systemic inflammation(Reference Repaci, Gambineri and Pasquali50). n-3 Fatty acid supplementation may be associated with a decrease in inflammation and in pro-inflammatory cytokine levels by stimulating the secretion of anti-inflammatory adipokines (such as adiponectin)(Reference Calder51). Moreover, these effects of n-3 fatty acids could be related to their influence on signalling pathways that regulate the expression of genes encoding pro-inflammatory cytokines(Reference Lo, Chiu and Fu52–Reference Novak, Babcock and Jho54). This regulation could be associated with NF-κB, one of the main transcription factors involved in up-regulation of the genes encoding pro-inflammatory cytokines, adhesion molecules and cyclo-oxygenase-2(Reference Babcock, Novak and Ong53). In fact, EPA decreases lipopolysaccharide-induced NF-κB activation in monocytes, and DHA reduces NF-κB activation in response to lipopolysaccharide in macrophages and dendritic cells(Reference Lo, Chiu and Fu52–Reference Novak, Babcock and Jho54). Besides, the influence of EPA and DHA on NF-κB activation involves PPAR-γ, resulting in inhibition of NF-κB activation and reduced production of the pro-inflammatory cytokines TNF and IL-6 due to lipopolysaccharide stimulation(Reference Calder51).
In women, adiponectin controls steroidogenesis of ovarian granulosa and theca cells, oocyte maturation and embryo development beyond insulin sensitising and known anti-inflammatory effects, which also modulates folliculogenesis and androgen synthesis in ovaries(Reference Barbe, Bongrani and Mellouk55). Its receptor was also found in endometrial and placental cells, suggesting that this adipokine might play a crucial role in fetal growth, trophoblast invasion and embryo implantation(Reference Barbe, Bongrani and Mellouk55). n-3 Fatty acids may improve insulin sensitivity by enhancing the production and secretion of anti-inflammatory adipokines, such as adiponectin, consequently reducing inflammation and proinflammatory cytokines(Reference Tortosa-Caparrós, Navas-Carrillo and Marín56). A previous meta-analysis that assessed the effectiveness and safety of n-3 fatty acids for patients with PCOS included nine RCT (n 591 patients) and observed that, compared with the control group, n-3 fatty acids may improve adiponectin levels (weighted mean difference 1·34; 95 % CI 0·51, 2·17; P = 0·002)(Reference Yang, Zeng and Bao25). Plasma adiponectin concentrations correlate negatively with body weight and BMI in women with or without PCOS. Moreover, hypoadiponectinaemia is associated with higher degrees of hyperinsulinaemia and IR; hence, it could be related not only to obesity but also to the metabolic alterations that characterise PCOS(Reference Ardawi and Rouzi57).
It has been reported that circulating levels of visfatin and its gene expression were increased in women with PCOS, compared with controls matched by BMI and age(Reference Dambala, Paschou and Michopoulos58). Visfatin has been identified as a protein of 52 kDa produced by the bone marrow, liver and muscle and, during pregnancy, by the epithelium of the amniotic membrane, chorionic trophoblast and decidua(Reference Dambala, Paschou and Michopoulos58). Years later, it was proven that visfatin is produced by adipocytes and has insulin-mimetic action(Reference Sun, Wu and Wei59). Visfatin has the ability to stimulate proinflammatory activity by enhancing TNF and IL-6 secretion, which further increases IR. On the other hand, the insulin-like effect of visfatin is not sufficient to counteract IR in conditions such as type 2 diabetes mellitus and PCOS, despite its high serum levels(Reference Sun, Wu and Wei59). In fact, a meta-analysis with seventeen studies (1341 subjects – 695 cases and 646 controls) showed that visfatin levels are higher in women with PCOS compared with non-PCOS controls; furthermore, the study did not indicate a correlation between high visfatin levels and BMI, homoeostatic model assessment for IR or total testosterone levels in PCOS patients when compared with controls(Reference Sun, Wu and Wei59). The changes in visfatin levels induced by n-3 fatty acids vary according to the type of dietary fat, supporting the hypothesis that visfatin up-regulation by EPA could be another mechanism by which n-3 fatty acids may improve insulin sensitivity(Reference Moreno-Aliaga, Lorente-Cebrián and Martínez60).
OS is characterised by the imbalance between the capacity of the body to neutralise free radical molecules, using antioxidant enzymes, and their production. Excessive reactive oxygen species (ROS) generation promotes inflammation by activation of redox and inflammatory signalling pathways such the NF-κB pathway(Reference Ardawi and Rouzi57). PCOS patients demonstrate OS due to hyperglycaemia, IR and chronic inflammation. Moreover, higher levels of NEFA lead to excess production of ROS(Reference Bannigida, Nayak and Vijayaragavan61). A cross-sectional study suggested that excess androgen increases the generation of ROS from leucocytes, p47phox gene expression and the formation of MDA. OS increases chronic inflammation and vice versa(Reference Deba, Jambale and Swamy62). Furthermore, OS and inflammation in the ovaries play an important role in the pathogenesis of PCOS and cause the development of atherosclerotic lesions in the ovary(Reference Chattopadhayay, Ganesh and Samanta63).
The mechanism by which OS could be reduced following n-3 fatty acid supplementation is still unclear, but it has been assumed that these effects may occur through immunomodulation and decreased leucocyte activation(Reference Mori, Puddey and Burke64,Reference Mas, Woodman and Burke65) . In this context, it is known that activated immune cells produce cytokines that consequently promote ROS generation. Moreover, EPA and DHA are effective as superoxide scavengers in an unsaturation-dependent manner, given the high unsaturation level of n-3 fatty acids(Reference Yang, Fernández-Galilea and Martínez-Fernández66).
PCOS patients have an increased risk of developing the MetS, which may be related to OS and cardiovascular events. While obesity can be a putative factor leading to the MetS, the relationship between the MetS and PCOS is attributed mainly to IR(Reference Mahalingaiah and Diamanti-Kandarakis67). A prospective controlled study evaluated whether the presence of the MetS in PCOS patients could influence endoplasmic reticulum stress markers, OS and leucocyte endothelium interaction. The data highlight that ROS production, and therefore OS, is enhanced in PCOS, and it is associated with the presence of the MetS, which can increase CVD risk. Moreover, PCOS subjects with the MetS exhibited enhanced levels of proinflammatory cytokines, and these cytokines were correlated with homoeostatic model assessment for IR, reinforcing the importance of the MetS to IR and inflammation in PCOS(Reference Bañuls, Rovira-Llopis and Martinez de Marañon68).
The n-3 fatty acids are also known to improve the TAC and various associated signalling pathways, probably suppressing lipid peroxidation, which is represented by MDA(Reference Mayyas, Alsaheb and Alzoubi69). MDA levels are significantly higher in PCOS patients and can be considered an important marker for OS(Reference Shahrokhi and Naeini70). In the present meta-analysis, no relationship was found between n-3 fatty acid supplementation and the decline of OS biomarker levels in PCOS. Another systematic review and meta-analysis that aimed to summarise the findings of RCT examining the effects of n-3 fatty acids on OS markers in healthy subjects, including thirty-nine trials (n 2875 participants), evidenced a significant increase of serum TAC and decrease of MDA in the intervention group when compared with the placebo group(Reference Heshmati, Morvaridzadeh and Maroufizadeh71). However, the study did not find significant results for NO and GSH, according to our results.
Although the literature search was conducted using multiple databases, without language restriction and following the protocol established in accordance with standardised recommendations, the present meta-analysis has some limitations. The lack of data on the actual consumption of n-3 fatty acids must be considered because it may influence inflammatory and OS markers. In addition, none of the studies included in the meta-analysis presented intention-to-treat analysis, a statistical approach that is usually associated with more conservative results. Moreover, only one study(Reference Amini, Bahmani and Foroozanfard33) showed a low risk of bias due to imprecision, suggesting that our results should have external validity.
In conclusion, the present systematic review with meta-analysis of RCT suggests that n-3 fatty acid supplementation in patients with PCOS was associated with a moderate increase in the concentrations of adiponectin and hs-CRP and no effect on the concentrations of visfatin or OS markers when compared with a placebo. Caution must be taken in interpreting these results because important sources of heterogeneity were found in the meta-analyses of n-3 fatty acid supplementation. Therefore, future RCT are necessary to confirm these findings.
Acknowledgements
This study was supported by Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) (protocol 306398/2017-6). K. B. G., F. M. R. and M. T. A. are grateful to Conselho CNPq for the research fellowship. J. A. G. T. is grateful to Coordenação de Aperfeiçoamento de Pessoal de Nível Superior - CAPES for the research fellowship.
The authors’ contributions were as follows: J. A. G. T., M. T. A. and K. B. G. designed and performed the study; J. A. G. T., M. T. A., V. E. A. and K. B. G. analysed the data; J. A. G. T., M. T. A., A. L. C., F. M. R., V. E. A. and K. B. G. wrote and reviewed the paper. All the other authors critically reviewed and improved the manuscript.
The authors declare that there are no conflicts of interest.
Supplementary material
For supplementary materials referred to in this article, please visit https://doi.org/10.1017/S0007114520003207