Introduction
With a continuous rise in human population size and industrial development, threats to ecosystem functioning and biodiversity are magnified by anthropogenic drivers around the globe (Bergmann et al. Reference Bergmann, Collard, Fabres, Gabrielsen, Provencher and Rochman2022; Callaghan et al. Reference Callaghan, Björn, Chernov, Chapin, Christensen and Huntley2004; Ims and Fuglei Reference Ims and Fuglei2005; Luck Reference Luck2007). Although the Arctic is considered one of the last pristine environments on Earth because few humans live there, it is nonetheless affected by human activities such as oil, gas, and mineral extraction (Tolvanen et al. Reference Tolvanen, Eilu, Juutinen, Kangas, Kivinen and Markovaara-Koivisto2019), and transport of pollutants to the Arctic by atmospheric or oceanic currents (Barrie Reference Barrie1986; Macdonald et al. Reference Macdonald, Barrie, Bidleman, Diamond, Gregor and Semkin2000; Zarfl and Matthies Reference Zarfl and Matthies2010). Moreover, most Arctic-nesting birds are migratory and spend the nonbreeding season in temperate or tropical regions where anthropogenic impacts are considerable (Bauer and Hoye Reference Bauer and Hoye2014; Moisan et al. Reference Moisan, Gravel, Legagneux, Gauthier, Léandri-Breton and Somveille2023). Therefore, events occurring in the temperate zone might affect breeding birds and other species living in the Arctic, with carry-over effects on the Arctic environment (Jefferies et al. Reference Jefferies, Rockwell and Abraham2004; Lamarre et al. Reference Lamarre, Legagneux, Gauthier, Reed and Bêty2017).
The Snowy Owl Bubo scandiacus, a top predator of the Arctic, is highly dependent on the abundance of small mammals, particularly lemmings (Lemmus and Dictrostonyx spp.), during the breeding season (Dorogoy Reference Dorogoy2017; Gilg et al. Reference Gilg, Sittler, Sabard, Hurstel, Sane and Delattre2006; Hagen Reference Hagen1952; Portenko Reference Portenko1972; Therrien et al. Reference Therrien, Gauthier, Pinaud and Bêty2014b). Some studies reported fading lemming population cycles and persistent low populations at some Arctic sites (Ims et al. Reference Ims, Henden and Killengreen2008; Kausrud et al. Reference Kausrud, Mysterud, Steen, Vik, Ostbye and Cazelles2008), possibly due to changing climatic conditions, which could negatively affect tundra predators like the Snowy Owl (Schmidt et al. Reference Schmidt, Ims, Høye, Gilg, Hansen and Hansen2012). Food abundance and distribution in time and space determine reproductive success and local breeding densities across its range (Robillard et al. Reference Robillard, Therrien, Gauthier, Clark and Bêty2016), so understanding the impact of humans on the habitat and food resources used by Snowy Owls is imperative for their conservation. The global population of Snowy Owls is particularly difficult to assess because the breeding range is restricted to remote circumpolar Arctic tundra and because individuals range widely during their annual dispersal and exhibit very low site fidelity (Doyle et al. Reference Doyle, Therrien, Reid, Gauthier and Krebs2017; Fuller et al. Reference Fuller, Holt, Schueck, Berthold, Gwinner and Sonnenschein2003; Robillard et al. Reference Robillard, Gauthier, Therrien and Bêty2018; Therrien et al. Reference Therrien, Gauthier, Pinaud and Bêty2014b). Furthermore, movements of individuals are often nomadic (i.e. irregular movement patterns that differ from year to year; Andersson Reference Andersson1980; Teitelbaum and Mueller Reference Teitelbaum and Mueller2019) and/or can be irruptive (i.e. migratory movements that only occur in some years and are linked to a fluctuating food supply; Newton Reference Newton2006, Reference Newton2010).
This species is classified as “Vulnerable” to extinction by the International Union for Conservation of Nature (IUCN) global Red List. IUCN assigns species to one of the nine categories of threat based on whether they meet any one of the criteria related to (1) population trend, (2) population size, and (3) structure and geographical range (IUCN 2012). The Snowy Owl was uplisted from “Least Concern” to “Vulnerable” in 2017 due to “an observed, estimated, inferred or suspected population size reduction of 30% over the last 10 years or three generations” based on reported population declines in North America (Rosenberg et al. Reference Rosenberg, Kennedy, Dettmers, Ford, Reynolds and Alexander2016) and likely in Europe and Russia (BirdLife International 2020). However, reported population declines were based on relatively informal and potentially biased assessments such as Christmas Bird Counts (National Audubon Society 2020) and eBird sightings (Fink et al. Reference Fink, Auer, Johnston, Strimas-Mackey, Robinson and Ligocki2021) and they did not quantitatively combine trend estimates from across the species’ range (BirdLife International 2024). Such methods did not exist until recently (McClure et al. Reference McClure, Rolek and Fleischer2023a,Reference McClure, Rolek and Fleischerb; Sherley et al. Reference Sherley, Winker, Rigby, Kyne, Pollom and Pacoureau2020). Further, although there are several long-term and broad-scale efforts to monitor Snowy Owls, the irruptive and nomadic ecology renders quantitative inference of population trends elusive.
This paper is an effort led by the International Snowy Owl Working Group (ISOWG) and researchers around the globe to quantitatively assess population trends and the current global status of the Snowy Owl. Based on the most recent literature we review basic life history traits and population dynamic metrics for the circumpolar population of Snowy Owls. To estimate population abundance and trends we use results from recent ecological and genetic studies and, in particular, breeding data from major monitoring sites across the Arctic. We account for the irruptive and nomadic ecology of this species while combining trends in data collected across its breeding range. Our results are interpreted in the context of the Snowy Owl’s global conservation status. We also assess threats faced by the species and conclude with recommendations to guide conservation efforts.
Distribution, ecology, and demography
The Snowy Owl has a circumpolar breeding distribution that is associated with treeless tundra and valley and plateaus across seven Arctic countries (Cramp and Brooks Reference Cramp and Brooks1985; Holt et al. Reference Holt, Larson, Smith, Evans, Parmelee and Poole2020; Portenko Reference Portenko1972). In an effort to fill in the knowledge gaps from the extant breeding range of the Snowy Owl, we compiled reported nest sites across the circumpolar Arctic over the last 50 years from the Arctic Monitoring and Assessment Program (1990–2018; n = 150 nests), eBird (1971–2022; n = 75 nests), GPS-tagged owls (2016–2021; – n = 19 nests), and nest locations from collaborators and from seven long-term monitoring sites (1987–2020; n = 560 nests) (Figure 1). Although uncommon, several long-term studies have or are still monitoring annual breeding density and productivity including sites in Alaska, Canada, Greenland, Fennoscandia, and Russia (Figure 1, Supplementary material Appendix S1). In North America, Snowy Owls still regularly breed in Alaska, Nunavut, and the northern parts of Yukon, Northwest Territories, and Québec (Doyle et al. Reference Doyle, Therrien, Reid, Gauthier and Krebs2017; Holt et al. Reference Holt, Maples, Petersen-Parret, Korti, Seidensticker and Gray2009; Miller et al. Reference Miller, Russell and Gunn1975; Therrien et al. Reference Therrien, Gauthier, Pinaud and Bêty2014b). In Greenland, Snowy Owls now seem restricted to breed only in the north-east and very few breeding attempts were recorded in recent decades, associated with irregular lemming population peaks (Gilg et al. Reference Gilg, Sittler, Sabard, Hurstel, Sane and Delattre2006, Reference Gilg, Sittler and Hanski2009). In Fennoscandia, Snowy Owls regularly bred when lemming peaks were steady, until the late 1980s (Ehrich et al. Reference Ehrich, Schmidt, Gauthier, Alisauskas, Angerbjörn and Clark2020; Jacobsen Reference Jacobsen2005). After a long period without lemming peaks and breeding owls, nests were found again in 2007, 2011, and 2015 (Jacobsen et al. Reference Jacobsen, Øien, Solheim and Aarvak2014; Øien et al. Reference Øien, Jacobsen, Aarvak, Solheim and Kleven2016), but rodent numbers might have collapsed again after the absence of a peak around 2019 (Jacobsen et al. Reference Jacobsen, Solheim, Øien and Aarvak2019). In Russia, Snowy Owls have been recorded breeding from the Yugorsky Peninsula, Novaja Zemlya, and Vaygach Island in north-east Europe to the Taimyr Peninsula in Siberia (Kharitonov et al. Reference Kharitonov, Volkov, Willems, Van Kleef, Klaassen and Nowak2008; Morozov et al. Reference Morozov, Rosenfeld, Rogova, Golovnyuk, Kirtaev and Kharitonov2020) and in eastern Siberia, the Lena delta, Anzhu Islands, and Wrangel Island (Litvin and Baranyuk Reference Litvin and Baranyuk1989; Menyushina Reference Menyushina1997, Reference Menyushina and Gruzdev2007). The longest running discrete study sites monitoring breeding activities, spanning the last ~30 years, include: Wrangel Island, Russia; Utqiagvik (formerly Barrow), Alaska, USA; Bylot Island, Nunavut, Canada; Fennoscandia; Karupelv Valley, Greenland.

Figure 1. Snowy Owl Bubo scandiacus confirmed breeding sites (red dots) within the known breeding range (grey outline; BirdLife International and Handbook of the Birds of the World 2021) across the circumpolar Arctic. Breeding sites include nests reported to the Arctic Monitoring and Assessment Program (1990–2018; n = 150), eBird (1971–2022; n = 75), from GPS-tagged owls (2016–2021; n = 19), and nest locations from collaborators and from seven long-term monitoring sites in Russia, USA, Canada, Greenland, and Fennoscandia (1987–2020; n = 560). Graphs show the annual number of Snowy Owl nests (y-axis) found at the five monitoring sites between 1988 and 2020.
During the nonbreeding season, the distribution expands to include the entire breeding range, coastal Arctic Sea ice, and some temperate regions south of the boreal forest and Arctic tundra (Holt et al. Reference Holt, Larson, Smith, Evans, Parmelee and Poole2020; Øien et al. Reference Øien, Aarvak, Jacobsen and Solheim2018; Portenko Reference Portenko1972; Therrien et al. Reference Therrien, Weidensaul, Brinker, Huy, Miller and Jacobs2017).
The Snowy Owl is a nomadic species exhibiting long-distance breeding dispersal annually (Therrien et al. Reference Therrien, Gauthier, Pinaud and Bêty2014b) with unpredictable and highly variable movements when searching for a suitable nesting site (Holt et al. Reference Holt, Larson, Smith, Evans, Parmelee and Poole2020; Therrien et al. Reference Therrien, Gauthier, Pinaud and Bêty2014b; Robillard et al. Reference Robillard, Gauthier, Therrien and Bêty2018). Satellite tracking of Snowy Owls in Canada indicated that individuals cover large distances (
![]() $ \overline{\mathrm{x}}\hskip0.24em $
= 828 ± 600 [SD] km, range 220–2433 km, n = 9 adult females) in spring when prospecting for potential nesting sites with sufficient prey resources (Therrien et al. Reference Therrien, Gauthier, Pinaud and Bêty2014b). After a nesting season, adult Snowy Owls radio-tagged in north-western Alaska flew west, ranging widely in far north-eastern Russia, then wintering proximate to northern Russia coastlines. Ultimately, these individuals returned to Alaska, flying past their previous nesting area and into the north-western Canadian islands for the next breeding season (Fuller et al. Reference Fuller, Holt, Schueck, Berthold, Gwinner and Sonnenschein2003). Satellite-tagged adult Snowy Owls breeding in Norway wandered back and forth between Norway and Russia, sometimes flying as far east as the Taimyr Peninsula in western Siberia during low lemming years, presumably searching for suitable nesting sites (Jacobsen et al. Reference Jacobsen, Solheim, Øien and Aarvak2010, Reference Jacobsen, Øien, Solheim and Aarvak2014; Øien et al. Reference Øien, Aarvak, Jacobsen and Solheim2018; Solheim et al. Reference Solheim, Jacobsen and Øien2008).
$ \overline{\mathrm{x}}\hskip0.24em $
= 828 ± 600 [SD] km, range 220–2433 km, n = 9 adult females) in spring when prospecting for potential nesting sites with sufficient prey resources (Therrien et al. Reference Therrien, Gauthier, Pinaud and Bêty2014b). After a nesting season, adult Snowy Owls radio-tagged in north-western Alaska flew west, ranging widely in far north-eastern Russia, then wintering proximate to northern Russia coastlines. Ultimately, these individuals returned to Alaska, flying past their previous nesting area and into the north-western Canadian islands for the next breeding season (Fuller et al. Reference Fuller, Holt, Schueck, Berthold, Gwinner and Sonnenschein2003). Satellite-tagged adult Snowy Owls breeding in Norway wandered back and forth between Norway and Russia, sometimes flying as far east as the Taimyr Peninsula in western Siberia during low lemming years, presumably searching for suitable nesting sites (Jacobsen et al. Reference Jacobsen, Solheim, Øien and Aarvak2010, Reference Jacobsen, Øien, Solheim and Aarvak2014; Øien et al. Reference Øien, Aarvak, Jacobsen and Solheim2018; Solheim et al. Reference Solheim, Jacobsen and Øien2008).
These extensive annual movements and low breeding site fidelity are in line with results from genetic analyses. Marthinsen et al. (Reference Marthinsen, Wennerberg, Solheim and Lifjeld2009) analysed the mitochondrial DNA of Snowy Owls from North America, Fennoscandia, and eastern Russia and found no phylogeographic genetic structure, suggesting a single panmictic population with unrestricted exchange of genetic material. More recently, Gousy-Leblanc et al. (Reference Gousy-Leblanc, Therrien, Broquet, Rioux, Curt-Grand-Gaudin and Tissot2023) investigated genetic differentiation using single nucleotide polymorphisms (SNPs)-based analyses of owls sampled across North America and found high genetic intermixing indicating a single Snowy Owl population within the continent.
Studies in Greenland and Nunavut, Canada, have both shown that Snowy Owls do not attempt to breed unless there is a consistent threshold density of about 2 lemmings/ha at snow melt (Gilg et al. Reference Gilg, Sittler, Sabard, Hurstel, Sane and Delattre2006; Therrien et al. Reference Therrien, Gauthier, Pinaud and Bêty2014b). However, a study in north-western Taimyr, Russia found Snowy Owls started nesting only when lemming abundance reached approximately 11 lemmings/ha (Kharitonov et al. Reference Kharitonov, Volkov, Willems, Van Kleef, Klaassen and Nowak2008). Telemetry studies confirmed little fidelity to a breeding site and revealed that Snowy Owls tend to nest wherever prey is available, regardless of the conditions at their previous breeding site (Doyle et al. Reference Doyle, Therrien, Reid, Gauthier and Krebs2017; Fuller et al. Reference Fuller, Holt, Schueck, Berthold, Gwinner and Sonnenschein2003; Watson Reference Watson1957). For example, Therrien et al. (Reference Therrien, Gauthier, Pinaud and Bêty2014b) found that adult females breeding in the Canadian Arctic dispersed on average 725 ± 517 [SD] km (range: 18–2,224 km, n = 12) between consecutive years, indicating a general lack of site fidelity. However, some Snowy Owls in Norway have appeared on the same breeding grounds as during former lemming peak several years previously (Jacobsen et al Reference Jacobsen, Øien, Solheim and Aarvak2011; Solheim et al Reference Solheim, Jacobsen and Øien2008). These large breeding dispersal distances impose strong limitations on our ability to reliably estimate population size worldwide as detailed below.
The exact age of sexual maturity is mostly unknown, especially in the wild, but Snowy Owls may reach sexual maturity in the first year of life (Holt et al. Reference Holt, Larson, Smith, Evans, Parmelee and Poole2020). Although Snowy Owls are thought to usually start breeding around three or four years old in the wild, one-year-old females were confirmed breeding in Norway in 2011 and 2015 (Solheim et al. Reference Solheim, Jacobsen, Øien and Aarvak2018). Experienced breeding females can apparently breed every year even if they need to move long distances to find a suitable nesting area (Therrien et al. Reference Therrien, Gauthier and Bêty2012).
Long-term nest monitoring suggests there might be core and peripheral breeding populations throughout the Arctic. Breeding densities under good conditions reported by Gilg et al. (Reference Gilg, Sittler, Sabard, Hurstel, Sane and Delattre2006) and Therrien et al. (Reference Therrien, Gauthier, Korpimaki and Bêty2014a,Reference Therrien, Gauthier, Pinaud and Bêtyb) can be up to 20 nests/100 km2. Falling within the “boom-and-bust” breeding strategy (Newton Reference Newton2006), Snowy Owls lay large clutches (e.g. 5–11 eggs,
![]() $ \overline{x} $
= 7.0 ± 2.1 eggs; Hagen Reference Hagen1960; Holt et al. Reference Holt, Larson, Smith, Evans, Parmelee and Poole2020; Portenko Reference Portenko1972; Therrien et al. Reference Therrien, Gauthier, Robillard, Lecomte and Bêty2015) during years when the abundance of small mammals in the Arctic tundra is high (i.e. “boom” years) (Robillard et al. Reference Robillard, Therrien, Gauthier, Clark and Bêty2016). In a 20-year study investigating the links between lemming abundance and Snowy Owl breeding success on Bylot Island, Therrien et al. (Reference Therrien, Gauthier, Robillard, Lecomte and Bêty2015) reported a high nesting success (96%; proportion of nests where at least one young survived until fledging) during years of high lemming density, similar to findings by Gilg et al. (Reference Gilg, Sittler, Sabard, Hurstel, Sane and Delattre2006) from north-east Greenland.
$ \overline{x} $
= 7.0 ± 2.1 eggs; Hagen Reference Hagen1960; Holt et al. Reference Holt, Larson, Smith, Evans, Parmelee and Poole2020; Portenko Reference Portenko1972; Therrien et al. Reference Therrien, Gauthier, Robillard, Lecomte and Bêty2015) during years when the abundance of small mammals in the Arctic tundra is high (i.e. “boom” years) (Robillard et al. Reference Robillard, Therrien, Gauthier, Clark and Bêty2016). In a 20-year study investigating the links between lemming abundance and Snowy Owl breeding success on Bylot Island, Therrien et al. (Reference Therrien, Gauthier, Robillard, Lecomte and Bêty2015) reported a high nesting success (96%; proportion of nests where at least one young survived until fledging) during years of high lemming density, similar to findings by Gilg et al. (Reference Gilg, Sittler, Sabard, Hurstel, Sane and Delattre2006) from north-east Greenland.
The Snowy Owl is a regular wintering bird in certain areas of the USA and Canada (Wiebe et al. Reference Wiebe, Bidwell and McCabe2023). Numbers of owls wintering on the Canadian Prairies are relatively stable despite some annual variation (Boxall and Lein Reference Boxall and Lein1982; Kerlinger et al. Reference Kerlinger, Lein and Sevick1985), but much lower and more variable in areas farther south, west, and east, where the species is more irregular and irruptive (Kerlinger and Lein Reference Kerlinger and Lein1988; Kerlinger et al. Reference Kerlinger, Lein and Sevick1985). Many adults remain in the Arctic throughout the winter, exploiting marine environments in ice-covered areas (Fuller et al. Reference Fuller, Holt, Schueck, Berthold, Gwinner and Sonnenschein2003; Øien et al. Reference Øien, Aarvak, Jacobsen and Solheim2018; Robillard et al. Reference Robillard, Gauthier, Therrien, Fitzgerald, Provencher and Bêty2017, Reference Robillard, Gauthier, Therrien and Bêty2018; Therrien et al. Reference Therrien, Gauthier and Bêty2011). In western North America, many individuals also remain in the Arctic but primarily use alpine non-forest areas (Doyle et al. Reference Doyle, Therrien, Reid, Gauthier and Krebs2017). Thus, Snowy Owls can use a wide variety of habitats (i.e. inland, coastal, oceans, alpine areas) during the nonbreeding season. In addition to the diversity of wintering habitats, there is also a dramatic change in overwintering population composition over time. Indeed, the high reproductive output during a good lemming year (Gilg et al. Reference Gilg, Sittler, Sabard, Hurstel, Sane and Delattre2006; Therrien et al. Reference Therrien, Gauthier, Korpimaki and Bêty2014a) means local individual density can increase dramatically at the end of those breeding seasons. This will cause a large influx of young of the year into the regular and irregular wintering ranges south of the boreal forest and explain the periodic irruptions of the species (Robillard et al. Reference Robillard, Therrien, Gauthier, Clark and Bêty2016; Santonja et al. Reference Santonja, Mestre, Weidensaul, Brinker, Huy and Smith2019).
Based on satellite tracking of 12 adult females marked in the Canadian Arctic, Therrien et al. (Reference Therrien, Gauthier and Bêty2012) found that the survival rate was relatively high over three years (estimated annual survival at 85–92%). Using capture–recapture of owls marked on the Canadian Prairies in winter over 15 years, Heggøy et al. (Reference Heggøy, Aarvak, Øien, Jacobsen, Solheim and Zazelenchuk2017) estimated apparent annual survival of adults (n = 13) at 70.4 ± 8.6%, which is probably biased low by permanent emigration. McCabe et al. (Reference McCabe, Therrien, Wiebe, Gauthier, Brinker and Weidensaul2022) estimated winter survival (4.5 month-long period) at 93% for adult females in temperate regions in North America and 98% for those wintering in the Arctic (n = 144 owl-winters). Survival of owls wintering in the Prairies (94%) was also greater than those wintering in eastern North America (81%) (n = 252 owl-winters), and winter survival estimates of first-year owls in non-irruptive years (100%) were also greater than in irruptive years (52%) (n = 93 owl-winters).
Historical population estimates and trends
Given the high mobility and apparent lack of fidelity to nesting areas, reliable estimates of population size throughout the circumpolar range are hard to obtain with traditional nesting density surveys. Previous population estimates, which were as high as 290,000 individuals worldwide (Rich et al. Reference Rich, Beardmore, Berlanga, Blancher, Bradstreet and Butcher2004) are now considered gross overestimates because they relied on a misconception that Snowy Owls bred regularly and uniformly across their entire breeding range and ignored large-scale annual breeding dispersal movements combined with low breeding site fidelity. An updated range map depicting at a finer scale the boundaries of the Snowy Owl range (i.e. breeding, nonbreeding, pre- and post-breeding migratory seasons) (Fink et al. Reference Fink, Auer, Johnston, Strimas-Mackey, Robinson and Ligocki2021; https://ebird.org/science/status-and-trends/snoowl1/) in North America portrays a more fragmented and restricted range than historical range maps. Other regions throughout the circumpolar Arctic (e.g. inland Greenland, some islands in Nunavut, Canada) are covered by ice caps or devoid of lemmings, suggesting a smaller range than historically presented.
Recently, various approaches were suggested to estimate Snowy Owl population size. Potapov and Sale (Reference Potapov and Sale2012) suggested a “loose boid” approach – estimating the probability of aggregations of Snowy Owls in a particular area and integrating this spatial probability for the entire range. This approach used historical breeding data from the literature, unpublished continental transects and aerial surveys, migration patterns, and observations reported by the Arctic Wader Study Group. With this approach, they estimated 14,000 pairs of Snowy Owls worldwide. Considering that the total global population can fluctuate with good or poor breeding conditions depending on the year, Potapov and Sale (Reference Potapov and Sale2012) suggested that a more conservative population estimate would be half of that estimate (7,000–8,000 pairs). However, many assumptions behind this approach (e.g. size and number of individual boids) remain vague and untested.
In northern Russia, an effort estimated population size based on counts conducted along aerial transects totalling 17,854 km in length in western Siberia in 2019 (Morozov et al. Reference Morozov, Rosenfeld, Rogova, Golovnyuk, Kirtaev and Kharitonov2020). Snowy Owls were detected within a 3,792 km2 area along these transects in the Taimyr and Yamal regions with 221 (217 and 4, respectively) individuals counted. Simultaneously, researchers monitored two sites on the ground in Taymir peninsula (130 and 120 km2). Based on the number of owls and nests counted (217 and 9, respectively) over the surveyed area, the authors estimated the current population for the whole Russian Arctic at 14,000 individuals. They also suggested that numbers might have declined since similar counts were conducted in the same region in the mid-1990s.
An alternative approach is to calculate a theoretical carrying capacity of the tundra habitat for breeding owls. Walker et al. (Reference Walker, Raynolds, Daniels, Einarsson, Elvebakk, Gould, Katenin, Kholod, Markon, Melnikov, Moskalenko, Talbot and Yurtsev2005) estimated the size of the non-glaciated Arctic tundra biome at 5,000,000 km2. If we assume (1) that only 20% of this area is suitable for owl nesting, a conservative estimate, and (2) that owls breed only in good lemming years, which have a typical recurrence of about four years (Gauthier et al. Reference Gauthier, Ehrich, Belke-Brea, Domine, Alisauskas and Clark2024), this means that about 250,000 km2 of Arctic tundra may offer suitable breeding conditions for owls every year. We can further assume that experienced owls breed every year (Therrien et al. Reference Therrien, Gauthier and Bêty2012) by moving to areas of high lemming abundance (Therrien et al. Reference Therrien, Gauthier, Pinaud and Bêty2014b). Finally, using a mean density of 0.1 pairs/km2 in good breeding years (Gilg et al. Reference Gilg, Sittler, Sabard, Hurstel, Sane and Delattre2006; Therrien et al. Reference Therrien, Gauthier, Pinaud and Bêty2014b), we could estimate that up to 25,000 pairs could breed annually worldwide (G. Gauthier, personal communication).
Genetic analyses can also provide information on the effective population size (N e, the number of breeding individuals in an idealised population that would maintain genetic variability; Lande and Barrowclough Reference Lande, Barrowclough and Soulé1987) of a species. Based on mitochondrial DNA analyses, Marthinsen et al. (Reference Marthinsen, Wennerberg, Solheim and Lifjeld2009) estimated the maximum effective population at 14,000 females worldwide. More recently, Gousy-Leblanc et al. (Reference Gousy-Leblanc, Therrien, Broquet, Rioux, Curt-Grand-Gaudin and Tissot2023) estimated the current North American effective population to be 15,792 individuals (10,850–28,950; 95% confidence interval (]CI]), using genetic methods based on nuclear SNP.
Butcher and Niven (Reference Butcher and Niven2007) found that Snowy Owls have undergone a small, statistically insignificant decline during the last 40 years in North America by combining data from Breeding Bird Surveys and Christmas Bird Counts. However, because these monitoring schemes cover a limited part of the wintering range of the species, they can likely miss the long-term trends of a nomadic species like the Snowy Owl. In a global analysis of North American bird fauna, Rosenberg et al. (Reference Rosenberg, Kennedy, Dettmers, Ford, Reynolds and Alexander2016) reported a 64% decline of Snowy Owls in North America over the period 1970 to 2014. However, the value is most likely inflated as this analysis may be mixing previous population estimates, which were considered overestimates (see above), with more recent ones. It is however clear that, since the Last Glacial Maximum 20,000 years ago, the North American Snowy Owl population has undergone a slow decline interspaced with accelerated decreases during warming events (Gousy-Leblanc et al. Reference Gousy-Leblanc, Therrien, Broquet, Rioux, Curt-Grand-Gaudin and Tissot2023). Since the genetic analysis used in that study cannot determine the trend in the last hundred years, we still must rely on alternative estimates such as long-term monitoring at their breeding sites (see below).
Current population dynamics and trend analyses
To estimate recent population trends of Snowy Owls, we used count data of nests from five of the seven long-term sites (i.e. Bylot Island Core, Fennoscandia, Karupelv Valley, Utqiagvik, and Wrangel Island) monitored annually (with one exception) during the breeding seasons of 1988–2020 (Figure 1, Appendix S1). Two other sites with shorter time-series (Igloolik and Hochstetter Forland) were excluded from the analyses as we required >10 years of monitoring for the trend analysis (see below for details). We assumed that the number of nests counted, instead of individuals, was a good proxy for the IUCN (2012) criteria considering each nest needs two mature individuals. We then used these results to assess the status of the species according to IUCN Red List Criteria (IUCN 2012).
A potential problem of our trend analysis is the cyclical pattern of owl presence at several sites (Figure 1), a consequence of the cyclicity in the populations of their main prey species, lemmings (Gauthier et al. Reference Gauthier, Ehrich, Belke-Brea, Domine, Alisauskas and Clark2024). To evaluate whether linear regression models could accurately estimate population trends when ignoring cyclicity, we simulated data and applied generalised linear models (GLMs) within a frequentist framework. Simulations indicated little bias and good coverage by CIs of estimated temporal trends, especially when time-series were >10 years and mean abundance was >0.5 (methods and results are presented in Appendix S2). Based on simulation results, we excluded sites not meeting these criteria (Igloolik and Hochstetter Forland).
We analysed Snowy Owl nest count data using a Bayesian hierarchical GLM, adjusting existing methodology (McClure et al. Reference McClure, Rolek and Fleischer2023a,Reference McClure, Rolek, Kemp and Wolterb) by implementing various distributional assumptions (Poisson, negative binomial, and zero-inflated Poisson; see detailed methods in Appendix S3) to account for an excess of zeros in the counts, which is typical of an irruptive species (Figure 1). We retained the distribution with the best goodness-of-fit. To obtain an overall population growth rate (λ) that was informed by these five sites while accounting for population size at each site, we calculated a weighted mean from posterior draws of abundance so that the contribution of each site was proportional to average counts at those sites (McClure et al. Reference McClure, Rolek and Fleischer2023a). Further, we did not need to account for differing areas surveyed among sites because our analysis was concerned with rates of change, not absolute numbers. The areas surveyed within four of the five monitoring sites (Fennoscandia is the exception) remained constant through time allowing us to interpret observed changes in counts as indices of population change. We converted population growth rates to per cent change over three generations using generation times of 8 and 10.7 years (i.e. 24 years and 32 years; see details in Appendix S3) to present results in the context of the IUCN Red List Criteria A2 to assess the conservation status of a species (IUCN 2012). Based on the length of our monitoring and the median annual per cent change detected, our analysis should have a reasonable statistical power according to White (Reference White2019) and Wauchope et al. (Reference Wauchope, Amano, Sutherland and Johnston2019).
Population growth rates averaged over the entire survey period (1988–2020) suggested negative trends (median λ = 0.98, 80% Highest Density Intervals [HDIs] = [0.96, 1.01], probability of direction [pd] = 0.80). Inter-annual population growth rates had significant declines during six years (2005, 2011, 2012, 2015, 2016, and 2018) (Figure 2) with 80% HDIs excluding zero. Trends at individual sites were stable except at Utqiagvik, which had a declining population trend (median = -0.73, 80% HDIs = [-1.50, -0.04]; Appendix S4).
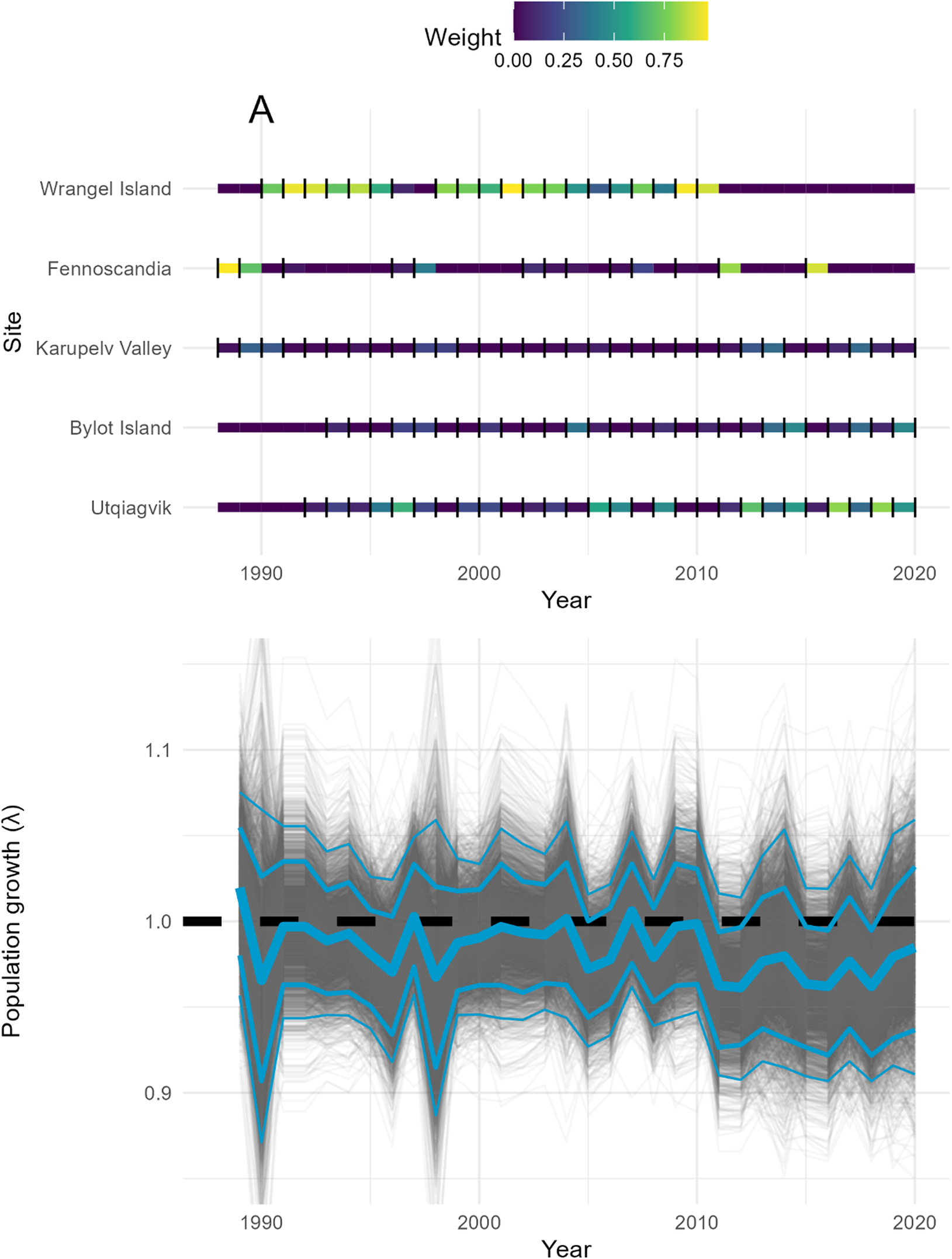
Figure 2. (A) Proportional weights assigned to each site to estimate the inter-annual population growth rate of Snowy Owls Bubo scandiacus. (B) Inter-annual population growth rates (λ) of Snowy Owls combining five long-term monitoring sites (Wrangel Island, Fennoscandia, Karupelv Valley, Bylot Island Core, and Utqiagvik). The median is depicted with a thick blue solid line, while the 80% and 95% highest density prediction intervals are depicted with medium and thin blue lines, respectively. Predictions from each posterior draw are depicted with grey lines (n = 4,000). A horizontal dashed line where λ = 1.0 depicts a stable population. Monitoring data spanned different time intervals for each site; therefore, we weighted these population growth rates so the contribution of each site is proportional to its population size (details in Appendix S3).
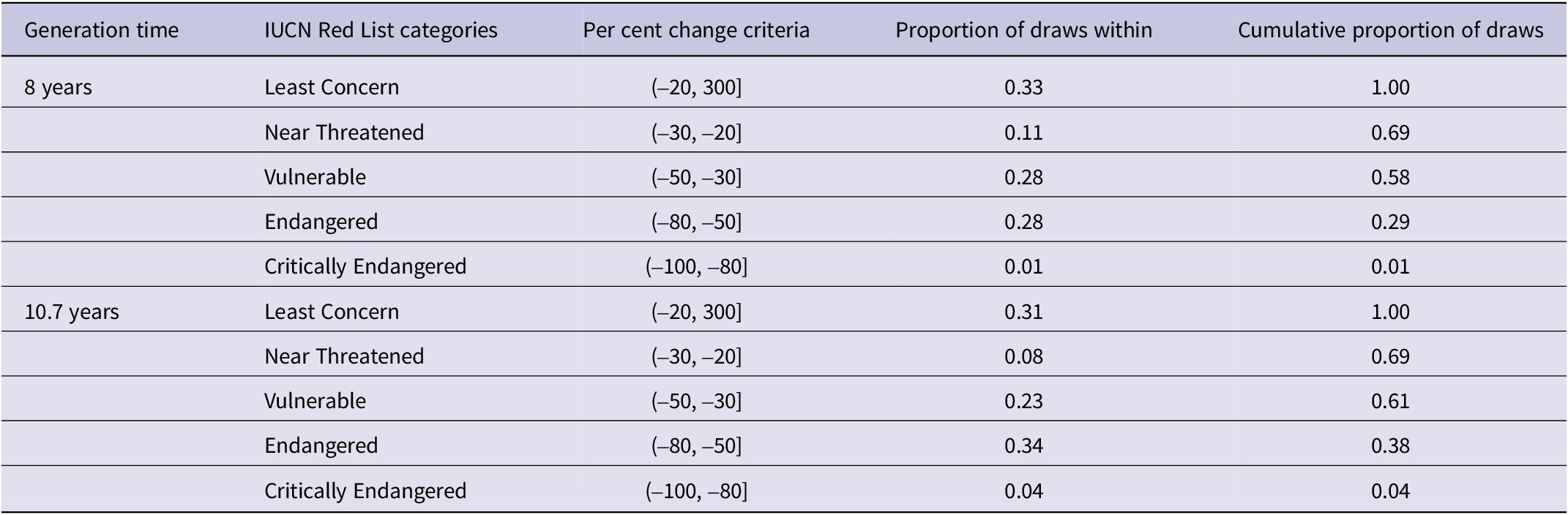
Population trends expressed as per cent change after three generations showed a significant negative trend using eight-year generations (median = -35.6%, 80% HDIs = [-74.9%, -1.2%], pd = 0.81), and a non-significant trend using 10.7-year generations (median = -41.0%, 80% HDIs = [-84.2%, 3.5%], pd = 0.80). Using eight-year generations, central tendencies of per cent change over three generations such as the mode (-45%) and median (-36%) all suggest that Snowy Owl should be considered Vulnerable according to the IUCN Red List Criteria, despite some uncertainty (Figure 3). Sixty-nine per cent of derived posterior draws of per cent change suggested “Near Threatened” status or worse, while 58% suggested “Vulnerable” or worse, and 29% suggested “Endangered” or worse (see Cumulative proportion of draws, Table 1). Using 10.7-year generations, central tendencies of per cent change over three generations such as the mode (-54%) and median (-41%) suggest that Snowy Owl should be considered Vulnerable or Endangered according to the IUCN Red List Criteria despite some uncertainty (Figure 3). Sixty-nine per cent of derived posterior draws of per cent change suggested Near Threatened status or worse, while 61% suggested Vulnerable or worse, and 38% suggested Endangered or worse (see Cumulative proportion of draws, Table 1).

Figure 3. Per cent change in the number of breeding Snowy Owls Bubo scandiacus at five monitoring sites in the Arctic over three generations using two generation times (8 and 10.7 years). Per cent change beginning in (A) 1996 and (B) 1988 as reference years to assess changes over three generations (24 and 32 years, respectively). Black solid lines depict the median, 80% and 95% highest density intervals (HDIs), and thin grey lines depict predictive posterior draws from the model (n = 4,000). (C) and (D) depict the total per cent change over three generations (1996–2020). The caterpillar plot depicts the median (point), 80% and 95% HDIs (vertical lines), and the grey polygon depicts density of estimates. Colours (dark green to red) illustrate IUCN Red List Criteria A2.
Table 1. Proportion of draws associated with different percentages of change in the number of breeding Snowy Owls Bubo scandiacus over three generations (1996–2020) at five monitoring sites in the Arctic. “Proportion of draws within” contains the proportion of posterior draws (n = 4,000) falling within each interval of IUCN Listing Criteria (“IUCN criteria”). “Cumulative proportion of draws” contains the cumulative sum of draws within each criterion and worse. “Per cent change criteria” describes IUCN Red List Criteria of per cent change over three generations. Square brackets indicate a value is included in the interval for IUCN criteria, while round parenthesis indicate the value is not included.
To evaluate the robustness of analyses to trends at individual sites, we omitted data from Utqiagvik, the only site with a significant decline. Trends were largely consistent with analyses presented here. Central tendencies of per cent change (median = -16% and mode = -41%) continued to suggest declines (Appendix S4). We implemented another analysis to evaluate robustness by including data from all sites with a random effect for site. This analysis largely agreed with those presented here suggesting declines (median = -25% and mode = -36%, Appendix S4. Combined, these two analyses demonstrate that results presented here are somewhat robust to the inclusion or exclusion of sites.
Main threats
Main potential threats to the Snowy Owl include: (a) climate change; (b) change in the abundance of prey species; (c) anthropogenic development and disturbance on the breeding and wintering areas; (d) anthropogenic mortality; (e) exposure to contaminants in the environment. We briefly address each of those separately below.
Mild winter temperatures and extended rainy periods associated with climate change will likely alter snow cover, stability of the microclimate during the breeding season, and plant growth, thereby negatively affecting the population cycles of lemmings and other rodents, the main food source of Snowy Owls during breeding (Domine et al. Reference Domine, Gauthier, Vionnet, Fauteux, Dumont and Barrère2018; Gilg et al. Reference Gilg, Sittler and Hanski2009; Kausrud et al. Reference Kausrud, Mysterud, Steen, Vik, Ostbye and Cazelles2008; Kharitonov et al. Reference Kharitonov, Bublichenko, Korkina, Volkov, Morozov and Sharikov2005). Therefore, a collapse in lemming populations would have a devastating effect on Snowy Owl reproduction, as reported in some regions of Greenland and Fennoscandia (Jacobsen et al. Reference Jacobsen, Solheim, Øien and Aarvak2019; Schmidt et al. Reference Schmidt, Ims, Høye, Gilg, Hansen and Hansen2012). However, while local and short-term cycle collapses can occur, there is yet little evidence that lemming population fluctuations have dampened globally across the Arctic or that populations are decreasing (Ehrich et al. Reference Ehrich, Schmidt, Gauthier, Alisauskas, Angerbjörn and Clark2020; Gauthier et al. Reference Gauthier, Ehrich, Belke-Brea, Domine, Alisauskas and Clark2024). Milder and wetter climates in the Arctic could increase the risk for detrimental black fly attacks on nestlings and breeding females in the low Arctic tundra (Lamarre et al. Reference Lamarre, Legagneux, Franke, Casajus, Currie and Berteaux2018; Solheim et al. Reference Solheim, Jacobsen, Øien, Aarvak and Polojärvi2013). Snowy Owls have also been documented hunting at the flow edge during the breeding season (N. Lecomte, personal communication) and along open channels in the sea ice during the nonbreeding season where large concentrations of waterfowl overwinter (Fuller et al. Reference Fuller, Holt, Schueck, Berthold, Gwinner and Sonnenschein2003; Gilchrist and Robertson Reference Gilchrist and Robertson2000; Hagen Reference Hagen1952; Therrien et al. Reference Therrien, Gauthier and Bêty2011). However, the ability for the Snowy Owl to efficiently capture waterfowl may decline if an expansion of these channels, as a result of climate change, disperse prey over a larger area, therefore reducing their concentrations and the availability of perching sites on the ice.
Survival, especially in first-year birds, is often influenced by prey availability and, in winter, the ability to avoid both natural and anthropogenic threats (e.g. disease, collisions with vehicles). Although we lack information to quantify survival rate during the post-fledgling and dispersal stages of juveniles, studies of other owl species provide insight. For example, Rohner and Hunter (Reference Rohner and Hunter1996) found that juvenile survival in Great Horned Owls Bubo virginianus in the Yukon, Canada, was just over 40% during the post-fledgling stage, with major causes of death including anaemia (33%), predation (28%), and collision with vehicles (15%). Similarly, fledgling mortality of northern Tawny Owls Strix aluco was highest just after leaving the nest and continued until dispersal, with starvation and predation being the primary causes of death (Overskaug et al. Reference Overskaug, Bolstad, Sunde and Øien1999).
Based on necropsies, the main causes of death identified for 383 wintering Snowy Owls in eastern North America were automobile collisions (18%), emaciation (16%), airplane collisions (9%), other types of collisions (8%), disease or parasites (6%), and electrocution (3%) (McCabe et al. (Reference McCabe, Therrien, Wiebe, Gauthier, Brinker and Weidensaul2022). In temperate western Canada, causes of death examined by Kerlinger and Lein (Reference Kerlinger and Lein1988) (n = 76 owls) and Chang and Wiebe (Reference Chang and Wiebe2016) (n = 225 owls) included various collisions with cars, powerlines, airplanes, and unknown objects (66% and 42%, respectively), emaciation (14% and 46%), shooting (13%), electrocution (6%), and entanglement in fishing equipment (<2%). The impact of wind turbines and the risks of collisions associated with these structures is unknown, but Snowy Owls are known to frequently use the highest available point in the terrain to perch and hunt (Solheim et al. Reference Solheim, Øien, Aarvak and Jacobsen2021), so wind turbines could be a potential threat.
Environmental pollutants examined in feathers of breeding birds (n = 5) collected in Finnmark, Norway in 2007 did not reveal elevated levels of pollutants although seven years earlier, analyses of feathers from a male Snowy Owl found dead in Norway showed elevated levels of polychlorinated biphenyls (PCBs) and persistent organic pollutants (POPs) (Jacobsen et al. Reference Jacobsen, Øien, Solheim and Aarvak2014). Nazneen et al. (Reference Nazneen, Jayakumar, Albeshr, Mahboob, Manzoor, Pandiyan, Krishnappa, Rajeswary and Govindarajan2022) found the presence of heavy metals in pellets of five owl species, demonstrating the need for additional studies on the influences of contaminants accumulating in owls and ultimately impacting their health and survival. Miller et al. (Reference Miller, Driscoll, Davison, Murphy, Bronson and Wack2015) conducted gross necropsies from Snowy Owls (n = 68) wintering in the mid-Atlantic USA in 2013–2014 and discovered that a few individuals had internal parasites (e.g. flukes, tapeworms, protozoans) and some individuals had been exposed to anticoagulant rodenticides, organochemicals (PCBs and DDE), and lead. However, the contribution of these factors to death is unknown. It is also reported that most owls turned in for necropsy in North America were in good body condition (Curk et al. Reference Curk, McDonald, Zazelenchuk, Weidensaul, Brinker and Huy2018), and most deaths were human caused (e.g. trauma from automobile and airplane collisions; McCabe et al. Reference McCabe, Therrien, Wiebe, Gauthier, Brinker and Weidensaul2022).
Conservation status and recommendations
The Snowy Owl was uplisted from Least Concern to Vulnerable in October 2017 (BirdLife International 2020) when increasing evidence (ISOWG 2017; Marthinsen et al. Reference Marthinsen, Wennerberg, Solheim and Lifjeld2009; Potapov and Sale Reference Potapov and Sale2012) suggested that worldwide populations were considerably lower than previous estimates (i.e. 200,000–300,000 individuals; Rich et al. Reference Rich, Beardmore, Berlanga, Blancher, Bradstreet and Butcher2004). The information summarised in this paper supports current population estimates (e.g. 14,000–28,000 breeding adults worldwide; BirdLife International 2020).
We suggest that previous reports of a strong recent decline in the Snowy Owl population (e.g. Rosenberg et al. Reference Rosenberg, Kennedy, Dettmers, Ford, Reynolds and Alexander2016) were an overestimation and the trend analysis that we present based on the best data available coming from the breeding ground supports this assertion. Our method formally, quantitatively, and reproducibly combines information from disparate time-series across the breeding range of the species and over a relatively long period (33 years). Unlike previous analyses, our method propagates the uncertainty associated with each population trend into the overall error (assessed by HDIs) associated with the global trend. We can thus estimate with a reasonable level of confidence the probability of the species having declined enough to qualify for each Red List category (Table 1). Results of our analysis suggest that breeding populations of Snowy Owls have indeed decreased globally by more than 30% over the past three generations, and therefore the species should continue to be categorised as Vulnerable under the IUCN Red List Criteria A2. However, we recognise the relatively large error associated with our estimates, something that could be improved with more data in the future. We emphasise that Snowy Owl monitoring sites in the Arctic are very scant and many parts of the breeding range (e.g. Siberia) are not well covered, which limits the robustness of the assessment presented here. Considering that genetic evidence suggests a single, panmictic population worldwide (Marthinsen et al. Reference Marthinsen, Wennerberg, Solheim and Lifjeld2009), Snowy Owl conservation must be addressed globally rather than regionally. Moreover, climate warming, changing prey availability, and increased anthropogenic pressure during winter are all factors that have a strong potential to negatively affect future population trends at a global scale.
In light of our current assessment, we make the following recommendations. We need to improve our knowledge on several aspects of Snowy Owl biology including: (a) better estimates of vital rates, especially survival rate in adults (e.g. seasonally) and in juveniles (i.e. nestling survival to fledging period and post-fledging survival) and age at first breeding; (b) improve international cooperation on conservation and research; (c) continue long-term monitoring of abundance on breeding grounds and increase the geographical coverage of monitoring including the far northern parts of their range to fill large gaps worldwide; (d) pursue necropsies of birds found dead and explore emerging methods for diagnostic testing and international sharing of information between pathologists. In addition, future research should incorporate traditional and local knowledge held by Indigenous peoples, including in-person interviews, surveys, and in-the-field participation of hunters, trappers, elders, and others.
Conclusions
Some aspects of the ecology of the Snowy Owl are still unknown (e.g. dispersal behaviour of juveniles, first-year survival, age-specific recruitment rate) and would benefit from additional research. Moreover, combining long-term monitoring of breeding and wintering ground surveys with individual tracking will help to better understand the movement ecology and demography of this elusive species and help link movement to the breeding success and survival probability. When assessing population size and trends, it is essential to consider the irruptive and nomadic behaviour of the species, something that has not received enough attention in the past. Considering the numerous threats faced by the species and uncertainties associated with population size and trends, we believe that the status of Vulnerable species is warranted for the Snowy Owl. Continued collaborative research is necessary for addressing knowledge gaps identified in the biology of the species and assessing future potential threats that could affect this charismatic and emblematic species of the Arctic wilderness.
Supplementary material
The supplementary material for this article can be found at http://doi.org/10.1017/S0959270924000248.
Acknowledgements
We dedicate this paper to the late G. Bortolotti, one of the founding members of the International Snowy Owl Working Group. We thank the Indigenous Communities across the Arctic for discussions, sharing observations, and allowing us to conduct research on native lands and in their communities; without their support and guidance, most of this data would not have been secured. We thank M. Nitze, F. Normann, R. Blöcher, P. Rapin, and M. Bos with nest searching on Karupelv Valley, all the field crews at the long-term monitoring sites, and all participants of long-term surveys (e.g. Christmas Bird Counts, Arctic Breeding Birds). We thank Project SNOWstorm collaborators and supporters and collaborators of the ISOWG. We are grateful for the valuable suggestions and feedback from S. Oppel, one anonymous reviewer, the Associate Editor A. Margalida and the Editor-in-Chief P. Atkinson. This is Project SNOWstorm contribution number 011 and Hawk Mountain contribution to conservation science number 399. This thorough analysis of the status and population trend of the Snowy Owl emerged from and was identified as a priority by the collaborative work of the ISOWG. JFT, GG, KOJ, OG, BS, JL, IJØ, TA, and RS contributed to the study conception and design. Data preparation and organisation, and analysis were performed by RAM, GG, BR, OK, and JFT. The first draft of the manuscript was written by RAM, GG, BR, and JFT, and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript. This work was supported by the Norwegian Snowy Owl Group, Project SNOWstorm donors, Hawk Mountain Sanctuary Association supporters including the Marshall–Reynolds Foundation, French Polar Institute (IPEV, Program Interactions-1036), Agence Nationale de la Recherche (Project PACS, grant number ANR-21-CE02-0024), Natural Sciences and Engineering Research Council of Canada (NSERC) (to GG and NL), NSERC Canada Research Chair Program (NL), NSERC Discovery Grant (KLW, grant number 203177), IBPN RAS grant number 123032000020-7 and 124050700005-0 (to OK), Canadian Network of Centres of Excellence ArcticNet (GG), Fonds Québécois de Recherche Nature et Technologies (GG), Polar Knowledge Canada (to GG and NL), Environment and Climate Change Canada (GG), Natural Resources Canada Polar Continental Shelf Program (to GG and NL), and the Garfield Weston Foundation (JFT).






