Chronic stress has been linked with a wide range of health disorders, including metabolic, cardiovascular, mood and cognitive( Reference Bose, Olivan and Laferrere 1 – Reference Tamashiro, Sakai and Shively 6 ). Although the exact mechanisms through which chronic stress may lead to negative health outcomes have not been well delineated, according to the allostatic load hypothesis, these pathological effects reflect ‘fatigue’ of physiological systems from repeating cycles of stress arousal, stress response and post-stress adaptation. The suboptimal performance of multiple physiological systems as a consequence of cumulative stress exposure over a lifetime is termed allostatic load (AL). Biomarkers representing the key physiological players in stress adaptation, namely neuroendocrine, cardiovascular, immune and metabolic systems, have been combined to form composite AL measures( Reference McEwen 7 – Reference Seeman, Singer and Rowe 9 ). These measures have been shown to predict incident physical decline( Reference Karlamangla, Singer and McEwen 10 ), cognitive dysfunction, CVD( Reference Seeman, Singer and Rowe 9 ), frailty( Reference Gruenewald, Seeman and Karlamangla 11 , Reference Szanton, Allen and Seplaki 12 ) and mortality( Reference Seeman, McEwen and Rowe 13 ).
Substantial variability in AL is evident during the middle age period in adult lifespan. This is the time when AL reaches its peak and when disparities in AL are especially pronounced( Reference Crimmins, Johnston and Hayward 14 , Reference Chyu and Upchurch 15 ). Therefore, identification of factors associated with AL during middle age may potentially advance our understanding of determinants of stress resilience and vulnerability. Previous studies of AL have primarily focused on social contextual and individual psychological predictors of AL, such as socio-economic status( Reference Gruenewald, Karlamangla and Hu 16 , Reference Seeman, Merkin and Crimmins 17 ), race( Reference Chyu and Upchurch 15 , Reference Deuster, Kim-Dorner and Remaley 18 , Reference Geronimus, Hicken and Keene 19 ), neighbourhood characteristics( Reference Bird, Seeman and Escarce 20 , Reference Merkin, Basurto-Davila and Karlamangla 21 ), social relationships( Reference Seeman, Singer and Ryff 22 , Reference Singer and Ryff 23 ), coping orientations( Reference Chen, Miller and Lachman 24 ) and stress perception( Reference Glei, Goldman and Chuang 25 ). However, there is limited research on the relationship of antioxidant dietary factors with AL.
Data from animal and human studies indicate that chronic stress is linked to oxidative stress, increased inflammation, and DNA and cellular damage( Reference Bauer, Jeckel and Luz 26 – Reference O'Donovan, Tomiyama and Lin 29 ). Thus, it is plausible that exposure to beneficial antioxidant and anti-inflammatory dietary substances may be associated with variations in AL vulnerability. Carotenoids are a major class of diet-derived antioxidants with known anti-inflammatory, DNA repair, cardio- and cancer-protective properties( Reference Agarwal, Parameswari and Vasanthi 30 – Reference Tapiero, Townsend and Tew 34 ). In several cross-sectional and prospective studies, low levels of carotenoids have been linked with certain types of cancer( Reference Miller and Snyder 35 ), CVD( Reference Willcox, Curb and Rodriguez 36 ) and mortality( Reference Shardell, Alley and Hicks 37 ). While the association of serum carotenoids with individual cardiovascular, metabolic and inflammatory biomarkers is established in the literature( Reference Andersen, Jacobs and Gross 38 – Reference Sugiura, Nakamura and Ikoma 44 ), little is known about the relationship of serum carotenoids with AL. Identification of nutrients that might contribute to AL will inform the design of prevention strategies to slow down multi-system physiological dysregulation and reduce the impact of chronic stress on morbidity and mortality( Reference Juster, McEwen and Lupien 45 ). The purpose of the current study was to examine the association of serum carotenoid concentrations and AL in a national sample of middle-aged adults (45–64 years) in the USA.
Methods
Participants
Data for the current study come from the Third National Health and Nutrition Examination Survey (NHANES III, 1988–1994). The survey is based on a nationally representative sample of the US population, with over-sampling of African-Americans, Hispanics and persons aged 60 years and older. Additional information about NHANES III can be found elsewhere( 46 ). The current study included middle-aged adults (45–64 years). Out of 4535 participants (2360 females and 2175 males) who were in the middle age category, 3945 had valid serum carotenoid data and 3914 had valid biological marker data. The final analysis sample was 3387 (1750 females and 1637 males).
Dependent variable
A composite AL score was created following the method described in a prior AL study( Reference Seeman, Merkin and Crimmins 17 ). The composite score includes cardiovascular (systolic and diastolic blood pressure, pulse rate), metabolic (total cholesterol, HDL-cholesterol, glycosylated Hb, sex-specific waist-to-hip ratio) and inflammatory (albumin and C-reactive protein (CRP)) indicators. Each of the nine indicators was dichotomized into ‘high = 1’ and ‘low = 0’ risk categories based on clinical criteria. Then, the scores from different indicators were summed to yield a composite AL score (range 0–9). In the composite measure each indicator was equally weighted. Total scores from 0 to 2 were designated as ‘low’ AL and scores ≥3 as ‘high’ AL. The composite score was calculated for participants with complete data on all nine indicators.
Total cholesterol (mg/dl), HDL-cholesterol (mg/dl), CRP (mg/dl), albumin (g/dl) and glycosylated Hb (%) were measured by contract laboratories using reference analytical methods (for details consult the Laboratory Procedures for NHANES III)( Reference Gunter, Lewis and Koncikowski 47 ). Waist-to-hip ratio, radial pulse (beats/min), and systolic and diastolic blood pressure (mmHg) were measured by trained examiners using standard protocols. Blood pressure was measured using a mercury sphygmomanometer (for additional information on these measurements, see NHANES III Examination Data File( 48 )). The arithmetic mean of three systolic and diastolic pressures was used in analysis.
Main independent variables
The main independent variables examined in the current study included serum levels of α-carotene, β-carotene, β-cryptoxanthin, lutein/zeaxanthin and lycopene (μmol/l). These carotenoids were measured in serum by isocratic HPLC, with chromatogram recording at wavelengths of 300, 325 and 450 nm (for details see Laboratory Procedures for NHANES III)( 49 ).
Covariates
Covariates examined in the current study included age, educational level, race/ethnicity, smoking status, alcohol consumption and physical activity. Age in years was measured as a continuous variable. Education level was categorized based on the US Census criteria (less than high school, high-school graduate, college graduate). NHANES III race/ethnicity categories included non-Hispanic White, non-Hispanic Black, Mexican-American and Other. Smoking status was defined based on serum cotinine levels. Previously reported serum cotinine cut-off points( Reference Caraballo, Giovino and Pechacek 50 ) were used to designate non-smokers (≤15·0 ng/ml (85·2 nmol/l)) and smokers (>15·0 ng/ml (85·2 nmol/l)). Serum cotinine was measured by MS analysis, after an initial screening by the enzyme immunoassay method. Alcohol consumption measure was based on participants’ responses about frequency of beer, wine and hard liquor intake in the past month. Summed responses were grouped into alcohol consumption categories ‘none’, ‘moderate’ and ‘excessive’, according to the previously described gender-based criteria( Reference Ford, Zhao and Tsai 51 ). For females, moderate alcohol consumption was defined as 1–30 drinks/month (the equivalent of ≤1 drink/d). For males, moderate alcohol consumption was defined as 1–60 drinks/month (the equivalent of ≤2 drinks/d). Physical activity was determined based on the frequency of participation in nine physical activities during the past month. The frequency of each activity was multiplied by its respective metabolic equivalent (MET)( Reference Ainsworth, Haskell and Whitt 52 ) to get an intensity-weighted score for each activity. Intensity-weighted scores for nine activities (walking, running/jogging, riding a bicycle, swimming, aerobic dancing, other dancing, calisthenics or exercise, gardening/yardwork, lifting weights) were then summed up and categorized as inactive (values ≤3·5) and active (values >3·5), as described in prior literature( Reference Zhu, St-Onge and Heshka 53 ).
Statistical analysis
Analyses were conducted using IBM SPSS Complex Samples version 20·0. All variance estimates were corrected for complex multistage design and population estimates were adjusted based on sample weights provided by NHANES III. P value of < 0·05 (two-sided) was set as the criterion of significance. The bivariate associations of continuous variables with sex-specific AL were examined using general linear models and the bivariate associations of categorical variables with AL using χ 2 tests. Binary logistic regression was used to model the relationship of individual serum carotenoids (α-carotene, β-carotene, β-cryptoxanthin, lutein/zeaxanthin, lycopene) with sex-specific AL, after adjusting for age, education, race/ethnicity, serum cotinine, alcohol consumption, physical activity and other carotenoids. In logistic analysis, serum carotenoid concentrations were categorized into quartiles based on the data in the analysis sample. General linear regression modelling was used to test the linear trends in the relationship between mean concentrations of serum carotenoids and the total AL score, after adjustment for covariates and other carotenoids. In that analysis, the regression of continuous serum concentrations for individual carotenoids v. the continuous total AL score was performed. Consistently with prior AL studies( Reference Chyu and Upchurch 15 , Reference Geronimus, Hicken and Keene 19 , Reference Seeman, Singer and Ryff 22 , Reference Mattei, Noel and Tucker 54 ), we did not control for medication use or doctor-diagnosed chronic conditions as our goal was to capture the existent degree of biological dysfunction.
Results
A total of 3387 middle-aged participants (1750 females and 1637 males) were included in the study. The mean participant age ranged from 53 to 55 years. The total AL score ranged from 0 to 8 in this sample (maximal possible range 0–9). The mean total AL score was 2·13 and 2·53 for females and males, respectively. The prevalence of high AL was 38·1 % for females and 46·8 % for males. Table 1 shows crude associations of AL with covariates. For both sexes, participants with high AL were significantly older (P < 0·001) and more likely to have lower educational status (P < 0·001). Race/ethnicity status was significantly associated with high AL among females only (P < 0·001). Among males, participants with high AL were significantly more likely to be smokers than their male peers with low AL (P = 0·011). Among females, the proportions of participants who reported moderate as well as excessive alcohol consumption were lower in the high AL group than in the low AL group. The proportion of female non-drinkers was also higher in the high AL group (P = 0·032). We also noted a statistically significant inverse association between physical activity status and AL for both females and males (P < 0·001 and P = 0·015, respectively; Table 1).
Table 1 Baseline characteristics by allostatic load (AL) status of middle-aged (45–64 years) participants, Third National Health and Nutrition Examination Survey, NHANES III (1988–1994)

*Data are weighted percentages, except for Age expressed as mean (in years).
†P values computed using complex-samples general linear model for continuous variables and χ 2 test of independence for categorical variables.
The unadjusted proportions of female and male participants at high risk for individual AL biomarkers are shown in Fig. 1. For both sexes, the leading risk factor was waist-to-hip ratio. This was also the only AL component for which there were more at-high-risk individuals than at-low-risk individuals (66 % of females and 92 % of males). The second leading risk factor for females was CRP (42 % at high risk) and for males, HDL-cholesterol (41 % at high risk).
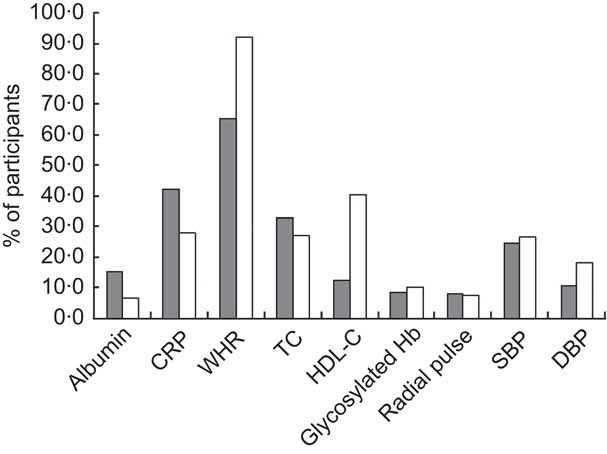
Fig. 1 Unadjusted proportions of middle-aged (45–64 years) participants (![]() , females;
, females; ![]() , males) at high risk for individual biomarkers of allostatic load from the Third National Health and Nutrition Examination Survey, NHANES III (1988–1994) (CRP, C-reactive protein; WHR, waist-to-hip ratio; TC, total cholesterol; HDL-C, HDL-cholesterol; SBP, systolic blood pressure; DBP, diastolic blood pressure)
, males) at high risk for individual biomarkers of allostatic load from the Third National Health and Nutrition Examination Survey, NHANES III (1988–1994) (CRP, C-reactive protein; WHR, waist-to-hip ratio; TC, total cholesterol; HDL-C, HDL-cholesterol; SBP, systolic blood pressure; DBP, diastolic blood pressure)
In unadjusted logistic regression analysis, we observed significant dose–response inverse associations of serum concentrations of α-carotene, β-carotene and β-cryptoxanthin with high AL in females and males (see Crude model in Tables 2 and 3). These significant trends persisted after adjusting for age, education, race/ethnicity, serum cotinine, alcohol consumption and physical activity (Partially adjusted model in Tables 2 and 3). However, when we included other carotenoids in the model, only serum concentration of β-carotene remained inversely and significantly associated with high AL for both sexes (Fully adjusted model in Tables 2 and 3). In the fully adjusted model, females and males in the lowest quartiles of β-carotene had a 2·94- and 2·90-fold increased odds of high AL, respectively, compared with their peers in the highest quartiles (95 % CI 1·74, 4·94 and 95 % CI 1·43, 5·89, respectively). After full adjustment, the linear trend for the association of serum β-carotene quartiles and high AL also remained significant for both sexes (P for trend = 0·001 and 0·018 for females and males, respectively).
Table 2 Logistic regression odds ratios with 95 % confidence intervals of high allostatic load by individual serum carotenoid quartiles in middle-aged (45–64 years) female participants, Third National Health and Nutrition Examination Survey, NHANES III (1988–1994)
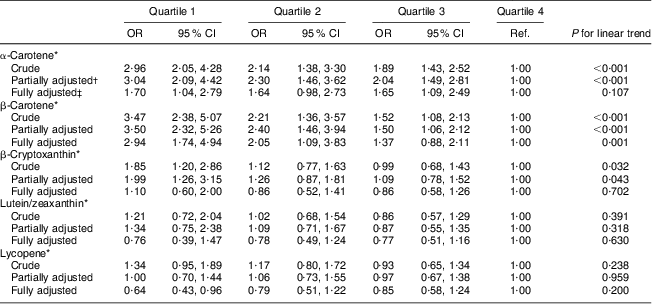
Ref., referent category.
*μmol/l; to convert from μmol/l to μg/l units divide α-carotene, β-carotene and lycopene serum concentrations by 0·001863; β-cryptoxanthin serum concentrations by 0·001809; and lutein/zeaxanthin serum concentrations by 0·001758.
†Adjusted for age, education, race/ethnicity, serum cotinine, alcohol consumption and physical activity.
‡Adjusted for age, education, race/ethnicity, serum cotinine, alcohol consumption, physical activity and other carotenoids.
Table 3 Logistic regression odds ratios with 95 % confidence intervals of high allostatic load by individual serum carotenoid quartiles in middle-aged (45–64 years) male participants, Third National Health and Nutrition Examination Survey, NHANES III (1988–1994)

Ref., referent category.
*μmol/l; to convert from μmol/l to μg/l units divide α-carotene, β-carotene and lycopene serum concentrations by 0·001863; β-cryptoxanthin serum concentrations by 0·001809; and lutein/zeaxanthin serum concentrations by 0·001758.
†Adjusted for age, education, race/ethnicity, serum cotinine, alcohol consumption and physical activity.
‡Adjusted for age, education, race/ethnicity, serum cotinine, alcohol consumption, physical activity and other carotenoids.
In the fully adjusted model, females in the lowest quartile for α-carotene had a 1·70-fold increased odds of high AL (95 % CI 1·04, 2·79, P for trend = 0·107), compared with females in the highest quartile. Females in the lowest quartile of lycopene had a 0·64-fold reduced odds of high AL (95 % CI 0·43, 0·96, P for trend = 0·200), compared with females in the highest quartile.
To rule out potential confounding of the serum carotenoids–AL relationship from the ongoing acute inflammatory processes, we repeated analyses after excluding participants with white blood cell count greater than 10 000 cells/μl and those who responded ‘Yes’ to a survey question ‘In the past few days have you had a cough, cold, or other acute illness?’ (excluded females n 542, males n 499). After exclusion, the fully adjusted odds of high AL for all carotenoids did not change appreciably for both sexes (results not shown).
To test whether individual serum carotenoid concentrations were associated with the total number of ‘high-risk’ AL components, i.e. total AL score, we performed a multiple linear regression analysis. For females, serum β-carotene concentration was inversely associated with the total AL score (P for trend < 0·001). Compared with females with a total AL score of 0, females with a total AL score ≥5 had a 31 % lower mean β-carotene concentration. For males, serum β-carotene concentration was inversely associated with the total AL score (P for trend = 0·004). Compared with males with a total AL score of 0, males with a total AL score of ≥5 had a 17 % lower mean β-carotene concentration (Table 4).
Table 4 Mean concentrations * of serum carotenoids by the number of ‘high-risk’ allostatic load (AL) components†, adjusted‡ for covariates, in middle-aged (45–64 years) participants, Third National Health and Nutrition Examination Survey, NHANES III (1988–1994)
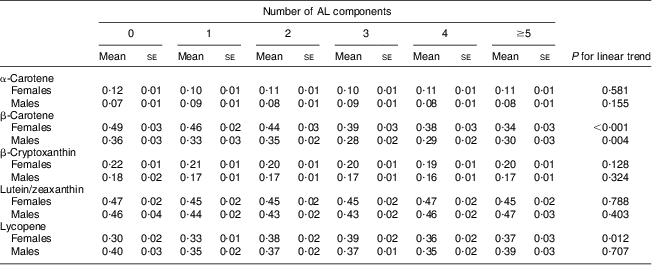
* μmol/l; to convert from μmol/l to μg/l units divide α-carotene, β-carotene and lycopene serum concentrations by 0·001863; β-cryptoxanthin serum concentrations by 0·001809; and lutein/zeaxanthin serum concentrations by 0·001758.
†The number of AL components in the high risk category, possible score range 0–9, sample score range 0–8; score categories 5–8 were combined because of low cell size.
‡Adjusted for age, education, race/ethnicity, serum cotinine, alcohol consumption, physical activity and other carotenoids.
Discussion
To our knowledge, the current study is the first one to examine the association of serum carotenoid concentrations with AL in a representative sample of the US middle-aged population. We found significant dose–response, inverse associations of serum β-carotene concentrations and high AL risk in females and males. These trends persisted after we accounted for age, education, race, smoking, alcohol consumption and physical activity, as well as evidence of ongoing or recent acute inflammation. Our results also demonstrated that mean serum β-carotene concentrations decreased in a linear fashion with increasing number of high-risk AL biomarker components.
The construct of AL represents a composite biomarker signature of multi-system ‘wear and tear’ from cumulative chronic stress exposure over a lifetime. Our findings, thus, may signify that β-carotene has broad biological significance across multiple physiological systems. It is likely that β-carotene supports optimal physiological performance though its various bioregulatory functions, including genetic modulation of oxidative stress pathways( Reference Da Costa, Badawi and El-Sohemy 55 , Reference Landrier, Marcotorchino and Tourniaire 56 ), upregulation of immune response( Reference Chew and Park 31 ) and pro-vitamin A activity( Reference Rao and Rao 33 , Reference Calder, Albers and Antoine 57 ). Alternatively, depletion of serum β-carotene levels may result in the setting of adverse physiological conditions, e.g. conditions of increased oxidative stress and inflammation( Reference Andersen, Jacobs and Gross 38 , Reference Jonasson, Wikby and Olsson 58 – Reference Quasim, McMillan and Talwar 60 ). Our findings are consistent with the results of other studies showing an inverse association of serum β-carotene with abnormalities in individual AL biomarkers, including abdominal obesity( Reference Ford, Mokdad and Giles 40 ), BMI( Reference Andersen, Jacobs and Gross 38 ), insulin resistance( Reference Sugiura, Nakamura and Ikoma 44 ), hypertension( Reference Ford, Mokdad and Giles 40 ) and CRP( Reference Erlinger, Guallar and Miller 39 , Reference Ford, Liu and Mannino 41 , Reference Kritchevsky, Bush and Pahor 43 ).
Biological interrelationships among carotenoids and various dietary antioxidants have been increasingly emphasized in the literature( Reference Shardell, Alley and Hicks 37 , Reference Kritchevsky 61 – Reference Kohlmeier, Kark and Gomez-Gracia 63 ). That is why all our analyses were adjusted for other individual carotenoids. Our results clearly demonstrate that carotenoids have a modulating influence on each other. For example, the association of α-carotene and β-cryptoxanthin with AL, found to be significant in the crude and partially adjusted analyses, ceased to be significant after adjustment for other carotenoids. In contrast, the inverse association of β-carotene with AL remained significant for both sexes even after adjustment for other carotenoids. This finding suggests that serum levels of β-carotene are more strongly determined by the physiological milieu than by the presence of other carotenoids. Additional research on physiological interaction of various carotenoids is warranted.
A noteworthy observation in the current study is that a disproportionately higher number of participants in this national sample of middle-aged adults were in the high risk category for central obesity and CRP than for any other component of AL. Weight gain has been characterized in the theoretical literature as a natural biological mechanism for adaption to environmental demands( Reference Peters and McEwen 64 , Reference Tremblay and Doucet 65 ). Empirical data linking stress and obesity( Reference Block, He and Zaslavsky 66 – Reference Vicennati, Pasqui and Cavazza 68 ) and epidemiological trends indicating that onset of obesity occurs earlier in modern US cohorts( Reference Lee, Pilli and Gebremariam 69 ) indeed suggest that metabolic mechanisms of stress adaptation may be predominant in the years leading to middle age. The role of obesity/inflammation in the development of AL across the lifespan needs to be examined in future longitudinal studies. Individual AL biomarker components, currently receiving equal weight in the composite AL measures, may need to be weighted differentially based on the timing and duration of risk factor exposure. Additionally, given the characteristics of the sample, it is possible that the observed inverse association of serum β-carotene with AL was heavily influenced by its known strong association with obesity( Reference Andersen, Jacobs and Gross 38 , Reference Kimmons, Blanck and Tohill 42 ) and CRP( Reference Erlinger, Guallar and Miller 39 , Reference Kritchevsky, Bush and Pahor 43 ). Previous studies suggest that the inflammatory environment( Reference Jonasson, Wikby and Olsson 58 – Reference Quasim, McMillan and Talwar 60 , Reference Chang, Chen and Ke 70 , Reference Curran, Sattar and Talwar 71 ) and obesity-linked oxidation of the lipoprotein carriers of β-carotene, specifically LDL( Reference Beck, Ferrucci and Sun 72 , Reference Weinbrenner, Schroder and Escurriol 73 ), may be related to low serum β-carotene levels.
The risk of high AL has been linked with lower educational status( Reference Seeman, Merkin and Crimmins 17 ), minority racial status( Reference Chyu and Upchurch 15 ), low physical activity( Reference Mattei, Noel and Tucker 54 , Reference Gay, Salinas and Buchner 74 , Reference Parente, Hale and Palermo 75 ) and smoking( Reference Gustafsson, Janlert and Theorell 76 ) in previous investigations. These associations have been confirmed in our study. In our crude analysis, we also noted that females with high AL were less likely to report drinking alcohol compared with their female counterparts with low AL. Although a similar association between reduced alcohol consumption and high AL has been observed in a study of middle-age and older Puerto Rican adults( Reference Mattei, Noel and Tucker 54 ), these are bivariate results and should be considered with caution. Considerable methodological differences across studies that examined the association of alcohol consumption with AL( Reference Mattei, Noel and Tucker 54 , Reference Parente, Hale and Palermo 75 , Reference Gustafsson, Janlert and Theorell 76 ) currently prevent drawing any conclusions about this relationship.
Generally, the association trends between serum carotenoids and AL were similar for both sexes. Two sex-based differences in the results are worth noting, however. First, the linear trends for the inverse association of serum β-carotene quartiles and high AL, and of mean serum β-carotene concentrations with the number of high-risk AL components, were more pronounced in females than in males. It is possible that gender differences in AL profile are responsible for these dissimilarities. As has been pointed out earlier, males in this sample were more likely to be centrally obese and have low HDL-cholesterol, while females were more likely to be centrally obese and have elevated CRP. Second, the lowest quartile of serum α-carotene was significantly associated with high AL risk in females only. Since female populations tend to have higher α- and β-carotene levels than male populations( Reference Brady, Mares-Perlman and Bowen 77 , Reference Rock, Thornquist and Kristal 78 ), it is possible that lower serum α- and β-carotene status is a more sensitive indicator of increasing physiological dysfunction in females than in males.
Our findings should be interpreted with caution. First, causality of the association between serum carotenoids and AL cannot be assumed based on the cross-sectional design of the study; however, our results point to an increased vulnerability to high AL in individuals with low serum β-carotene levels. Second, theoretical refinement of the AL construct is still needed to aid interpretations of AL studies. For example, the cut-off point for designating high AL was based on the example of previous AL studies. No strong theoretical justification for this cut-off point exists, however.
Our study has several strengths. First, our study contributes to a still limited research on the relationship of antioxidant status and the measure of cumulative stress exposure, AL. Second, the study utilized a nationally representative sample of middle-aged adults, which enhances the generalizability of our findings. Third, we examined the middle age population, which exhibits significant variation in AL levels yet has received relatively less attention in AL studies.
Conclusion
Our results demonstrate a strong inverse association of serum β-carotene with the measure of cumulative stress exposure, AL. Further research is needed to determine whether chronic stress is the mechanism linking low levels of serum β-carotene with adverse health outcomes.
Acknowledgements
Sources of funding: The work of K.E. on this manuscript was partially supported by the Robert Wood Johnson Foundation Nurse Faculty Scholar Program (Grant # 71249). The funder made no contributions to the study design, analysis or interpretation. Conflicts of interest: There are no conflicts of interest, including specific financial interests or affiliations relevant to the subject of this manuscript. Ethics statement: This research has been determined as exempt by the University of Illinois at Chicago Institutional Review Board. Authors’ contributions: N.R. designed the study, performed data analysis and wrote the manuscript. C.G.P. assisted with statistical analysis and interpretation of data. K.E. supervised the study design, analysis and interpretation, and made significant contributions to the manuscript preparation. All authors contributed to the development of the manuscript.







