Highlights
A high-fat diet and/or the addition of a certain amount of cholesterol or sugar induces neuropsychiatric disorders in rats and mice.
A high-fat diet and/or the addition of a certain amount of cholesterol or sugar exacerbates the neuropsychiatric disorders observed in animal models of depression.
The mechanisms by which diet induces anxiety/depressive-like behaviour may involve neuroinflammation, neurotransmitters/neuromodulators, neurotrophins and the gut–brain axis.
Future research should be focused on identifying the contribution of diet and diet-induced metabolic risk to neuropsychiatric disorders, which can serve as the basis for future clinical dietary intervention strategies for neuropsychiatric disorders.
The massive but largely unrecognised burdens of neuropsychiatric disorders are obvious throughout the world, while the most common neuropsychiatric disorders are anxiety and depression disorders. Globally, an estimated 4·4 % of the global population suffers from depression and 3·6 % from anxiety(Reference Vos, Allen and Arora1,Reference Murray and Lopez2) . In most countries, the lifetime incidence of depression is 8–12 %(Reference Andrade, Caraveo-Anduaga and Berglund3). Similarly, anxiety is a serious neuropsychiatric disorder that often coexists with other neuropsychiatric disorders, especially major depressive disorder(Reference Craske and Stein4). In Europe, Africa, Asia and the USA, the lifetime incidence of anxiety is between 9 and 29 %(Reference Simpson, Neria and Lewis-Fernández5,Reference Kessler, Berglund and Demler6) . Shockingly, suicide has been linked to neuropsychiatric disorders and is now the third leading cause of death among young people in Western countries(Reference Mullins, Bigdeli and Børglum7). Unfortunately, we still know very little about anxiety and depression, and a large proportion of patients are treated with drugs targeting the central nervous system that have poor therapeutic effects and serious side effects(Reference Wong, Siah and Lo8).
Currently, chronic metabolic diseases such as obesity, type 2 diabetes mellitus (T2DM), non-alcoholic fatty liver disease (NAFLD), atherosclerosis and the metabolic syndrome are also major health problems. In 2016, more than 1·9 billion adults were overweight and more than 650 million adults were obese, representing 39 and 13 % of the world’s adult population, respectively(Reference Blüher9). T2DM worldwide has increased from 108 million in 1980 to 422 million in 2014(Reference Sarwar, Gao and Kondapally Seshasai10) and is largely the result of overweight and sedentary lifestyles. In addition, NAFLD is closely related to diabetes and obesity and affects one-quarter of the world’s population(Reference Younossi, Anstee and Marietti11). However, there is still a lack of effective medications for NAFLD(Reference Eslam, Sanyal and George12). Although genetics plays a role in regulating the metabolic responses of body weight, lipids and glucose to food intake in humans and animals, genetics cannot account for the climb in metabolic diseases worldwide in a short time, which may result from individual differences in genetic susceptibility to dietary factors. Diets rich in fat, cholesterol and/or sugar can cause a series of metabolic diseases in humans and animals(Reference Zheng, Ley and Hu13–Reference Santhekadur, Kumar and Sanyal16).
Individual metabolic status is highly correlated with diet. Diets rich in fat not only induce metabolic disorders in humans and animals but also induce chronic metabolic diseases. Recent studies have shown that individuals with anxiety or depression frequently co-occur with multiple co-morbidities, especially obesity, T2DM, NAFLD and its various risk factors in the metabolic syndrome(Reference Roy and Lloyd17,Reference Schmitz, Deschênes and Burns18) . Obese individuals have a higher risk of depression and anxiety, and diet is a risk factor for the development of depression(Reference Jebeile, Gow and Baur19). Clinically, one in four patients with T2DM has significant depression. Depression not only increases the risk of developing T2DM but also increases the risk of hyperglycaemia, insulin resistance and microvascular and macrovascular complications(Reference Semenkovich, Brown and Svrakic20,Reference Vancampfort, Correll and Galling21) . Two studies recently found that depression is independently associated with NAFLD in adults(Reference Kim, Yoo and Li22), and the association is stronger after adjusting for covariates such as age, sex and insulin resistance(Reference Jung, Park and Oh23). Therefore, diet is a risk factor for anxiety and depression in addition to altered metabolic status(Reference Adan, van der Beek and Buitelaar24). Recent studies have shown that there are clear links between diet and vulnerability to or protection from neuropsychiatric disorders, including anxiety and depression, but the mechanisms are not yet understood(Reference Cryan, O’Riordan and Sandhu25). In fact, some patients with anxiety and depression have metabolic diseases and the therapeutic effect of drugs targeting the central nervous system is not as satisfactory(Reference Semenkovich, Brown and Svrakic20). Furthermore, an estimated 50 % of patients with depression is inadequately treated by available interventions. This condition can be called treatment-resistant depression, which is associated with a variety of factors, including metabolic risks of CVD and past depressive episodes(Reference Akil, Gordon and Hen26). At the same time, some scholars have pointed out that immune-metabolic depression is a subtype of chronic depression(Reference Vogelzangs, Beekman and Boelhouwer27–Reference Lamers, Milaneschi and Penninx29). If depression is combined with the metabolic syndrome, depressive symptoms can worsen and the risk of depression recurrence increases nearly 3-fold. Thus, metabolic disorders are important risk factors for depression(Reference Leite, Leite and Rasini28). While the determining factors of neuropsychiatric disorders are complex, preclinical and clinical studies have shown that there is a strong link between diet, metabolic state and neuropsychiatric disorders, including anxiety and depression(Reference Oliver and Wardle30–Reference Dallman32). Undoubtedly, rodent models have become an important tool for understanding preclinical research on anxiety and depression(Reference Gururajan, Reif and Cryan33). With the widespread use of transgenic animals, a great deal of research has focused on transgenic animal models of neuropsychiatric disorders such as anxiety and depression(Reference Cryan and Holmes34). Although transgenic animals have greatly aided our understanding of the interactions between genes and neuropsychiatric disorders, relatively little attention has been given to dietary risk factors in animal models(Reference Hintze, Benninghoff and Cho35,Reference Hollander, Cory-Slechta and Jacka36) . In fact, exposure to a high-fat diet (HFD) and/or adding a certain amount of cholesterol or sugar to the diet of rats or mice induces metabolic disorders that can lead to some of the hallmark symptoms of depression, including anxiety, anhedonia and despair, and exacerbate neuropsychiatric disorders observed in animal models of depression. Diet and its associated metabolic disorders have important implications for the development of neuropsychiatric disorders in rodent models. Therefore, this article focuses on a rodent model of chronic consumption of HFD, and/or the addition of a certain amount of cholesterol or sugar, meanwhile, summarising the pattern of diet that induces anxiety/depressive-like behaviour and explores the mechanisms that have been proposed for neuropsychiatric disorders induced by dietary patterns, especially a diet rich in fat.
Behavioural assessment of animal models
Testing for anxiety-like behaviours
In an attempt to model human pathological anxiety in rodents, a wide range of behavioural testing paradigms have been developed. The social interaction test was the first ethologically based anxiety model that used natural behaviour as a dependent variable(Reference File and Seth37). Once a social partner is present, the latency and time spent with the partner are measured and the ratio of the time spent in the zone with and without the social partner is used as an indicator of anxiety. The open field test is a common measure of exploratory behaviour and general activity in rodents, where both the quality and quantity of the activity can be measured(Reference Fernandez, Misilmeri and Felger38). Some outcomes, particularly defecation, centre time and activity within the first 5 min, likely gauge some aspects of emotionality, including anxiety. The elevated plus maze is a widely used behavioural assay for rodents, and it has been validated to define brain regions and mechanisms underlying anxiety-related behaviour(Reference Walf and Frye39). A decrease in open arm activity (duration and/or entries) in which rodents are exposed to the plus maze for 5 min reflects anxiety behaviour. The light/dark transition test is a characteristic method used in the assessment of anxiety: the apparatus consists of a simple chamber divided into dark and light compartments. Rodents are allowed to move freely between the two chambers(Reference Takao and Miyakawa40). The number of entries into the bright chamber, the duration of time spent there and the related exploratory behaviours, detected via a video tracking system, are reliable parameters for assessing anxiolytic effects.
Testing for depressive-like behaviours
The clinical diagnosis of depression requires the presence of several ‘core’ symptoms (depressed mood, decreased pleasure), and these signs can be followed with behavioural assessments in different animal models of depressive states. The forced swim test is the most widely used tool for assessing anti-depressant activity preclinically(Reference Petit-Demouliere, Chenu and Bourin41). Investigators measure the amount of time between when the animal is placed in an inescapable cylinder of water and the onset of immobility. Rodent models of depression exhibit a decrease in the time spent trying to escape. The tail suspension test is a reliable test procedure for antidepressants in which a mouse is suspended by the tail from a lever, and the movements of the animal are recorded(Reference Steru, Chermat and Thierry42). During this test, typically 6 min in duration, the resulting escape-oriented behaviours are quantified. The sucrose preference test is a reward-based test used as an indicator of anhedonia(Reference Eagle, Mazei-Robison and Robison43). Anhedonia, or the decreased ability to experience pleasure, represents one of the core symptoms of depression. In general, this test measures the amount of a sweet-tasting solution that the animal ingests across a fixed period. The reduced preference for sweet solution in the sucrose preference test represents anhedonia.
High-fat diet-induced neuropsychiatric disorders
Diets rich in fat not only induce metabolic disorders in humans but also cause animal metabolic disorders. In animal models, as in humans, metabolic disorders can be assessed by criteria based on metabolic abnormalities in body weight, blood glucose, insulin levels, TAG, total cholesterol, LDL-cholesterol and HDL-cholesterol levels(Reference Hariri and Thibault44,Reference Willebrords, Pereira and Maes45) . A growing body of research in humans and in animals has found that HFD not only causes these metabolic phenotypes but can also induce or increase the risk of neuropsychiatric disorders. In recent years, research on diet-induced neuropsychiatric disorders in animal models has increased dramatically. This section focuses on dietary patterns and summarises the literature of the past 10 years to explore the mechanisms that have been proposed for neuropsychiatric disorders induced by diets rich in fat and the possibility of reversing diet-induced neuropsychiatric disorders in animal models.
Dietary fat content
In a previous review, low-, medium- and high-fat diets were defined as <20, 20–35 and >35 % of total energy, respectively(Reference Hintze, Benninghoff and Cho35). Currently, feeding a HFD leads to metabolic disorders and cognitive and neuropsychiatric disorders compared with feeding a low-fat diet in a rodent model. Chudasama & Bhatt’s research team(Reference Chudasama and Bhatt46) first fed male Sprague–Dawley rats a HFD containing 60·29 % kcal fat, 17·85 % kcal carbohydrate and 20·15 % kcal protein for 5 weeks. The results showed for the first time that the body weight and systolic blood pressure of the rats increased significantly, and the immobility time in the 5-min forced swim test, reflecting depression-like behaviour, increased meaningfully. On the other hand, the immobility time in the 6-min tail suspension test, which reflects anxiety-like behaviour, also increased significantly. Then, the number of obese rats that passed the 5-min open field test, which reflects anxiety-like behaviour (an indicator that indirectly reflects immobility time), was also significantly reduced. These results suggest that a HFD and its related metabolic characteristics are positively correlated with depression and anxiety. In the literature included in this study (Table 1), most of the animal model studies on anxiety- and depression-like behaviour induced by HFD used 45 % of energy from fat(Reference Aslani, Vieira and Marques47–Reference de Noronha, Campos and Abreu53), even up to 60 % or higher of the energy from fat(Reference Chudasama and Bhatt46,Reference Agusti, Moya-Pérez and Campillo54–Reference Yamada, Katsuura and Ochi70) . The control diet is generally below 20 % of energy from fat. However, there are several studies(Reference Wu, Liu and Kalavagunta71,Reference Pan, Liu and Wan72) that use male C57BL/6 mice and fed a medium-fat diet (23·7 % kcal fat) compared with a control low-fat diet (13·3 % kcal fat) for 3–6 weeks. The mice in the medium-fat diet group showed significant weight gain and decreased glucose tolerance. Because most neurobehavioural performance tests, including the open field test, tail suspension test, forced swim test and sucrose preference test, were abnormal, these results suggest that the medium-fat diet can also induce depression-like behaviour. In fact, several studies have used a HFD and/or adding a certain amount of cholesterol or sugar to intervene in rodents and observed the effects of high cholesterol on anxiety/depression-like behaviour. A fat-rich diet (>20 %) and adding 0·2–2·5 % cholesterol can induce anxiety/depressive behaviour in male mice and rats(Reference Wu, Lv and Pan73–Reference Hu, Xu and Liu77). A low-fat diet with 0·2–10 % cholesterol(Reference Strekalova, Evans and Costa-Nunes78–Reference Metwally, Rashad and Mahmoud80) can also induce anxiety/depressive behaviour in female mice but no anxiety/depressive behaviour in male rats(Reference Hu, Xu and Liu77). There are sex differences in the role of cholesterol in the diet in anxiety and depression, and its detailed mechanism needs to be further studied. The high-sugar diet is associated with an increased consumption of simple sugars, typically in the form of sucrose- or fructose-derived sweeteners. Several studies(Reference Rebolledo-Solleiro, Roldán-Roldán and Díaz81–Reference Gancheva, Galunska and Zhelyazkova-Savova89) have used a high-sugar diet (or ‘high-sugar and high-fat’ or ‘Western diet’) to intervene in rodents and observed the effects of anxiety/depression-like behaviour (also shown in Table 1). There is a study that has particularly attracted attention; male BALBc mice (an inbred immune-deficient mouse) were fed a high-sugar diet (60 % sugar) for 9 weeks, with a significant increase in body weight and total cholesterol, and the mice showed anxiety-like behaviour abnormalities. Importantly, the authors showed that fat and sucrose affect behaviour differently and sometimes oppositely, and thus, the proportion of fat and sugar in the diet should be given more attention when designing behavioural studies(Reference Pyndt Jørgensen, Hansen and Krych90). These effects of the HFD inducing neuropsychiatric behaviour are often attributed to the high dietary content of fat. Therefore, the fat content of HFD may be the major cause of anxiety and depression-like behaviour(Reference Nakajima, Fukasawa and Gotoh91).
Table 1 Most commonly used high-fat diets to induce anxiety/depressive-like behaviour in rodent models

–, Not reported; ↑, increase v. control diet; TC, total cholesterol; LDT, Light and dark test; OFT, open field test; SPT, sucrose preference test; FST, forced swim test; EZM, elevated zero maze; EPM, elevated plus-maze; WT, wild-type; KI, knock-in; AST, aspartate aminotransferase; ALT, alanine aminotransferase; T2DM, type 2 diabetes mellitus; ORT, object recognition test; NSFT, novelty suppressed feeding test; NAFLD, non-alcoholic fatty liver disease; SD, Sprague–Dawley; SPBT, Shock-probe/burying test; ETM, elevated T-Maze; TST, tail suspension test; SLA, spontaneous locomotor activity score; SIT, social interaction test; HBT, hole-board test.
*P < 0·05 was considered significant.
Diet exposure period
Most of the included studies used medium- and long-term HFD to induce neuropsychiatric disorders, most commonly at 8–32 weeks. In some studies, male Wistar rats or mice were fed a HFD (60 % kcal from fat) for 8 weeks(Reference Yang, Liu and Jiang55,Reference Hassan, Mancano and Kashofer66) or a HFD (45 % kcal from fat) and HSD (10 % sugar water) for 8 weeks(Reference Gancheva, Galunska and Zhelyazkova-Savova89). They found that a HFD led to metabolic disorders and triggered similar anxiety- and depressive-like phenotypes, such as decreased social interaction. This strongly suggests that a HFD can lead to anhedonia. Furthermore, female C57BL/6 mice were fed a HFD (60 % kcal from fat) for 32 weeks, and body weight, fasting blood glucose and oral glucose tolerance tests increased significantly in HFD-induced mice. The error time in the Y-maze test was significantly higher than that in the control group, and the sucrose consumption rate in the sucrose preference test was significantly reduced. A long-term HFD not only leads to depression-like behaviour but also affects spatial memory damage(Reference Wu, Guo and Zhang57). In addition, whether short-term (1–4 weeks) high-fat feeding induces anxiety-like and depression-like behaviour remains controversial. Several studies involving short-term feeding of rodent models with a HFD (60 % kcal from fat) for 1–4 weeks have found that anxiety- and depression-like behaviour is significantly changed(Reference Alonso-Caraballo, Hodgson and Morgan58,Reference Gainey, Kwakwa and Bray62,Reference Vagena, Ryu and Baeza-Raja69,Reference Wu, Liu and Kalavagunta71,Reference Kaczmarczyk, Machaj and Chiu104) . However, evidence from rodent models suggests that the consumption of a HFD for 3 weeks can have anti-depressant and anxiolytic effects(Reference Finger, Dinan and Cryan107). Sweeney’s study(Reference Sweeney, O’Hara and Xu56) sought to determine whether the duration of exposure to a HFD (60 % kcal from fat) had a significant effect on anxiety-related behaviour. Their results showed that HFD feeding had a time-dependent biphasic effect on anxiety-related behaviours, and the level of weight gain and the degree of decreased glucose tolerance were positively correlated with the severity of anxiety. Anxiety-like behaviour was reduced in mice fed a short-term HFD (60 % kcal from fat) for 5 weeks. Interestingly, after 15 weeks of HFD feeding, mice exhibiting metabolic symptoms of obesity (significant weight gain and reduced glucose tolerance) showed an increase in anxious behaviour. Taken together, these findings suggest that metabolic abnormalities, such as weight fluctuations, glucose tolerance and blood lipids, promote anxiety-related behaviour in animals fed a HFD and that the feeding duration is likely to affect the progression of this relationship. It is certain that, in particular, long-term HFD (over 8 weeks) and their induced metabolic disorders may produce persistent depressive/anxiety behaviours.
Species and genetic background
Similar to other animal models of disease, Sprague–Dawley rats, Wistar rats and C57BL/6 mice are currently used to observe metabolic abnormalities and neuropsychiatric disorders induced by a HFD. Wistar Han rats(Reference Aslani, Vieira and Marques47), Long Evans Rattus rats(Reference Blaisdell, Lau and Telminova108) and Swiss albino mice(Reference Nakajima, Fukasawa and Gotoh91,Reference Kurhe and Mahesh95–Reference Kurhe, Mahesh and Devadoss97) have also been used. In addition, several studies using rodents with specific genetic backgrounds have produced interesting results. In a recent study(Reference Alonso-Caraballo, Hodgson and Morgan58), two types of obesity-prone and obesity-resistant Sprague–Dawley rats were selected. After feeding a HFD for 8 weeks, the weight and insulin level increased significantly and anxiety-like behaviours were enhanced in obese-susceptible rats. However, anxiety-like behaviour remained unaffected in obesity-resistant rats, despite a significant increase in weight and insulin levels. Therefore, obesity-prone mice may be one of the appropriate models to explore the mechanism of HFD-induced anxiety-like behaviour. The tryptophan hydroxylase 2 R439H knock-in mouse line harbours a partial loss-of-function mutation in the brain 5-hydroxytryptamine (5-HT) synthesis enzyme tryptophan hydroxylase 2(Reference Beaulieu, Zhang and Rodriguiz109). Homozygous knock-in animals from this line have 60–80 % less brain 5-HT than their homozygous wild-type littermates(Reference Beaulieu, Zhang and Rodriguiz109,Reference Jacobsen, Siesser and Sachs110) . Tryptophan hydroxylase 2 R439H knock-in mice with brain 5-HT deficiency were fed a HFD (39·7 % kcal from fat) for 22 weeks, and it was shown that a HFD could increase body weight and anxiety-like behaviour and decrease the depression-like behaviour of wild-type mice. However, in tryptophan hydroxylase 2 R439H knock-in mice with brain 5-HT deficiency, HFD intervention did not significantly affect weight, anxiety-like behaviour or depression-like behaviour. This preliminary study suggests that 5-HT deficiency in the brain has a significant effect on HFD-induced behaviour and the molecular response(Reference Karth, Baugher and Daly94). Suarez’s study(Reference Suárez, Rivera and Aparisi Rey101) used transgenic mice to assess the effects of cannabinoid type 1 receptor knockout on behavioural and molecular changes induced by a HFD (60 % kcal from fat) after long-term feeding for 26 weeks. The results showed that a HFD could significantly increase depression-like behaviour in wild-type mice but not in cannabinoid type 1 receptor knockout mice.
In 2012, Del Rosario et al. (Reference Del Rosario, McDermott and Panee93) used male mice with close homology to human genes and a CD-1 genetic background to conduct research. Fed a HFD (45 % kcal from fat) for 8 weeks, body weight was significantly increased by 14 %, the anxiety level increased significantly and the depression-like behaviour was reduced significantly, which implied that the HFD had an anti-depressant effect on the mice. Interestingly, the study found that the effects of a HFD on anxiety- and depression-like behaviour in male mice with a CD-1 genetic background were inconsistent. To date, most of the included studies have used mouse models and a few have used rat models. Careful consideration of the baseline traits of the strain in both rats and mice should be considered before experimentation(Reference Jacobson and Cryan111), although there is no systematic literature to study the advantages and disadvantages of various rodent models induced by HFD. More importantly, several studies using rodents with specific genetic backgrounds have yielded some interesting results, and they may represent appropriate models to explore the mechanism of HFD-induced mood disorders. Furthermore, non-human primates have been used to model mood disorders for several decades. The success of this paradigm is related to the fact that there are comparable cognitive skills, brain morphology and social complexity in adult monkeys and humans(Reference Nelson and Winslow112). Therefore, in addition to rodent models, this field could benefit from additional studies of non-human primate models.
Sex and age
Although depression is more common in women than men (almost 2:1), the vast majority of preclinical studies have been conducted in male animals(Reference Salk, Hyde and Abramson113). Only a limited number of studies have been conducted in female rodent models. There is little evidence supporting sex differences in mood disorders induced by a HFD. C57BL/6 mice were fed a HFD (60 % kcal from fat) for 12 weeks. Both male and female mice gained significantly more weight, but only males showed an increase in anxiety-like behaviour, not females(Reference Bridgewater, Zhang and Wu64). In another study(Reference Sweeney, O’Hara and Xu56), after feeding mice a HFD (60 % kcal from fat) for 15 weeks, anxiety-like behaviour and body weight increased and glucose tolerance decreased in both female and male C57BL/6J mice. HFD (59 % kcal from fat) exposure for 18 weeks led to glucose intolerance, anxiety/depression-like behaviour and neuroinflammation in male mice, with similar but non-significant trends in females(Reference Feng, Crowley and Patel106). Wu et al. (Reference Wu, Guo and Zhang57) also fed C57BL/6 female mice a HFD (60 % kcal from fat) and showed depression-like behaviour 32 weeks later. Therefore, there are significant sex differences in anxiety-like behaviours induced by a HFD, and male mice seem to be more sensitive to the anxiety-inducing effects of a HFD than female mice(Reference Bridgewater, Zhang and Wu64). The importance of sex as a biological variable must be emphasised in future similar studies.
Although almost all studies adopt 4- to 8-week-old adult rodents, it is important to point out that a research group(Reference Ogrodnik, Zhu and Langhi67) used 32-week-old elderly male C57BL/6J mice fed a HFD (60 % kcal fat) for 8 weeks and found that obesity results in the accumulation of senescent glial cells in proximity to the lateral ventricle, a region in which adult neurogenesis occurs. Furthermore, senescent glial cells exhibit excessive fat deposits, a phenotype termed ‘accumulation of lipids in senescence.’ Clearing senescent cells from high-fat-fed or leptin receptor-deficient obese mice restored neurogenesis and alleviated anxiety-related behaviour. Current animal literature supports the programming effect of maternal nutrition (Western style diet) on negative-valence behaviours (including depression and anxiety) of offspring(Reference Baker, Loughman and Spencer114,Reference Sullivan, Nousen and Chamlou115) . The next stage is not only to study the relationship between diet and neuropsychiatric disorders in adult mice but also to consider related research in offspring and elderly mice. Because of different ages, the pathogenesis may be different.
Mechanisms of high-fat diet-induced neuropsychiatric disorders
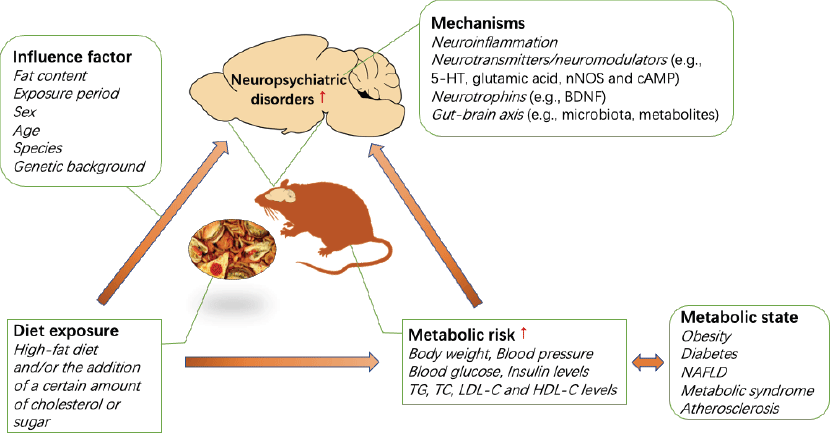
In this section, the discussed mechanisms include elevated neuroinflammation, changes in neurotransmitters/neuromodulators (e.g. 5-HT, glutamic acid, neuronal nitric oxide synthase and 3′,5′-cyclic AMP (cAMP)), neurotrophins (e.g. brain-derived neurotrophic factor (BDNF)) and the gut–brain axis (e.g. microbiota, metabolites), which may be involved in the pathogenesis of diet-induced neuropsychiatric disorders.
Neuroinflammation
A number of studies support the idea that HFD-induced neuropsychiatric disorders may be related to inflammatory responses in the brain(Reference Dutheil, Ota and Wohleb59,Reference Almeida-Suhett, Graham and Chen63,Reference Bruce-Keller, Salbaum and Luo65,Reference Wu, Lv and Pan73,Reference Suárez, Rivera and Aparisi Rey101,Reference Feng, Crowley and Patel106) , referred to as neuroinflammation, and to peripheral inflammatory responses(Reference Huang, Zhang and Ding61,Reference Wu, Lv and Pan73) . Two animal models examining short-term HFD-induced depression-like behaviour noted increased serum inflammatory factors, including IL-6, IL-1β, TNFα and IL-6(Reference Huang, Zhang and Ding61,Reference Kaczmarczyk, Machaj and Chiu104) . Furthermore, a long-term HFD significantly increased the expression of pro-inflammatory cytokines (TNFα, IL-1β, IL-6, IL-2 and IL-17A) in the hippocampus, amygdala and prelimbic cortex(Reference Almeida-Suhett, Graham and Chen63,Reference Wu, Lv and Pan73,Reference Feng, Crowley and Patel106) and down-regulated the expression of anti-inflammatory cytokines (IL-10 and IL-4) in the hippocampus(Reference Wu, Lv and Pan73). Increased anxiety-like behaviour was positively correlated with high expression of IL-1β in the amygdala(Reference Dutheil, Ota and Wohleb59,Reference Almeida-Suhett, Graham and Chen63,Reference Suárez, Rivera and Aparisi Rey101) . Four-month HFD feeding leads to anxiety and anhedonic behaviour associated with TLR expression and pro-inflammatory cytokine production (IL-6, IL-1β and TNFα)(Reference Dutheil, Ota and Wohleb59). Another experiment found that adipocyte-specific cannabinoid type 1 receptor influences obesity-related depression-like behaviour concomitantly with neuroinflammation in the hippocampus and hypothalamus(Reference Suárez, Rivera and Aparisi Rey101). In addition, obesity drives senescence in glial cells in the lateral ventricle of mouse brains, and clearing senescent cells from HFD-fed or leptin receptor-deficient obese mice restores neurogenesis and alleviates anxiety-related behaviour(Reference Ogrodnik, Zhu and Langhi67).
Neurotransmitters/neuromodulators
As a monoamine neurotransmitter, serotonin, also named 5-HT, is widely involved in the mediation of various life activities in the central and peripheral regions and its regulatory effect is related to the diversity of receptor subtypes(Reference Hoyer, Hannon and Martin116). As an ion-gated channel receptor, the 5-HT receptor is widely expressed in the hippocampus, amygdala and posterior polar region(Reference Enge, Fleischhauer and Gärtner117). In recent years, research on improving neuropsychiatric disorders induced by HFD has focused on 5-HT and its receptors(Reference Sumaya, Bailey and Catlett118). A number of preclinical studies have shown that a HFD regulates the metabolism of 5-HT in the brain and in the periphery and that 5-HT receptor antagonists may have anti-depressant and anti-anxiety effects(Reference Fakhfouri, Rahimian and Dyhrfjeld-Johnsen119). First, several studies have demonstrated that a HFD regulates peripheral 5-HT metabolism. Male C57BL/6 mice were fed a HFD for 4 weeks, which significantly increased circulating 5-HT levels(Reference Pan, Liu and Wan72). Female adult C57BL/6 mice were fed a HFD for 10 months, which led to decreased serum 5-HT and induced depression(Reference Wu, Guo and Zhang57). An untargeted plasma metabolomic analysis showed that a HFD that induces depressive-like behaviour could affect peripheral tryptophan metabolites (kynurenine pathway)(Reference Abildgaard, Elfving and Hokland105). Several studies have focused on how a HFD regulates 5-HT metabolism in the brain. Male C57BL/6 mice were fed a HFD for 14 weeks, which caused hippocampal neuron loss and decreased the gene expression of BDNF, 5-HT1A, 5-HTT and IDO2(Reference Wu, Lv and Pan73). Three studies from Kurhe’s team(Reference Kurhe and Mahesh95–Reference Kurhe, Mahesh and Devadoss97) confirmed that a HFD decreased the concentrations of cAMP, BDNF and 5-HT in the hippocampus, leading to anxiety and depression. Additionally, depressive- and anxiety-like behaviours increased in HFD-induced obese mice, the number of 5-HT- and TPH-positive cells decreased, and 5-HT1A and 5-HTTP protein expression decreased in the dorsal raphe(Reference Park, Lee and Cho68). Finally, two recent studies by Zemdegs’ team(Reference Zemdegs, Quesseveur and Jarriault51,Reference Zemdegs, Martin and Pintana52) systematically demonstrated the mechanism of HFD-induced anxiety and depression-like behaviour. The increased body weight, hyperglycaemia, impaired glucose tolerance and insulin resistance accompanied by elevated circulating levels of branched-chain amino acids in response to HFD were correlated with anxiogenic-like/depressive-like symptoms. Moreover, this phenotype was associated with decreased extracellular 5-HT levels in the hippocampus, which may result from increased sensitivity of the dorsal raphe 5-HT1AR.
Glutamic acid is considered to be the most abundant neurotransmitter in the brain, and its excitability plays a crucial role in brain structure and function. The two studies found that a decrease in hippocampal glutamate transporters may play a critical role in the pathogenesis of metabolic disorder-related depression. On the one hand, a HFD disturbs the function of hippocampal astrocytic neuroplasticity-related protein, GLAST, glutamate transporter 1 and connexin-43 and induces depression-like behaviours in mice(Reference Tsai, Wu and Chen60). On the other hand, high-lard/high-sucrose diets induced anxiety-like behaviour and an increase in glutamate transporters and a decrease in glutamate receptor mRNA expression in the prefrontal cortex(Reference Nakajima, Fukasawa and Gotoh91). Neuronal nitric oxide synthase is a key regulator of emotional behaviour. Some experiments(Reference Gainey, Kwakwa and Bray62,Reference Tomiga, Yoshimura and Ito102,Reference Tomiga, Yoshimura and Ra103) found that anxiety caused by a HFD was associated with increased levels of neuronal nitric oxide synthase in the hippocampus and cerebral cortex. The content of glutathione in the blood is used as a biomarker to reflect the redox state of mice(Reference Masood, Nadeem and Mustafa120). A HFD led to a more than 60 % reduction in total glutathione levels in the blood, which may have contributed to the increased anxiety levels in the mice(Reference Tomiga, Yoshimura and Ito102). Consumption of a HFD selectively induced the accumulation of palmitic acid in the hypothalamus, suppressed the cAMP/protein kinase A signalling pathway and increased the concentration of free fatty acid receptor 1. Deficiency of phosphodiesterase 4A, an enzyme that degrades cAMP and modulates stimulatory regulative G protein (Gs)-coupled receptor signalling, protected animals from either a genetic- or dietary-induced depression phenotype(Reference Vagena, Ryu and Baeza-Raja69).
Neurotrophin
BDNF, one of the major neurotrophic factors, plays an important role in the maintenance and survival of neurons and in synaptic plasticity. Several lines of evidence suggest that the expression of BDNF is decreased in depressed models. BDNF concentrations in the hippocampus were significantly lower in diet-induced obese mice, and leptin administration significantly increased hippocampal BDNF concentrations in CD mice(Reference Yamada, Katsuura and Ochi70). HFD rapidly impacts dopamine metabolism in the brain, appearing to trigger anxiety-like behaviours and learning/memory impairments prior to the onset of weight gain and/or pre-diabetes. Examination of the mouse cortex, hippocampus and hypothalamus for dopamine and its metabolites demonstrated increased homovanillic acid concentrations in the hippocampus and cortex that were associated with decreased cortical BDNF gene expression(Reference Kaczmarczyk, Machaj and Chiu104).
Gut–brain axis
Research into the role of the microbiota in modulating brain function has rapidly increased over the past 10 years. Increasing clinical and preclinical evidence implicates the microbiome as a possible key susceptibility factor for neurological disorders(Reference Cryan, O’Riordan and Sandhu25,Reference Valles-Colomer, Falony and Darzi121,Reference Sherwin, Dinan and Cryan122) . In fact, HFD consumption generally leads to a decrease in Bacteroidetes and an increase in Firmicutes. These alterations have been associated with obesity and the subsequent development of chronic diseases(Reference Murphy, Velazquez and Herbert123). First, a HFD damages markers of intestinal barrier function and increases circulating endotoxin levels. The evaluation of brain homogenates revealed that the HFD-shaped microbiota increased neuroinflammation and disrupted cerebrovascular homoeostasis(Reference Bruce-Keller, Salbaum and Luo65). Second, prolonged HFD-induced depression-like behaviour in mice was associated with significant changes in IM, brain metabolome, the NPY system and DPP-4 activity(Reference Hassan, Mancano and Kashofer66). Finally, a HFD increased the concentration of plasma leptin and faecal corticosterone and significantly decreased the relative expression of the leptin receptor OB-R in the hippocampus and small intestine. On the other hand, dopamine and noradrenaline levels in the small intestine and adrenaline levels in the hypothalamus were reduced. More importantly, the concentration of 5-HT in the hippocampus of mice was significantly reduced, and the relative expression of TLR2 in the small intestine and hippocampus was significantly increased, leading to the occurrence of anxiety and depression(Reference Agusti, Moya-Pérez and Campillo54). Intervention with Bifidobacterium pseudocatenulatum CECT 7765 for 14 weeks can adjust the endocrine and gut–brain axis and plays a role in obesity co-morbid with depressive behaviour. Diet is perhaps one of the greatest factors influencing microbiota composition(Reference Sandhu, Sherwin and Schellekens124). Where research has shown that there are clear links between diet and neuropsychiatric disorders, assessing what role, if any, the microbiome has in terms of causality will be important(Reference Cryan, O’Riordan and Sandhu25). The effects of both dietary components and microbial-generated metabolites on host physiology and health are gaining attention, which will be important for moving therapeutic approaches forward.
Diet exacerbates neuropsychiatric disorders observed in animal models of depression
Finally, some literature supports that not only does diet induce or increase the risk of anxiety and depression-like behaviour, but, more importantly, it may also aggravate anxiety- and depression-like behaviour in rodent models of neuropsychiatric disorders (Table 2).
Table 2 Diet exacerbates neuropsychiatric disorders observed in animal models of depression

–, Not reported; ↑, increase v. control diet; EPM, elevated plus-maze; OFT, open field test; ORT, object recognition test; LDT, light and dark test; FST, forced swim test; TST, tail suspension test; SLA, spontaneous locomotor activity score; SIT, social interaction test; SPT, sucrose preference test; TC, total cholesterol; AST, aspartate aminotransferase; ALT, alanine aminotransferase.
*P < 0·05 was considered significant.
Chronic social defeat stress paradigm-induced depression model
The chronic social defeat stress (CSDS) paradigm induces depression by repeatedly exposing naïve mice to aggressor mice. After 7–15 d in this emotionally stressful environment, mice display robust depressive phenotypes, which are characteristic of human symptoms(Reference Allen and Badcock125). Male C57BL6/J mice (8–10 weeks old) were subjected to CSDS for 10 d and then fed a HFD (42 % kcal from fat) for 30 d. Mice subjected to CSDS and then fed a HFD for 30 d display significantly severe depression-related behaviour (greater social avoidance than mice receiving regular chow) accompanied by redistribution of body fat and increased serum leptin levels(Reference Chuang, Krishnan and Yu126). Male Wistar rats (21 d old) were subjected to social isolation during the prepubertal period for 7 d and then fed a HFD (42 % kcal from fat) for 70 d. They found that both social isolation and HFD induced depressive-like behaviour. These findings showed that brief social isolation and chronic HFD during a sensitive developmental period cause depressive-like behaviour in adulthood(Reference Arcego, Toniazzo and Krolow127). Eight-week-old male C57BL/6 mice were subjected to CSDS for 10 d and then fed a HFD (60 % kcal from fat) for 2 h or 24 h(Reference Otsuka, Shiuchi and Chikahisa128). HFD intervention for 2 h increased the anxiety-like behaviour of the CSDS mice. However, HFD intervention for 24 h can reduce the anxiety of CSDS mice. It is suggested that a HFD may reduce the burden of stress and this benefit is probably related to the metabolism of cholesterol in the liver.
Chronic unpredictable mild stress-induced depression model
Chronic unpredictable mild stress (CUMS) is currently the most commonly used, reliable and effective rodent model of depression(Reference Willner, Towell and Sampson129). Experimental mice underwent CUMS exposure for approximately 14 consecutive days with exposure each day to at least one stressor that was randomly chosen from nine different stressors, and stressors were given in a random order to ensure unpredictability(Reference Willner130). A HFD and/or the addition of a certain amount of cholesterol or sugar exacerbates the neuropsychiatric disorders observed in animal models of depression. Six-week-old female and male C57BL/6 mice were fed a HFD (60 % kcal from fat) for 12 weeks and then subjected to CUMS for 18 d. Male mice were more vulnerable to the anxiogenic effects of the HFD, and obese male mice showed decreased locomotion activity in response to stress, whereas obese female mice did not. These results revealed distinct sex differences in the impacts of obesity and stress on anxiety-like behaviours in mood disorders(Reference Bridgewater, Zhang and Wu64). Male Wistar Han rats were fed a HFD (45 % kcal from fat) for 19 weeks and subjected to CUMS for 6 weeks. Specifically, animals fed a HFD displayed depressive- and anxious-like behaviours that were only present in the normal diet group upon exposure to CUMS. Of note, these mood impairments were not further aggravated when the HFD animals were exposed to CUMS, which suggests a ceiling effect(Reference Aslani, Vieira and Marques47). Male Wistar rats were fed a HFD (60 % kcal from fat) for 8 weeks and repeatedly exposed to CUMS for 3 weeks. The rats exhibited the most severe depression-like and anxiety-like behaviour. Depressive- and anxiety-like behaviours as well as cognitive impairment were positively correlated with the highest weight, plasma leptin levels and LepRb protein and mRNA levels in the hippocampus and hypothalamus(Reference Yang, Liu and Jiang55). Several studies(Reference Magdy, El-Kharashi and Nabih74,Reference Khedr, Elmelgy and El-Kharashi76) used a diet containing 2 % cholesterol to feed rats for 8–12 weeks and exposed them to chronic restraint stress for 6–8 weeks. In addition to abnormal liver lipids, atherosclerosis and other metabolic disorders, with the high cholesterol intervention, the rats later developed depression-like behaviours, cognitive impairment and diminished object recognition memory as well. In addition, sitagliptin is recommended in the management of NASH, especially when associated with depression(Reference Magdy, El-Kharashi and Nabih74), and metformin, which is commonly used to treat hepatogenic diabetes, can ameliorate resistance to fluoxetine in depression(Reference Khedr, Elmelgy and El-Kharashi76).
Genetic animal model of depression
Flinders Sensitive Line rats were, through selective breeding techniques, bred to be more sensitive to the anticholinesterase diisopropyl fluorophosphate(Reference Overstreet and Russell131), and Flinders Sensitive Line rats have been widely described and highly validated as a genetic animal model of depression(Reference Overstreet, Friedman and Mathé132). Abildgaard et al. used Flinders Sensitive Line rats fed with a HFD (60 % kcal from fat, mainly lard) for 10 weeks and subjected them to behavioural testing and metabolic assessment. They found that the HFD increased weight, insulin levels and fasting blood glucose levels. At the same time, HFD consumption led to exacerbation of the depressive-like behaviour of Flinders Sensitive Line rats in the forced swim test, which is a depression screening tool, and diminished social interaction as well(Reference Abildgaard, Solskov and Volke133).
Conclusions
The association between the development of metabolic disorders and chronic consumption of a HFD has been well demonstrated. Interestingly, emerging evidence indicates that HFD and metabolic risk are also associated with an increased risk of neuropsychiatric disorders, including anxiety and depression, in humans and in animals. A HFD and/or the addition of a certain amount of cholesterol or sugar can induce anxiety- or depression-like behaviours, which may vary based on the diet exposure period, sex, age, species and genetic background of the animals used. In addition, diet significantly aggravates anxiety- or depression-like behaviour in animal models of neuropsychiatric disorders. The effects of a HFD on anxiety are critically linked to the duration of HFD exposure. Prolonged exposure to a HFD promotes anxiety and depression in animals that are susceptible to HFD-induced metabolic disorders, and diets with a high fat percentage may accelerate the progression of this relationship in animal models of depression. In addition, there are significant sex differences in anxiety-like behaviours induced by a HFD and/or the addition of a certain amount of cholesterol or sugar. However, there have been a very limited number of studies involving female mice, and the direction of the sex differences in any individual behavioural test has not been well described. Future studies should investigate the extensive contributions of sex to neurobehavioural outcomes. It is not clear how diet affects anxiety- and depression-related behaviours, while the strong foundation of the literature supports continued investigation of the mechanisms of diet-induced neuropsychiatric behaviour. In addition to diet, metabolic conditions can increase the risk of neuropsychiatric diseases. Individual metabolic status are highly correlated with diet, so it is difficult to distinguish their contribution to individual neuropsychiatric disorders. Currently, epidemiological studies are complicated by individual differences in diet and nutrition, but the dietary pattern and exposure period can be precisely controlled in animal models. To date, most studies have used rodent models, yielding some interesting results. The mechanisms by which diet induces anxiety/depressive-like behaviour may involve neuroinflammation, neurotransmitters/neuromodulators (e.g. 5-HT, glutamic acid, neuronal nitric oxide synthase and cAMP), neurotrophins (e.g. BDNF) and the gut–brain axis (e.g. microbiota, metabolites) (see Fig. 1). Further studies are needed to determine the cellular and neural circuit mechanisms governing the complex relationship between diet, metabolic state and neuropsychiatric behaviour. Furthermore, non-human primates have been used to model mood disorders for several decades. The success of this paradigm is related to the comparable cognitive skills, brain morphology and social complexity between adult monkeys and humans(Reference Nelson and Winslow112). Therefore, in addition to rodent models, this field could benefit from additional studies of non-human primate models. Altogether, the presented literature has provided reliable evidence for the association between diet and neuropsychiatric disorders, and future research should be focused on elucidating the mechanism and identifying the contribution of diet and diet-induced metabolic risk to neuropsychiatric disorders, which can form the basis for future clinical dietary intervention strategies for neuropsychiatric disorders.
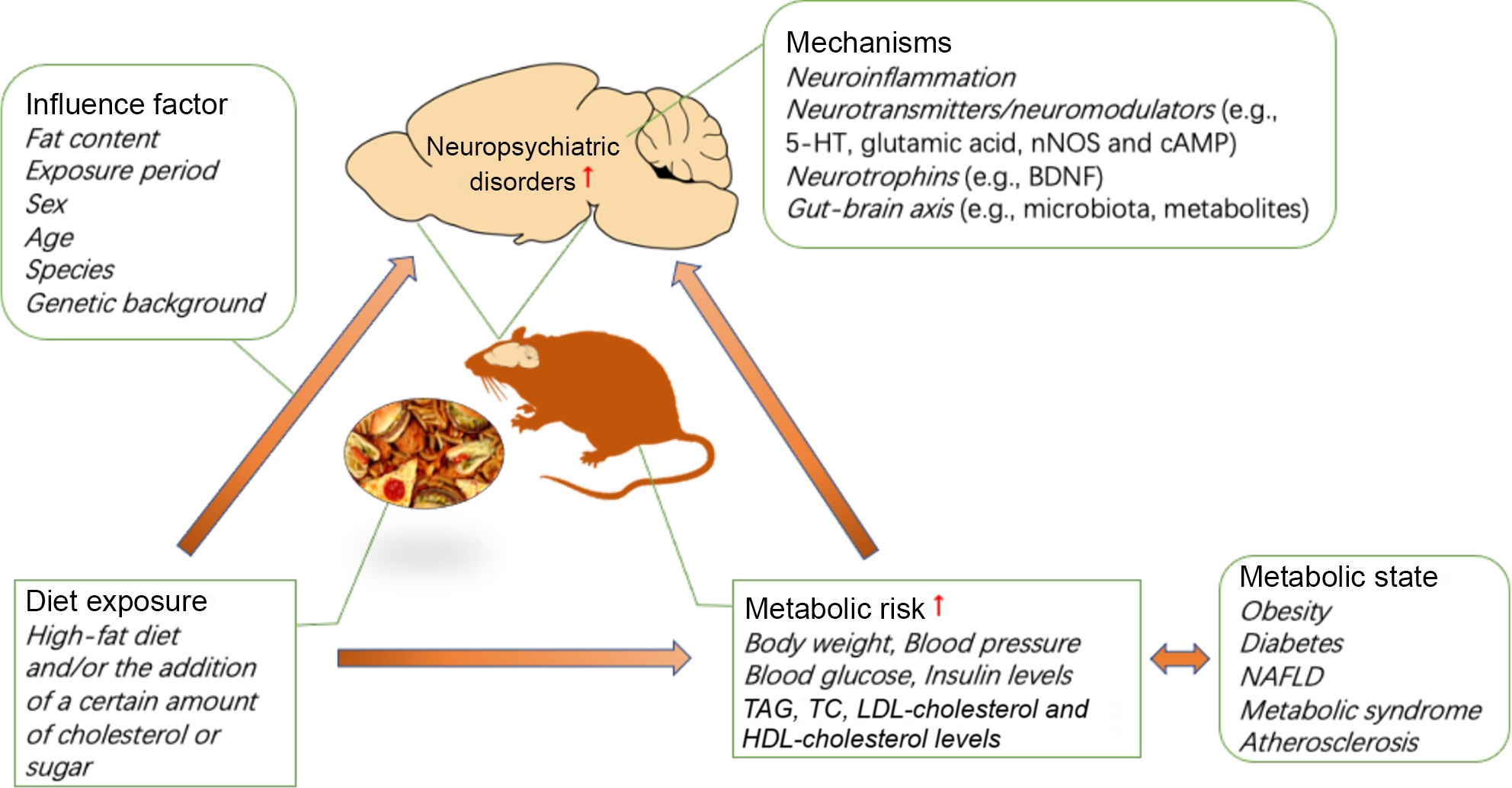
Fig. 1. Impact of diet and metabolic risk on neuropsychiatric disorders. BDNF, brain-derived neurotrophic factor; cAMP, 3′,5′-cyclic AMP; HFD, high-fat diet; 5-HT, 5-hydroxytryptamine; NAFLD, non-alcoholic fatty liver disease; nNOS, neuronal nitric oxide synthase; TC, total cholesterol.
Acknowledgements
This work was supported in part by the Traditional Chinese Medicine Bureau of Guangdong in China (no. 20161065 and 20201075), the National Health and Family Planning Commission of Guangdong in China (no. A2016583, A2017228, A2017140, A2020137 and A2021374), the Natural Science Foundation of Guangdong in China (no. 2016A030313824), the Undergraduate Training Programs for Innovation and Entrepreneurship of Jinan University in China (no. CX20157 and CX20145), the ‘Challenge Cup’ Undergraduate Academic Science and Technology Curriculum of Jinan University in China (no. 19113028), the 22nd Batch of Teaching Reform Research Projects of Jinan University (JG2020080) and Teaching Quality and Teaching Reform Project of Undergraduate University of Guangdong in China (2017 and 2020).
Y. L. and C. L. designed the study; L. Z. and Y. T. analysed data; S. Z. and X. C. edited for English; Y. L. wrote the manuscript, and C. L. revised the manuscript. All the authors read and approved the manuscript.
The authors declare that there are no conflicts of interest.