Delusional parasitosis is an uncommon diagnosis with an estimated incidence of 1.9 per 100 000 person years.Reference Bailey, Andersen, Lowe, Pittelkow, Bostwick and Davis 1 The diagnosis of delusional parasitosis is often delayed as patients are frequently evaluated by multiple physicians before seeing a psychiatrist. Patients with delusional parasitosis are often reluctant to accept psychiatric input as they steadfastly believe that their symptoms have a physical aetiology.Reference Lepping, Freudenmann, Huber, Bewley, Taylor, Reichenberg and Magid 2 There are numerous publications on delusional parasitosis, which have been comprehensively reviewed by Freudenmann et al Reference Freudenmann and Lepping 3 and Trabert.Reference Trabert 4 This case is unique in that it was identified by a consultation–liaison psychiatrist during a medical hospital stay. The consultation–liaison psychiatrist continued treating the patient on an out-patient basis following the hospital stay and provides a longitudinal perspective on the first 3 years of treatment. The successes and pitfalls of the treatment process are reviewed, and possible implications for an expanded role of consultation–liaison psychiatrists are discussed.
Case report
Mr T. was a 65-year-old South American man who presented to the emergency department with dyspnoea and lethargy and was admitted for an exacerbation of congestive heart failure and acute kidney injury. His medical history included: atrial fibrillation, coronary artery disease, diabetes mellitus, chronic kidney disease, hypertension, obesity, remote cerebrovascular accident (CVA) and obstructive sleep apnoea. He acknowledged to his medical team that he had not been compliant with his out-patient medications which included: carvedilol, digoxin, furosemide, glipizide, insulin aspart, insulin glargine, metolazone, pantoprazole, potassium chloride, rivaroxaban and spironolactone. His wife also informed the admitting physician that his activity level was markedly decreased and that he had become preoccupied with the idea that there were bugs on his skin, though she had never seen any bugs on his body or in their house. This was the patient's second hospital stay in the course of a month, and his eighth hospital stay in a 3-year period. Psychiatry had not been consulted during any of the previous hospital stays, as the patient had never endorsed any psychiatric symptoms. However, during this admission psychiatry was consulted to evaluate for depression and comment on a potential psychogenic aetiology for his dermatological concerns.
On consultation, the patient expressed that there was nothing psychiatrically wrong with him and felt that his activity level was normal for a man of his age and health status. He stated that he did not see a reason to continue taking his medications as he had little hope for improvement, but he was not suicidal. On further questioning, he endorsed a full vegetative–affective cluster of depressive symptoms that had been present for more than 1 year and were getting progressively worse. He reported that he had never previously been depressed, nor had he ever experienced symptoms of mania.
When asked about his dermatological condition, he described seeing and feeling small black bugs on his skin that caused pruritic skin lesions, though no bugs were visible to the examiner. He revealed numerous excoriations located diffusely on his extremities and trunk. He acknowledged feeling extremely discouraged about attempting to get treatment for the skin lesions and felt that physicians did not believe him, noting that he had been dismissed from one dermatologist's practice. He denied any other recent or past psychotic symptoms.
In obtaining collateral information from his wife, it was learned that Mr T. had been reporting having bugs on his skin for the past year. Each day he would scratch his skin, take extended hot showers and examine the skin he removed under a microscope. It was later revealed that he retained boxes of removed skin fragments and attempted to treat his condition by acidifying his blood by drinking lemon and grapefruit juices. Observation of the skin lesions was documented in the hospital record 10 months before this admission, but it was noted that this was being evaluated in the out-patient setting. His wife stated that he was examined by 11 different dermatologists, and none of them was able to find a cause for the skin lesions. She reported that he had always been a ‘pessimistic person’, but that his mood and activity level began to decline 7 years earlier, when he was initially diagnosed with cardiovascular disease. His mood had become particularly depressed in the time leading up to his concerns about bugs on his skin and had further worsened since. She also noted, and Mr T. confirmed, that he had no other previous history of mental illness, psychiatric treatment or suicide attempts. He had a 40 pack-year smoking history, but reduced his smoking significantly when he was diagnosed with cardiovascular disease. He reported only modest alcohol use and no use of illicit drugs. For many years he owned a successful construction company, but because of his illnesses he had largely relinquished control of the business to his son. He had attended some college, and there was never any suspicion of learning disorder. He reported no family history of mental illness.
On examination, his vital signs were within normal limits, and there were no focal neurological deficits. He displayed significant psychomotor retardation, was intermittently tearful and had a dysphoric affect. Cognitive testing revealed mildly impaired attention (as assessed by digit span), though he was fully oriented and his recent and remote memory were intact. Laboratory testing revealed normal urinalysis, negative urine drug screen, normal vitamin B12, normal folate, normal thyroid stimulating hormone and nonreactive rapid plasma reagin. A complete blood count was unremarkable, and a metabolic panel revealed an elevated blood urea nitrogen (106) and creatinine (2.3), which was increased from his baseline creatinine of 1.3–1.8. Computed tomography without contrast of his head showed no acute findings; however, mild to moderate small vessel ischemic disease and a remote left-sided parietal lobe infarction were noted, both of which were unchanged from a previous study (5 years earlier). No abnormalities in the occipital or temporal lobes were reported.
The patient was diagnosed with secondary delusional parasitosis because of major depressive disorder (MDD) with psychotic features by DSM-IV-TR criteria. He was started on escitalopram 5 mg daily and haloperidol 1 mg at bedtime, which were increased to 10 and 2 mg, respectively, after 3 days. On hospital day 5, the patient had improved significantly from a cardiovascular and renal standpoint and was discharged. His psychiatric symptoms were unchanged at the time of discharge, but he was agreeable to out-patient follow-up.
At the time of his first out-patient visit, 1 month after the initial consultation, he reported a significant improvement in his mood, noted no residual neurovegetative symptoms of depression and had returned to work. While he estimated that he had between 200 and 300 skin lesions at the height of his condition, he now had only four lesions. He reported that he discarded several boxes of removed skin fragments and discontinued the practice of acidifying his blood. At his second follow-up visit, 4 months after the initial consultation, his mood remained euthymic. He developed significant insight into his psychotic depression and openly discussed how this negatively affected his quality of life. For example, he typically enjoyed spending time with his grandchildren, but he actively avoided them when his skin lesions were present, fearing that he would infect them. Also, he felt that people in public places avoided him, fearing that they would become infected.
The patient presented for his 6-month follow-up appointment, remained asymptomatic and asked if he could discontinue his psychotropic medications. After a discussion of risks and benefits, he was advised that it was reasonable to taper off of haloperidol, but that he should continue to take escitalopram. He did not present for his next follow-up appointment and was lost to follow-up for the next 8 months. Fourteen months after the initial consultation, Mr T. was readmitted to the hospital with confusion and ataxia. He was found to be in a hyperosmolar hyperglycemic state, with a blood glucose level of 889 and a haemoglobin A1C of 16. As his blood glucose normalised, his mental status readily improved. A psychiatric consultation was requested for suspected depression. On interview, it was determined that Mr T. had become depressed approximately 2 months earlier after stopping escitalopram the month before. He then stopped taking his insulin. He endorsed a complete vegetative–affective cluster consistent with recurrent MDD, but on this occasion denied the presence of psychotic features. Ultimately, he agreed to restart escitalopram, 10 mg daily, and resumed out-patient follow-up.
Following an increase of escitalopram to 20 mg at month 15, he displayed a complete remission of his depressive episode at his 16-month out-patient visit. He was again lost to follow-up between months 16 and 27, following which he presented for an out-patient visit with a recurrence of MDD and delusional parasitosis. Symptoms reportedly began 2 months earlier despite adherence to escitalopram. He was restarted on haloperidol 2 mg to address delusional parasitosis. During month 28, he was medically hospitalised twice for congestive heart failure exacerbations accompanied by a hypoglycaemic episode, in the first admission, and an episode of acute kidney injury, in the second admission. He was followed by psychiatry during both admissions. Haloperidol was increased to 3 mg for ongoing delusional parasitosis, and trazodone 25 mg was added for insomnia on an as needed basis. He presented for an out-patient visit at 29 months with no improvement in symptoms of MDD or delusional parasitosis. Consequently, he was changed from haloperidol to aripiprazole 10 mg daily. At 30 months, he demonstrated a full remission of MDD and delusional parasitosis, and no longer required trazodone for sleep. During months 31–36, he consistently attended out-patient visits and demonstrated no recurrent symptoms of MDD or delusional parasitosis. The course of Mr T.'s treatment in the 36 months following the initial consultation is summarised in Table 1.
Table 1 Overview of treatment course

| Time (Months) | IP/OP | MDD | Delusional parasitosis | Medications | Medication changes |
|---|---|---|---|---|---|
| Hospitalised for congestive heart failure exacerbation and acute kidney injury at the time of consultation | |||||
| 0 | IP | Yes | Yes | None | Start escitalopram 10 mgStart haloperidol 2 mg |
| 1 | OP | No | No | Escitalopram 10 mg, haloperidol 2 mg | None |
| 3 | OP | No | No | Escitalopram 10 mg, haloperidol 2 mg | None |
| 6 | OP | No | No | Escitalopram 10 mg, haloperidol 2 mg | Stop haloperidol |
| Lost to follow-up between months 6 and 14 | |||||
| Hospitalised for hyperosmolar hyperglycemic state at month 14 | |||||
| 14 | IP | Yes | No | None | Start escitalopram 10 mg |
| 15 | OP | Yes | No | Escitalopram 10 mg | Increase escitalopram to 20 mg |
| 16 | OP | No | No | Escitalopram 20 mg | None |
| Lost to follow-up between months 16 and 27 | |||||
| 27 | OP | Yes | Yes | Escitalopram 20 mg | Start haloperidol 2 mg |
| Hospitalised for hypoglycaemia and congestive heart failure exacerbation at month 28 | |||||
| 28 | IP | Yes | Yes | Escitalopram 20 mg, haloperidol 2 mg | Increase haloperidol to 3 mg Start trazodone 25 mg prn |
| Hospitalised for congestive heart failure exacerbation and acute kidney injury at month 28.5 | |||||
| 28.5 | IP | Yes | Yes | Escitalopram 20 mg, haloperidol 3 mg, trazodone 25 mg prn |
None |
| 29 | OP | Yes | Yes | Escitalopram 20 mg, haloperidol 3 mg, trazodone 25 mg prn |
Stop haloperidol Start aripiprazole 10 mg |
| 30 | OP | No | No | Escitalopram 20 mg, aripiprazole 10 mg, trazodone 25 mg prn |
Stop trazodone |
| 31 | OP | No | No | Escitalopram 20 mg, aripiprazole 10 mg | None |
| 32 | OP | No | No | Escitalopram 20 mg, aripiprazole 10 mg | None |
| 33 | OP | No | No | Escitalopram 20 mg, aripiprazole 10 mg | None |
| 34 | OP | No | No | Escitalopram 20 mg, aripiprazole 10 mg | None |
| 35 | OP | No | No | Escitalopram 20 mg, aripiprazole 10 mg | None |
| 36 | OP | No | No | Escitalopram 20 mg, aripiprazole 10 mg | None |
IP, in-patient; OP, out-patient.
Discussion
Delusional parasitosis is characterised by a fixed and persistent belief of having a pathogenic infection, despite definitive evidence to the contrary. Along with the delusion of having bugs on their body, patients can also experience visual and tactile hallucinations or illusions of seeing and feeling the bugs.Reference Freudenmann and Lepping 3 , Reference Hinkle 5 Patients describe the pathogens in various ways including: bugs, insects, worms, viruses, bacteria or fibres. Patients with delusional parasitosis tend to engage in repeated self-examinations and laborious cleansing practices in an endeavour to remove the causative pathogen. These practices result in excoriations and skin lesions, ultimately reaffirming the belief of an infection.Reference Freudenmann and Lepping 3
Delusional parasitosis that presents in isolation and cannot be explained by any other condition is referred to as primary delusional parasitosis, that is, delusional disorder somatic type. Secondary delusional parasitosis can result from: psychiatric disorders, general medical conditions, therapeutic or recreational substances or organic brain disease.Reference Freudenmann and Lepping 3 Other authors have detailed aetiologies of secondary delusional parasitosis (Table 2).Reference Lepping, Freudenmann, Huber, Bewley, Taylor, Reichenberg and Magid 2 , Reference Freudenmann and Lepping 3 , Reference Beach, Kroshinsky and Kontos 6 Freudenmann et al Reference Freudenmann and Lepping 3 indicated that the relative predominance of primary or secondary delusional parasitosis has not been established. Delusional parasitosis can be episodic or chronic, and the duration can vary from days to decades, with mean duration of approximately 3 years.Reference Freudenmann and Lepping 3 Primary delusional parasitosis tends to have a chronic course. Depending on the aetiology, secondary delusional parasitosis may have a chronic course, for example, if it is because of a chronic medical condition, or a very brief course, for example, if it is substance-induced.Reference Freudenmann and Lepping 3 Freudenmann et al Reference Freudenmann and Lepping 3 reported that cases of secondary delusional parasitosis associated with MDD-recurrent type with psychotic features are usually characterised by an episodic course with intermittent asymptomatic periods.
Table 2 Potential aetiologies of secondary delusional parasitosisReference Lepping, Freudenmann, Huber, Bewley, Taylor, Reichenberg and Magid 2 ,Reference Freudenmann and Lepping 3 ,Reference Beach, Kroshinsky and Kontos 6
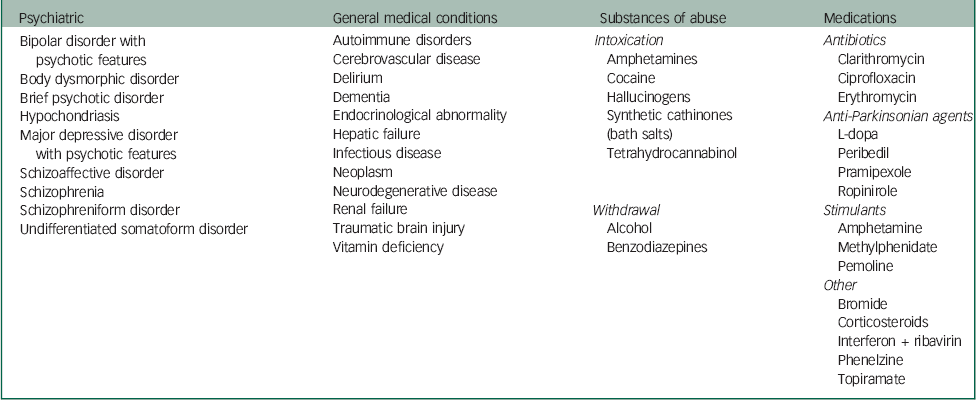
| Psychiatric | General medical conditions | Substances of abuse | Medications |
|---|---|---|---|
| Bipolar disorder with psychotic features Body dysmorphic disorder Brief psychotic disorder Hypochondriasis Major depressive disorder with psychotic features Schizoaffective disorder Schizophrenia Schizophreniform disorder Undifferentiated somatoform disorder |
Autoimmune disorders Cerebrovascular disease Delirium Dementia Endocrinological abnormality Hepatic failure Infectious disease Neoplasm Neurodegenerative disease Renal failure Traumatic brain injury Vitamin deficiency |
Intoxication
Amphetamines Cocaine Hallucinogens Synthetic cathinones (bath salts) Tetrahydrocannabinol Withdrawal Alcohol Benzodiazepines |
Antibiotics
Clarithromycin Ciprofloxacin Erythromycin Anti-Parkinsonian agents L-dopa Peribedil Pramipexole Ropinirole Stimulants Amphetamine Methylphenidate Pemoline Other Bromide Corticosteroids Interferon + ribavirin Phenelzine Topiramate |
Affective psychosis is not a common aetiology of secondary delusional parasitosis; these patients only accounted for 9% of all cases (including primary and secondary delusional parasitosis) in Trabert's meta-analysis.Reference Trabert 4 However, a single-centre study of patients with delusional parasitosis indicated that 74% had a history of depression, suggesting that these conditions may commonly co-occur.Reference Foster, Hylwa, Bury, Davis, Pittelkow and Bostwick 7 As Freudenmann et al Reference Freudenmann and Lepping 3 notes, there are patients for whom delusional parasitosis appears to precipitate depression, as well as patients for whom depression precipitates delusional parasitosis. Therefore, obtaining a careful longitudinal history has important implications in terms of determining the aetiology of delusional parasitosis and initiating appropriate treatment.
Antipsychotic medications have been the mainstay of treatment for delusional parasitosis. Although first-generation antipsychotics have frequently been used,Reference Trabert 4 current literature suggests that second-generation antipsychotics are efficacious for primary and secondary delusional parasitosis.Reference Freudenmann and Lepping 8 Freudenmann et al Reference Freudenmann and Lepping 8 noted a greater and more rapid response to treatment in secondary as opposed to primary delusional parasitosis. A systematic literature review revealed that both first- and second-generation antipsychotics are similarly efficacious in treating primary delusional parasitosis.Reference Lepping, Russell and Freudenmann 9 It has been postulated that serotonergic agents can be effective in treating primary and secondary delusional parasitosis.Reference Cupina and Boulton 10 , Reference Hayashi, Oshino, Ishikawa, Kawakatsu and Otani 11
In the case of Mr T., delusional parasitosis was interpreted as a secondary manifestation of MDD with psychotic features, and the patient was started on a combination of escitalopram and haloperidol. Escitalopram was chosen because of the low likelihood of drug–drug interactions.Reference Bareggi, Mundo, Dell'Osso and Altamura 12 Haloperidol was chosen over second-generation antipsychotics to reduce the risk of metabolic side effects. The decision to discontinue haloperidol following 6 months of remission of delusions was motivated by patient preference and the potential for side-effects. Previous publications have suggested treating delusional parasitosis with antipsychotic medications for at least 3 months, and that secondary delusional parasitosis tends to respond to treatment more rapidly than primary delusional parasitosis: 3 weeks as compared with 10 weeks on average.Reference Foster, Hylwa, Bury, Davis, Pittelkow and Bostwick 7 Treatment algorithms for secondary delusional parasitosis have supported simultaneously prescribing an antidepressant and an antipsychotic for delusional parasitosis associated with depression.Reference Lepping and Freudenmann 13
Mr T. experienced a second major depressive episode approximately 1 year after the resolution of the first episode, though he did not experience a recurrence of psychotic symptoms. The potential reasons for this relapse are numerous, but may include: his wife being diagnosed with cancer, poor adherence to escitalopram, poor blood glucose control and worsening cardiovascular and renal disease.
Mr T. experienced a third episode of MDD that began approximately 25 months after the initial consultation and was accompanied by delusional parasitosis. Haloperidol was restarted when Mr T. returned to treatment at month 27, but was ultimately changed to aripiprazole at month 29. This change was made because of the persistence of delusional parasitosis after 2 months of treatment with haloperidol, and the potential added benefit of aripiprazole on MDD symptoms.Reference Wen, Wang, Liu, Huang, Liu and Hu 14 Within 1 month of the medication change, he displayed remission of MDD and delusional parasitosis.
One important limitation of this case is that it cannot be definitively proven that MDD with psychotic features was the most accurate diagnosis for Mr T. Visual and tactile hallucinations typically are not of psychiatric origin, though both have been associated with delusional parasitosis.Reference Freudenmann and Lepping 3 , Reference Hinkle 5 Nihilistic delusions are much more common than somatic delusions in MDD with psychotic features. Although Mr T. expressed pessimistic thoughts (e.g. regarding his prognosis and his perception that people were avoiding him), his delusions were predominantly somatic. Although Mr T. and his family reported that worsening depressive symptoms preceded delusional parasitosis, recall bias cannot be excluded as delusional parasitosis began 1 year before evaluation. If delusional parasitosis developed before the onset of MDD, it is possible that the initial depressive episode was provoked by delusional parasitosis or that two separate processes were occurring. Since the patient was lost to follow-up on two occasions, it is difficult to draw conclusions about the relative impact of nonadherence and psychosocial factors on his second and third depressive episodes.
A conclusive diagnosis was further obscured by the presence of comorbid medical issues including congestive heart failure, diabetes mellitus and renal disease, all of which have been associated with secondary delusional parasitosis.Reference Freudenmann and Lepping 3 , Reference Herbert, Cameron and Battistella 15 , Reference Fruedenmann 16 Since exacerbations of these conditions can incite delirium, which can also cause secondary delusional parasitosis,Reference Freudenmann and Lepping 3 this diagnosis was carefully considered during the initial hospital stay. Delirium was ruled out clinically as Mr T.'s awareness and cognition were consistently intact. Although he did have a mild deficit in attention, it was not clear that this represented an acute change. An electroencephalogram would have provided objective evidence to rule out delirium,Reference Jacobson, Leuchter, Walter and Weiner 17 but was not obtained. However, even if delirium had been present during the hospital stay, the duration of his delusional parasitosis symptoms would have suggested against a causal relationship. It is also possible that the patient's prior CVA, involving the left parietal cortex, could have played a role in his delusional parasitosis. The absence of a brain MRI to provide greater anatomical detail of the parietal lesion is a limitation of this report. The relationship between cerebrovascular disease and delusional parasitosis is incompletely understood. Striatal lesions have been observed in patients with secondary delusional parasitosis due to medical causes, and it has been suggested that striato-thalamo-parietal circuitry may be pertinent.Reference Huber, Karner, Kirchler, Lepping and Freudenmann 18 More recently, measurable differences in multiple areas of cortex have been observed in patients with delusional parasitosis relative to healthy controls.Reference Hirjak, Huber, Kirchler, Kubera, Karner and Sambataro 19
Additionally, the patient was born and raised in South America, and it is possible that cultural factors played a role in how he experienced and reported his symptoms. One international study of the relationship between somatic symptoms and depression found that the two participating South American countries had among the highest rates of medically unexplained somatic symptoms.Reference Simon, VonKorff, Piccinelli, Fullerton and Ormel 20
This case demonstrates the integral role that consultation–liaison psychiatry services can play in the care of medically hospitalised patients. After the patient was seen by psychiatry, diagnosed and treated, he displayed a nearly complete remission of MDD and delusional parasitosis within 1 month. His social and occupational function improved, and he was only hospitalised once in the following calendar year. Treating his depression was particularly important as depression is a known risk factor for medication nonadherence, which had been a recurrent problem for this patient.Reference Bet, Penninx, van Laer, Hoogendijk and Hugtenburg 21
It is possible that initially seeing the psychiatrist in a medical setting, receiving reassurance that his symptoms were real, and being able to follow up with the same psychiatrist as an out-patient made the involvement of psychiatry more acceptable to the patient. Mr T. is a good example of a patient who might benefit from ongoing follow-up with a consultation–liaison psychiatrist. Patients who are uncomfortable or unfamiliar with mental health treatment, have significant medical comorbidities and have symptoms with physical and psychiatric overlap may fall into this category. Also, given the increasing interest in multi-specialty medical homes, consultation–liaison psychiatrists may have an opportunity for an expanded out-patient role in the future.Reference Katon and Unutzer 22
Patients with delusional parasitosis are not commonly seen by hospital-based consultation–liaison psychiatrists, likely because they are typically treated on an out-patient basis and present to physicians of other specialties. In this case, Mr T. was hospitalised for seemingly unrelated medical problems, and his dermatological and psychiatric concerns were uncovered only by a thorough review of systems and examination by the admitting physician. The consultation–liaison psychiatry team was able to effectively collaborate with the medical team and initiated definitive treatment during the medical hospital stay. This is particularly significant given the medical, psychiatric and cultural complexities of this case, and the likelihood that the patient might not have otherwise sought psychiatric treatment. Mr T. exemplifies one type of patient who might benefit from ongoing treatment from a consultation–liaison psychiatrist following hospital discharge.





eLetters
No eLetters have been published for this article.