Isotopes are elements with identical chemical and functional properties but different in atomic mass due to having different number of neutrons in the nucleus. The difference in mass makes it possible for the isotopes to be analytically set apart from each other( Reference Wilkinson 1 ). Thus the metabolic fate of each isotope can be traced when introduced into a biological system. Stable isotopes refer to non-radioactive isotopes occurring naturally, but elements or compounds can be synthesised that are enriched compared with the naturally occurring amount. Stable isotope techniques have been used in studies of human nutrition for over 50 years( 2 ). With the development of MS and infrared spectroscopy techniques coupled with wide commercial availability of tracers labelled with stable isotopes including 13C, 15N, 2H and 18O, detailed metabolic pathways can be investigated( Reference Bequette, Sunny and El-Kadi 3 ).
In nutrition, stable isotopes can be used to measure the amount of water or other nutrients in the body or the amount of an ingested nutrient that is absorbed and metabolised or excreted. They can be applied to determine the rate of absorption, utilisation or synthesis of proteins, fats or carbohydrates. Stable isotope techniques are non-invasive, and do not involve any radiation hazard. In nutritional assessments, stable isotope labelled compounds are given orally, often as part of a test meal, or intravenously and after a period of time urine, saliva, blood or breath samples are collected and the enrichment of the administered isotope in the compound of interest is measured using MS or infrared spectroscopy techniques.
Application of stable isotopes in nutrition assessments
Examples of applications of stable isotope techniques of relevance to the public health agenda include: assessment of breast-feeding practices, body composition, energy expenditure and energy requirements, evaluation of food-based approaches to improve nutrient intake, assessment of nutrient bioavailability and gut function. The first three of these involve the use of labelled water. Water is composed of isotopes of hydrogen and oxygen. Natural water is composed mainly of 1H and 16O, but contains a very small amount of 2H and 18O. However, water can be made to contain a much higher proportion of 2H or oxygen-18 compared with natural water, hence the water is said to be enriched. 2H oxide (2H2O or D2O) is enriched water in which 99·8 % of the hydrogen atoms are in the form of 2H. Water enriched to contain 10 % of the oxygen atoms in the form of oxygen-18 is also available for use in nutrition studies, but D2O is the most commonly used form, because it is about 100 times cheaper than H2 18O( 2 ).
Breast-feeding practices
The WHO recommends exclusive breast-feeding (EBF) of infants for the first 6 months of life followed by the introduction of nutritionally complete and safe complementary foods, with continued breast-feeding for up to 2 years and beyond( 4 ). The benefits of breast-feeding to both the infant and the mother are well established( Reference Victora, Bahl and Barros 5 ). Up to 823 000 deaths of children could be averted each year through universal breast-feeding( 6 ). EBF guarantees the optimal nutrient intake for the infant and is associated with reduced morbidity and mortality. In addition, EBF may result in fewer conduct disorders later in life( Reference Rochat, Houle and Stein 7 ). Global EBF rates are low with only marginal increase over the years (from 25 % in 1993 to 36 % in 2013 in low and middle income countries)( Reference Rollins, Bandari and Hajeebhov 8 ). Stable isotope techniques can help determine if a baby is exclusively breastfed or not, as well as how much human milk the baby consumes. The conventional method (test-weighing) to determine the quantity of milk consumed by a baby is time-consuming, and disturbs normal feeding pattern, as it requires that the baby is weighed before and after each feed. 2H2O dose-to-mother, technique, which was introduced by Coward and co-workers in 1982( Reference Coward, Cole and Sawyer 9 ) is presently the only method to objectively and non-invasively determine whether a baby is exclusively breastfed or not( 10 ).
Breast milk intake
The dose-to-mother technique for assessment of breast milk intake is described in detail in International Atomic Energy Agency (IAEA) Human Health Series No. 7, 2010( 11 ). A lactating mother drinks a dose of D2O. which is distributed throughout her body within a few hours and is incorporated into her milk. The baby receives 2H only during breast-feeding. The saliva and urine of both the mother and child is enriched with 2H and this can be measured using an isotope ratio mass spectrometer (IRMS) or Fourier transform infrared spectrometer. Fig. 1 presents the typical evolution of 2H enrichment in saliva in both mother and infant over 14 d. Saliva is the primary body fluid collected when Fourier transform infrared is used as urine may cloud the optical lens. Over a period of 14 d, samples of saliva are collected from the mother and child. Changes in isotope concentration reveal the quantity of human milk consumed by the baby and whether the baby has consumed water from other sources. Infants who consume less than 25 ml water/d from sources other than human milk are classified as exclusively breastfed( Reference Haisma, Coward and Albernaz 12 ). The technique also provides information on the body composition of the mother.
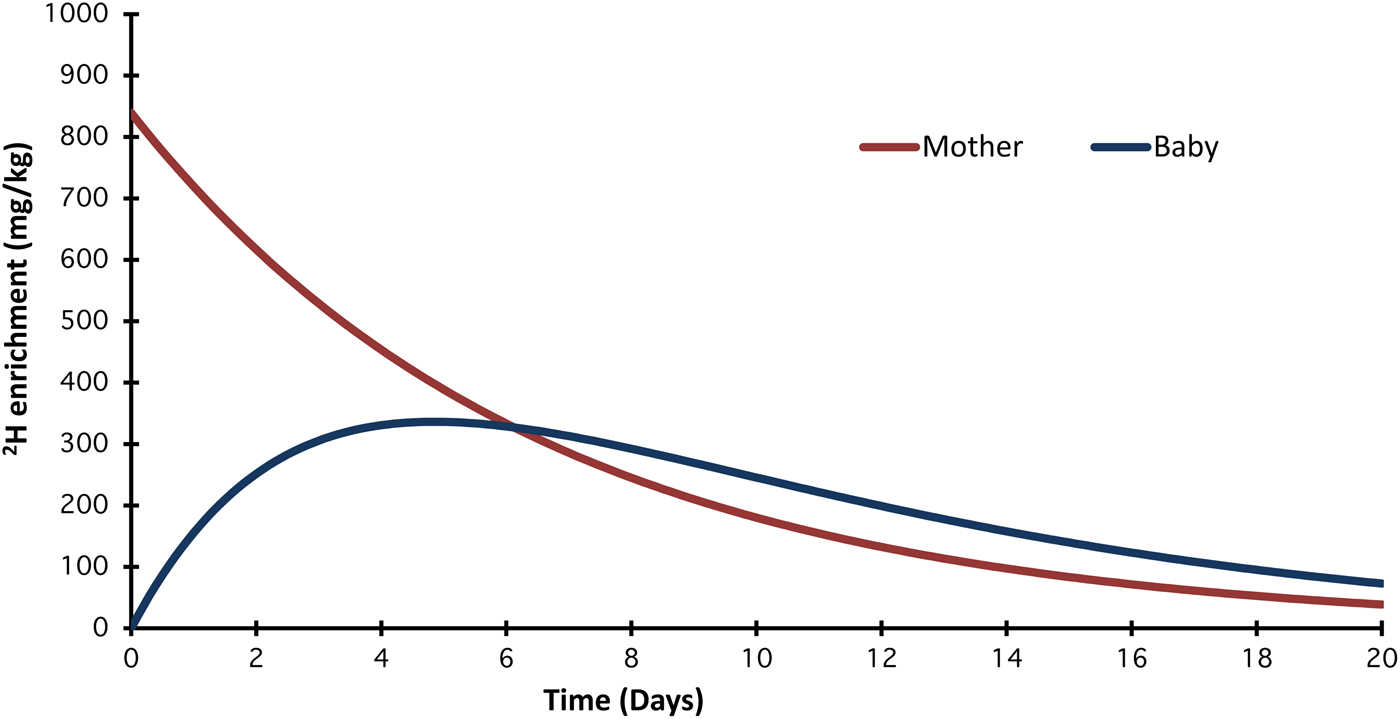
Fig. 1. (Colour online) Typical evolution of 2H enrichment in saliva for mother and infant over 14 d. As the 2H is eliminated from the mother's body, the enrichment in her milk declines and therefore the enrichment in the baby's body also falls. A mathematical model is used to determine how much of the 2H given to the mother appears in the baby's saliva. Milk and non-milk water volumes are calculated.
A comprehensive review of studies applying stable isotopes to measure breast milk intake was published in 2010( Reference da Costa, Haisma and Wells 13 ). According to the review, the overall mean breast milk intake was 0·78 (95 % CI 0·72, 0·84) kg/d, and the age-specific estimates indicated that intake increased over the first 3–4 months and remained above 0·80 kg/d until 6–7 months.
Exclusive breast-feeding
The World Health Assembly target is to increase the rate of EBF in the first 6 months up to at least 50 % by 2025. Hitherto data on EBF patterns are derived from maternal recall during routine demographic health surveys. Recent work in IAEA member states (Sri Lanka( Reference Bandara, Hettiarachchi and Liyanage 14 ), Cameroon( Reference Medoua, Sajo Nana and Ndzana 15 ), Kenya( Reference Oiye 16 ), Morocco( Reference Choua, El Kari and El Haloui 17 ), South Africa( Reference Mulol and Coutsoudis 18 ) and Guatemala( Reference Mazariegos, Slater and Ramirez-Zea 19 ) used the dose-to-mother technique to assess EBF compared with maternal recall (based on listing of liquid and food items (normally 11–12 items) and asking the mother whether the infant received any of these since birth or in the 24 h preceding the survey). The studies revealed that compared with the objective deuterium dilution (DD) method, mothers’ own recall of EBF is biased, tending to overestimate by about 40 % at 3–6 months of age (Table 1).
Table 1. Report compared with objectively measured exclusive breast-feeding (EBF) practices
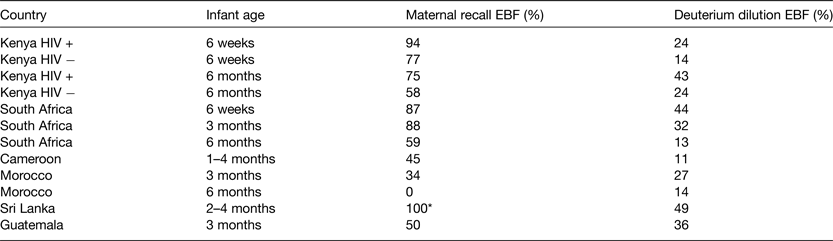
* In Sri Lanka, reported EBF was recruitment criterion for the study.
Impact of introduction of complementary foods
Breast milk continues to provide substantial amounts of key nutrients well beyond the first year of life, especially protein, fat and most vitamins( Reference Dewey 20 ). However, from age 6 months overall nutrient requirements increase and breast milk needs to be complemented with other foods. In addition to breast milk, energy requirements from complementary foods are estimated to be 836·8 kJ/d (199·9 kcal/d), 1255·2 kJ/d (299·9 kcal/d) and 2092 kJ/d (499·8 kcal/d) at age 6–8 months, 9–11 months and 12–24 months, respectively( Reference Dewey and Brown 21 ). In most low and middle income countries, plant-based complementary foods are the main source of nutrients; they are typically bulky and dense and are mostly served in the form of thin gruels with low energy and nutrient density( Reference Mensah and Tomkins 22 ). As such the child must consume high volumes of the foods in a manner likely to reduce the ability of the child to breastfeed adequately. Several studies have applied the dose-to-mother technique to assess the effect of complementary foods optimised to have high energy and nutrient density on breast milk intake from age 6 months. Although the studies differed in design and setting, most of them( Reference Owino, Bahwere and Bisimwa 23 – Reference Kumwenda, Dewey and Hemsworth 26 ), except one( Reference Islam, Peerson and Ahmed 27 ), found no significant reduction in the quantity of breast milk intake with the provision of complementary foods.
Body composition
Body composition refers to the components that comprise an individual's body weight. Most commonly, a two-compartment model is used that assumes that the body is composed of fat and lean tissue. The appropriate balance between fat and lean is essential for good health, and this changes as children grow and their bodies mature. Adult women have more body fat than men, and the distribution of body fat varies with sex and ethnicity, and is related to health outcomes. Although relatively easy to measure, body weight is a relatively poor indicator of health and nutritional status and needs to be combined with other indicators. Changes in body composition can be used to monitor interventions, such as programmes to prevent all forms of malnutrition, and response to treatment. Excess body fat is associated with physiological changes that can lead to non-communicable diseases, such as diabetes, CVD and some cancers. On the other hand, inadequate food intake or increased demand for certain amino acids caused by chronic inflammation can result in muscle wasting and ultimately death( 2 ).
The concept of body composition and its measurement by DD is comprehensively described in IAEA Human Health Series No. 12( 2 ). The DD technique for body composition assessment measures the amount of water in the body (total body water), which includes both intracellular water and extracellular water. The two-component model for body composition assessment assumes that the body mass is composed of fat mass and fat-free mass (FFM)( 2 ). It is assumed that water occurs only in the FFM. It should be noted that fat mass is not the same as adipose tissue, and FFM is not exactly the same as lean body mass. At birth, the body contains 70–75 % water, but as the body matures, this proportion decreases to 50–60 % in lean adults and to less than 40 % in obese adults. FFM can be determined by measuring total body water and then using an appropriate hydration factor to calculate FFM. The FFM is approximately 73 % water in adults. Body fat mass is the difference between body weight and FFM.
Body water can be sampled in the form of urine, saliva or plasma. In community settings, urine or saliva sampling is preferred, depending on the method available for analysis of 2H enrichment. Any matrix can be used if 2H enrichment is measured by IRMS, but saliva is preferred for analysis by Fourier transform infrared spectrometry. The dose of 2H required also depends on the sensitivity of the method of analysis available and ranges from 0·05 g/kg body weight for adults with analysis by IRMS to 0·5 g/kg body weight with analysis by transmission Fourier transform infrared spectroscopy.
In the DD technique, a pre-dose sample is collected from a person before s/he drinks an accurately weighed amount of D2O. Further saliva or urine samples are collected 3–5 h post-dose. Equilibration of 2H in saliva is faster (3 h) compared with urine (5–6 h). Equilibration takes longer in older persons because of their slower water turnover rate.
An advantage of this technique is that it can be used to assess longitudinal changes in body composition before and after an intervention. Other field methods to estimate total body water include bioelectrical impedance analysis and predictions from anthropometry (skinfold thickness, weight, height, sex, age). These are less accurate and require prediction equations to be derived for particular population groups by comparison with a reference method, such as DD.
The following are examples of IAEA funded work that assessed body composition to answer different research questions in Africa. A South African study( Reference Mulol and Coutsoudis 28 ) showed that infants who were exclusively breastfed for 6 months had a significantly higher per cent FFM at 12 months compared with infants who were not exclusively breastfed for 6 months. A study from Kenya( Reference Oiye 16 ) found no significant difference in FFM between HIV-unexposed and HIV-exposed (HIV-negative) infants and 6 weeks and 6 months of age. Workers in the Democratic Republic of Congo( Reference Bahwere, Balaluka and Wells 29 ) observed no difference in FFM post-recovery in children treated for severe acute malnutrition using two different ready to use therapeutic food formulations (standard peanut-based ready to use therapeutic food with milk v. milk-free pulse-based ready to use therapeutic food formulation). On-going work supported by the IAEA in this area includes development of reference body composition data for children from birth to age 2 years. Additionally, a new African regional project involving eight countries is evaluating medium to long-term risk for obesity among individuals (age 2–30 years) who were treated for severe acute malnutrition or moderate acute malnutrition in childhood. The DD method has previously been used to validate body measurement by bioelectric impedance analysis among HIV-infected Ethiopian adults( Reference Fufa, Umeta and Akalu 30 ) and to assess body composition changes following food supplementation among HIV-infected adults initiating anti-retroviral treatment in Ethiopia( Reference Olsen, Abdissa and Kæstel 31 ) and Zambia( Reference Zulu, Byrne and Munthali 32 ) and to assess body composition of HIV-infected breast-feeding women in South Africa( Reference Kindra, Coutsoudis and Esposito 33 ).
In Latin America, the focus had mainly been on establishing capacity to evaluate interventions designed to combat the double burden of malnutrition that exists in these areas( Reference Vásquez, Salazar and Andrade 34 – Reference Salazar, Vio and García 36 ).
Energy expenditure and physical activity
Knowledge of energy expended by an individual is an important pre-requisite to accurate determination of individual energy intake requirements. Using water labelled with two stable isotopes, 2H (2H2O) and oxygen-18 (H2 18O) in a technique known as the double-labelled water method, researchers can obtain an estimate of total daily energy expenditure in individuals undergoing their normal daily living activities. Total energy expenditure is also used to determine a person's physical activity level( 10 ). This technique is comprehensively described in the IAEA Human Health Series No. 3( 37 ). The method is non-invasive, precise and accurate and measures energy expenditure in free-living people, providing estimates of habitual expenditure over a time period of 7–20 d.
A person drinks a dose of double-labelled water, which is distributed throughout their body water. Every time the person breathes or exercises, some of the labelled oxygen and hydrogen is lost in their urine, sweat and breath. 2H is lost only in water, whereas oxygen-18 is lost in both water and carbon dioxide. The difference in the elimination rates of 2H and oxygen-18 is a measure of carbon dioxide production rate, from which energy expenditure can be calculated. Urine samples over a period of 7–14 d (depending on their age) are analysed using an IRMS to reveal the rate of decline in the concentration of the introduced isotopes. A very slow decline indicates low energy expenditure, while a sharper, faster decline indicates high energy expenditure( 37 ). The double-labelled water technique is ideal for measuring total daily energy expenditure in normal, daily living conditions and is being used by the IAEA in projects designed to address childhood obesity and quality of life in the elderly( 10 ). The global target is to halt the increase in childhood obesity by 2025( 38 ).
Evaluation of food-based approaches to improve nutrient intake
In addition to adequate macronutrient (carbohydrates, protein and fat) intake, human subjects require sufficient levels of crucial vitamins and minerals that allow them to be mentally and physically healthy. Micronutrient deficiencies affect as many as two billion people globally( Reference Bailey, West and Black 39 ), accounting for an estimated 7·3 % of the global disease burden, with iron and vitamin A deficiencies ranking among the top fifteen leading causes of the global disease burden( 10 ). For example, anaemia presently affects 533 million women of reproductive age (15–49 years) and the global target is to reduce this burden by 50 % by 2025( 38 ). Understanding how to measure and interpret biochemical indicators along with clinical and functional indicators is key to elucidate the extent of the global burden of micronutrient deficiencies( 10 , Reference Tontisirin, Nantel and Bhattacharjee 40 ) and to evaluate the success of interventions to address the problem.
Present strategies to address hidden hunger include individual supplementation, staple food fortification, biofortification of crops and improved dietary diversification. Micronutrient supplementation provides one or more micronutrients daily or periodically in liquid, tablet or capsule form. For example, high dose vitamin A supplements are given every 6 months to children between 6 and 59 months to prevent mortality in areas where vitamin A deficiency is prevalent. Food fortification can be achieved by adding micronutrients to staple foods that are regularly eaten by the population, so that the micronutrient is consumed frequently in amounts chosen to prevent deficiencies, while avoiding the likelihood of excessive amounts, which are also unhealthy, will be consumed. This provides an efficient distribution system for staple foods that are processed at just a few sites and distributed widely, for example large grain mills or the principal producers of cooking oils.
Biofortification is the process by which the nutritional quality of staple crops is enhanced by the accumulation of higher levels of minerals and vitamins in seeds and roots during growth( 10 ). Another essential strategy is the promotion of dietary diversification or consumption of a wide variety of foods across nutritionally distinct food groups. Dietary diversification( 10 , Reference Tontisirin, Nantel and Bhattacharjee 40 ) or modification strategies at the community or household level aim to enhance the availability, access and utilization of foods with a high content and bioavailability of micronutrients throughout the year.
The IAEA provides support for the use of stable isotope techniques to investigate absorption and retention of iron or zinc from fortified or biofortified foods fed to adults or children, from mixed diets that contain enhancers and inhibitors of absorption or from modified dietary practices, for example using traditional household methods such as fermentation, germination and soaking to reduce phytic acid. These techniques enable accurate measurement of bioavailability and bioconversion of provitamin A, using isotope labelled β-carotene; absorption and retention of iron and zinc, using their least common stable isotopes from fortified or biofortified foods, or from diverse diets that contain enhancers and inhibitors of absorption( 10 ). Additionally, the absorption of protein from plant foods can be done using isotope labelled amino acids and protein. Most recently, the IAEA has supported work on assessing vitamin A safety in large-scale nutrition intervention programmes( Reference Tanumihardjo, Mokhtar and Haskell 41 ) such as twice annually megadose of vitamin A for children aged below 5 years and widespread fortification of food commodities including sugar, oil and starch.
Several IAEA-supported studies have evaluated the benefit of food fortification and biofortification in improving micronutrient status during early life. In Rwanda( Reference Petry, Egli and Gahutu 42 ), investigators used iron stable isotopes to study iron absorption from beans to determine which chemicals in beans should be the focus of crop breeding programmes to improve the absorption of iron from beans. Beans contain phytic acid and polyphenols that reduce iron, calcium and zinc absorption. The investigators concluded that phytic acid inhibited iron absorption by women so substantially that there would be little or no benefit in increasing iron or decreasing the polyphenol content of beans without also decreasing the phytic acid content.
A zinc absorption study in Bangladesh( Reference Islam, Woodhouse and Hossain 43 ) showed that zinc-biofortified rice contained more zinc, but it was less efficiently absorbed, so did not substantially improve the total amount of zinc retained by children compared with control rice. In India( Reference Kodkany, Bellad and Mahantshetti 44 ), iron and zinc from biofortified pearl millet were well absorbed in amounts that meet the requirements for young children.
Protein bioavailability
The last WHO/Food and Agriculture Organization/United Nations University Expert Consultation on the Protein and Amino Acid Requirements of Man in 2007 determined that the requirements of indispensable amino acids were higher than previously thought( 45 ), in particular isoleucine, leucine, lysine, valine, threonine, phenylalanine and tyrosine. Protein quality in terms of indispensable amino acids content has a great importance in meeting the nutritional needs of populations across the globe and throughout the life course. The Food and Agriculture Organization released a report on Dietary Protein Quality Evaluation in Human Nutrition in 2013( 46 ). The IAEA held a Consultants’ Meeting in October 2013 to discuss stable isotopic methods to measure protein quality in human subjects( 46 ). A Food and Agriculture Organization Expert Working Group Meeting in March 2014 in Bangalore discussed research methodologies to measure protein digestibility and utilisation in human subjects( Reference Tomé, Jahoor and Kurpad 47 ).
Dispensable and indispensable amino acids play key roles in achieving healthy growth in early life. The faster the growth rate the greater the need for indispensable amino acids; however, even non-essential amino acids play key roles in promoting lean body mass gain and linear growth in particular by modulating the secretion of hormones (i.e. arginine stimulates insulin secretion thus lowering serum glucose, which in turn drives growth hormone and lean mass growth including height gain). These amino acids are also responsible for stimulating insulin-like growth factors, which promote length gain, specific proteins in milk and other animal foods are relevant in this process( 48 ).
Fetal development, and child development during early life, is critically dependent on the appropriate amount of dietary proteins, for both appropriate growth and body composition, that may determine their human capacity and potential in adulthood. Protein synthesis rates in early gestation are associated with birth length( Reference Uauy 49 , Reference Duggleby and Jackson 50 ). Meeting population nutrient needs must also relate to the requirements of living under real life conditions such as repeated infections, poor environmental sanitation and psychosocial stress, all of which increase amino acid losses and prevent a strong anabolic response leading to recovery. Additionally, the effect of food processing and heating may alter absorption and utilisation of some amino acids such as lysine, which may be already limiting protein quality of many predominantly cereal-based diets.
Estimates of amino acid digestibility based on analysis of faeces relative to foods ingested do not represent the fraction of amino acid absorbed. Ideally, amino acid bioavailability should be measured from oro-ileal balances (the difference between ingested amino acids and the amino acids leaving the ileum), since absorption occurs only in the small intestine and colonic bacteria can recycle nitrogen. However this is virtually impossible to measure non-invasively in healthy human subjects.
The IAEA has a Coordinated Research Project, which started in 2015 and aims to develop and validate novel, minimally invasive approach to assessing protein quality based on the use of 15N, 13C and/or 2H labelled amino acids or intrinsically-labelled proteins. Intrinsically 2H labelled local varieties of grain legumes are being grown in collaboration with local agriculture institutes. Indispensable amino acids become labelled within the food matrix and following preparation and ingestion of the meal, the appearance of labelled amino acids in the blood gives a unique measure of bioavailability. If a labelled test meal is accompanied by a trace quantity of a differently labelled reference protein, such as 13C-labelled single cell protein of known digestibility, the ratio of the appearance of these two tracers in plasma amino acids, compared with the ratio in the meal is an index of its digestibility/bioavailability. MS instrumentation using newer sensitive methods (GC-pyrolysis-IRMS, liquid chromatography–MS), allows for minimally invasive sampling, such as the use of saliva.
Evaluating the impact of environmental factors on nutritional status
All known nutritional interventions combined, at 90 % coverage, only amount to partial (one third) reduction in stunting rates( Reference Bhutta, Ahmed and Black 51 ). The influence of environmental factors including living in unsanitary conditions, exposure to persistent organic pollutants and heavy metals and microbial toxins including aflatoxins, is yet to be fully understood. Living in poor sanitary conditions may induce gut dysfunction( Reference Keusch, Rosenberg and Denno 52 , Reference Mbuya and Humphrey 53 ), referred to as environmental enteric dysfunction (EED). Retarded growth, altered gut microbiota and decreased vaccine responsiveness are considered the most important consequences of EED.
Environmental enteric dysfunction
A technical meeting on EED organised and hosted by the IAEA in Vienna in October 2015 identified gaps including the need for a clear classification and understanding of causal pathways underpinning EED. The meeting recommended the development of practical, simple and affordable tools that include stable isotope techniques to diagnose and characterise EED to allow better targeting of interventions in vulnerable populations( Reference Owino, Ahmed and Freemark 54 , Reference Ross, Kosek and Krebs 55 ). A follow up technical meeting held from 31 May–3 June 2016 identified three priority areas where stable isotope techniques may initially be used in addressing EED: (1) dietary intake; (2) tracking of microbial translocation through the intestinal epithelial membrane and measuring attendant inflammatory activity; (3) human host response in terms of hormonal changes linked to growth and the risk for non-communicable diseases.
Characterising breastmilk-borne exposure to endocrine disruptors in infants and young children
The double burden of malnutrition characterised by co-existence of undernutrition, micronutrient deficiencies, overweight, obesity and diet-related chronic diseases is now a major global problem( Reference Shrimpton 56 ). Interventions to address this phenomenon focus mainly on infant and young child feeding practices, water, hygiene and sanitation and to a lesser extent, physical activity. One missing link is the role of persistent organic pollutants, which have been strongly associated with endocrine disruption( Reference Wohlfahrt-Veje, Audouze and Brunak 57 ) that may result in abnormal growth patterns and increased risk for obesity( Reference Vafeiadi, Georgiou and Chalkiadaki 58 – Reference Etzel and Balk 60 ). Other effects are low birth weight, motor and visual defects and delayed cognitive development( Reference Chen, Wang and Li 61 ). Persistent organic pollutants being lipophilic, preferentially bio-accumulate in breast milk( Reference Vigh, Colombo and Benfenati 62 ) and are transferred from the mother to the infant during breast-feeding( Reference Chen, Wang and Li 61 ). The DD technique can be used to quantify breast milk intake to allow characterisation of the magnitude of exposure to persistent organic pollutants via breast-feeding and to assess changes in body composition as an indicator of nutritional status and healthy growth.
Urea breath test to diagnose Helicobacter pylori
Helicobacter pylori (H. pylori) is present in all countries the world over. More than 50 % of the world's population harbour H. pylori in their upper gastrointestinal tract. It can negatively influence nutrition by affecting the uptake of iron and zinc and by increasing susceptibility to diarrhoeal disease. Beyond that, H. pylori is also a major cause of stomach diseases like chronic gastritis, and elevates the risk of developing stomach cancer( Reference DI Rienzo, D'Angelo and Ojetti 63 ).
The carbon-13 urea breath test is a quick and non-invasive diagnostic test to detect the presence of H. pylori and has been applied to related nutritional outcomes over the last 15 years in over twenty-five countries with IAEA support. The patient drinks urea labelled with stable carbon isotope (13C) that is dissolved in orange juice or citric acid to make sure it coats the entire surface of the stomach, thereby improving the test's accuracy. If H. pylori is present, it metabolises the urea and, after 30 min, produces carbon dioxide labelled with the stable carbon isotope (13CO2), which can be detected in the breath( Reference DI Rienzo, D'Angelo and Ojetti 63 ).
Present work includes assessing the effect of H. pylori infection on gastric acid secretion and on iron and zinc absorption in asymptomatic individuals from low and middle income countries. Gastric acid is essential for the conversion and absorption of micronutrients such as iron and zinc. One study showed that H. pylori infection is not associated with anaemia in six Latin American countries( Reference Santos, Boccio and Davidsson 64 ).
Conclusion
Stable isotopes have been applied over the past five decades to elucidate metabolic pathways essential to human health. This review is limited to the stable isotope techniques that the IAEA is promoting for the evaluation of nutrition interventions in its member states. These techniques remain invaluable in the tracking of global targets on EBF childhood obesity and anaemia among women. Better understanding of macronutrient metabolism and the effect of environmental exposures, including EED and persistent organic pollutants is required. Efforts are underway to make nuclear techniques more affordable, field-friendly, less invasive and to develop less sophisticated but precise equipment. The shift from MS to infrared spectroscopy techniques for analysis of 2H in saliva specimens for breast milk intake and body composition assessment and carbon-13 in breath samples by non-dispersive infrared spectroscopy for the assay of gut function is a big stride in this direction. Concerted advocacy for the wide adoption of the techniques remains a huge challenge.
Acknowledgements
All IAEA-supported projects whose work is cited in this manuscript are highly appreciated. Special thanks to the research teams from Guatemala, South Africa, Kenya and Sri Lanka for contributing most recent data on EBF.
Financial Support
V. O. travel expenses to ANEC VII conference in Marrakech, Morocco was fully funded by the IAEA.
Conflict of Interest
All authors are employees of the IAEA.
Authorship
All authors have reviewed and approved submission of the manuscript. V. O. drafted the manuscript and wrote the sections on breast-feeding practices and assessment of environmental aspects. C. S. wrote the sections on body composition, energy expenditure, protein bioavailability and urea breath test for H. pylori and reviewed the entire manuscript. C. L. contributed the section on evaluation of food-based approaches, reviewed the manuscript and provided overall editorial oversight.





