Article contents
Essential oils and essential oil compounds in animal production as antimicrobials and anthelmintics: an updated review
Published online by Cambridge University Press: 04 July 2023
Abstract
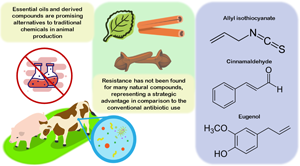
Several countries have shown an increased prevalence of drug resistance in animal production due to the indiscriminate use of antibiotics and antiparasitics in human and veterinary medicine. This article aims to review existing methods using naturally occurring essential oils (EOs) and their isolated compounds (EOCs) as alternatives to antimicrobials and antiparasitic compounds in animal production and, consequently, to avoid resistance. The most-reported mechanism of action of EOs and EOCs was cell membrane damage, which leads to the leakage of cytoplasmic content, increased membrane permeability, inhibition of metabolic and genetic pathways, morphologic changes, antibiofilm effects, and damage to the genetic material of infections. In parasites, anticoccidial effects, reduced motility, growth inhibition, and morphologic changes have been reported. Although these compounds regularly show a similar effect to those promoted by traditional drugs, the elucidation of their mechanisms of action is still scarce. The use of EOs and EOCs can also positively influence crucial parameters in animal production, such as body weight gain, feed conversion rate, and cholesterol reduction, which also positively impact meat quality. The application of EOs and EOCs is enhanced by their association with other natural compounds or even by the association with synthetic chemicals, which has been found to cause synergism in their antimicrobial effect. By reducing the effective therapeutical/prophylactic dose, the chances of off-flavors – the most common issue in EO and EOC application – is greatly mitigated. However, there is very little work on the combination of EOs and EOCs in large in vivo studies. In addition, research must apply the correct methodology to properly understand the observed effects; for example, the use of only high concentrations may mask potential results obtained at lower dosages. Such corrections will also allow the elucidation of finer mechanisms and promote better biotechnologic use of EOs and EOCs. This manuscript presents several information gaps to be filled before the use of EOs and EOCs are fully applicable in animal production.
- Type
- Review Article
- Information
- Copyright
- Copyright © The Author(s), 2023. Published by Cambridge University Press
References
- 8
- Cited by



