Introduction
Citrus crops (Rutaceae) are attacked by several pests which threaten the health of the trees and fruit quality through direct feeding damage or transmission of pathogens. The important pests include psyllids, leafminers, scales, whiteflies, mealybugs, weevils, and mites. The armored scale insects are one of the most important pests with abroad host range across agricultural crops, including citrus (Rosen, Reference Rosen1990; Mellado, Reference Mellado2012; Smith-Pardo, et al., Reference Smith-Pardo, Evans and Dooley2012; Ouvrard et al., Reference Ouvrard, Kondo and Gullan2013; Amouroux et al., Reference Amouroux, Crochard, Correa, Groussier, Kreiter, Roman, Guerrieri, Garonna, Malausa and Zaviezo2019). Among these, Florida red scale Chrysomphalus aonidum (L.) (Hemiptera: Diaspididae) is a polyphagous pest of about 192 plant genera of 77 different families with a high preference for Citrus and Eucalyptus (Hlavjenkova and Sefrova, Reference Hlavjenkova and Sefrova2013). It infests foliage and fruit and is of serious concern to citrus production in North America both for citrus produced under protective screens and open production systems (Mathis, Reference Mathis1947; Smith-Pardo et al., Reference Smith-Pardo, Evans and Dooley2012; Ahmed and Miller, Reference Ahmed and Miller2018).
The adult male is small, gnat-like, and free flying. The adult female is sessile about 2.0–2.2 mm in diameter and produces 50–150 bright yellow eggs under the armor (Miller and Davidson, Reference Miller, Davidson and Rosen1990; Gill, Reference Gill1997). The armor of C. aonidum is made of three concentric rings and is dark reddish brown with a conspicuous light brown center (Futch et al., Reference Futch, McCoy and Childers2001). The eggs hatch below the female armor giving rise to first instar nymphs known as ‘crawlers’, which migrate and seek for suitable feeding sites on leaves, twigs, or fruits, where they establish, feed, develop, and reproduce under the armor (Uygun and Elekcioglu, Reference Uygun and Elekcioglu1998). The head and thorax are indistinguishable, and the abdominal segments are fused into a condensed area called pygidium (Ahmed and Miller, Reference Ahmed and Miller2018). Female has piercing-sucking mouthparts and feed by sucking the sap from the plant tissues (Campolo et al., Reference Campolo, Malacrinò, Laudani, Maione, Zappalà and Palmeri2014). The injurious effects of C. aonidum include foliage yellowing, leaf drop, fruit deformity, and drop and twig dieback (Campolo et al., Reference Campolo, Malacrinò, Laudani, Maione, Zappalà and Palmeri2014). The duration of the life cycle as well as the number of generations depend on the temperature and humidity (Gill, Reference Gill1997). Campolo et al. (Reference Campolo, Malacrinò, Laudani, Maione, Zappalà and Palmeri2014) reported that the C. aonidum female developmental time (from crawler emergence to adult) was 92 days at 15°C and significantly decreased to 26 days at 35°C. The development time of the male was 101 days at 15°C and only 30 days at 35°C. Depending on the temperature and humidity, the number of generations in armored scales can range from two to as high as five or six per year (Beardsley and Gonzalez, Reference Beardsley and Gonzalez1975).
There is an alarming increase in the application of insecticides in Florida by the growers to control various agricultural pests particularly the Asian citrus psyllid, Diaphorina citri Kuwayama (Hemiptera: Liviidae), which is responsible for vectoring the causal pathogens of the devastating huanglongbing or citrus greening disease (Qureshi et al., Reference Qureshi, Kostyk and Stansly2014; Qureshi and Stansly, Reference Qureshi and Stansly2020). Most of these applications also target other pests such as leafminers, mites, and aphids. The frequent and repeated application of pesticides in the field resulted in the development of pests’ resistance, besides having adverse effects on natural enemies (Kanga et al., Reference Kanga, Eason, Haseeb, Qureshi and Stansly2015). Chemical control of the C. aonidum is usually difficult due to the natural protection offered by the armor, particularly for the spray material to reach inside the armor (Rehman et al., Reference Rehman, Browning, Nigg and Harrison2000). Rehman et al. (Reference Rehman, Browning, Nigg and Harrison2000) reported an increase in the C. aonidum population in Florida and associated that with the application of insecticides which negatively impacted populations of its parasitoid Aphytis holoxanthus. Biological control is also a better alternative to chemical control which besides impacting the natural enemies contaminates environment and results in pest resistance.
Ladybeetles (Coleoptera: Coccinellidae) are known for their remarkable predatory activity. Therefore, they have been used intensively as biological control agents in agricultural ecosystems and crops including citrus (Biddinger et al., Reference Biddinger, Weber and Hull2009; Obrycki et al., Reference Obrycki, Harwood, Kring and O'Neil2009; Qureshi and Stansly, Reference Qureshi and Stansly2009, Reference Qureshi and Stansly2011; Khan et al., Reference Khan, Qureshi, Afzal and Stansly2016). They are known for variability in their foraging behavior (changing from extensive to intensive searching behavior), ability to disperse and broadening the prey or host range (Kalushkov, Reference Kalushkov1999; Hodek, Reference Hodek, Hodek, van Emden and Honek2012). The predation rate and efficiency depend on the food quality and other factors, such as adult longevity and reproduction rate (Obrycki and Orr, Reference Obrycki and Orr1990; Michaud, Reference Michaud2000). Therefore, implementation of the biological control requires thorough investigation. Curinus coeruleus (Mulsant) was introduced to Hawaii in early 1920s to control several coconut pests, such as scales and mealybugs (Showier, Reference Showier1995; Soemargono et al., Reference Soemargono, Ibrahim, Ibrahim and Osman2008), and 20–30 years later, it was introduced to Florida from Mexico (Michaud et al., Reference Michaud, McCoy and Futch2002). Thereafter, in the 1980s–1990s, this ladybeetle was introduced to several south Asian countries to control Leucaena psyllids Heteropsylla cubana (Wagiman et al., Reference Wagiman, Mangoendihardjo and Maahrub1989). C. coeruleus was frequently found in eastern and southern Florida (Michaud et al., Reference Michaud, McCoy and Futch2002) and showed remarkable success to reduce the populations of the Asian citrus psyllid D. citri (Qureshi and Stansly, Reference Qureshi and Stansly2009). However, we are not aware of any studies of its evaluation against C. aonidum. There are fewer studies regarding the predation behavior of coccinellids against scale insects. Lopez et al. (Reference Lopez, Kairo and Irish2004) investigated the predation behavior of Cryptognatha nodiceps Marshal (Coleoptera: Coccinellidae) on Aspidiotus destructor Signoret (Hemiptera: Diaspididae). They observed significant predation and reproduction of C. nodiceps on A. destructor. Chilocorus bipustulatus (L.) (Coleoptera: Coccinellidae) was shown to be a good predator against C. aonidum with less preference toward armored scales (Yinon, Reference Yinon1969). The larva of C. bipustulatus consumed on average 80 C. aonidum during their development and adult consumed 5.2 per day (Yinon, Reference Yinon1969). Uygun and Elekcioglu (Reference Uygun and Elekcioglu1998) studied the predation behavior and development of C. bipustulatus on three different diaspidid armored scales; Aspidiotus nerii Bouche (Oleander scale), Aonidiella aurantii (Maskell), and Pseudaulacaspis pentagona (Targioni). They reported strong predation activity of C. bipustulatus on A. nerii characterized by short life cycle and low mortality under laboratory conditions.
To our knowledge, there has been no previous effort to test the predation of C. aonidum by C. coeruleus. Therefore, we evaluated adults and larvae of this ladybeetle species for their predatory potential on C. aonidum provided to them with armor intact or removed, followed by experiments to evaluate their predation potential by altering them between diets of C. aonidum with or without armor. Under natural conditions, C. aonidum are covered with the armor except for crawlers, however, to test for the effect of armor on beetle consumption we introduced a comparative treatment of scales without armor by manually removing the armor. Knowledge of C. aonidum consumption by adults and larvae of C. coeruleus is important in determining the usefulness of this predator for managing this armored scale particularly when both are present in the target region such as urban environment and commercial citrus groves in Florida.
Materials and methods
Stock colony of C. aonidum
Colony of C. aonidum was maintained on potted plants of Valencia (Citrus sinensis) on Swingle sweet orange in a screenhouse at the University of Florida's Indian River Research and Education Center (IRREC), Fort Pierce, FL. These plants got infested with feral population in the area in 2018. Temperature and relative humidity in the screenhouse averaged 27°C and 72%, respectively.
Stock colony of C. coeruleus
C. coeruleus were collected from a sweet orange grove in Fort Pierce, Florida, and the colony was maintained under optimal conditions of photoperiod (16:8 h, light:dark), humidity (50–55%), and temperature (25°C) in the laboratory at IRREC. Adults and larvae of the C. coeruleus were reared on an ad libitum supply of aphids and frozen eggs of the Mediterranean Flour Moth Ephestia kuehniella (Zeller) (Lepidoptera: Pyralidae) (Beneficial Insectary, Redding, CA, USA; Koppert Biological Systems, Romulus MI, USA). Food was provided three times a week. The adults were reared in groups of 20–30 individuals inside a 3 liter ventilated cubical plastic cage. Water was provided using a moist cotton wick in a small plastic cup fixed to the base of the cage (Qureshi and Stansly, Reference Qureshi and Stansly2011). Shoots from orange trees and rolled paper towel were provided to the beetles as substrates for oviposition every other day, when old materials were removed from the cage. On the same days, beetle eggs were collected and kept in ventilated plastic vials (2.5 cm diameter and 13 cm long). Larvae were reared individually in plastic vials (2.5 cm diameter and 13 cm long). The frozen eggs of E. kuehniella were provided to the larvae until they pupated. Upon emergence, the adult beetles were removed from the vials and were transferred into a 3 liter ventilated cubical plastic cage.
C. coeruleus consumption of C. aonidum with and without armor
The citrus leaves infested with C. aonidum were obtained from the colony maintained at IRREC. The upper and lower surfaces of the leaves were cleaned with a tissue paper to remove all except for ten fully developed female scales retained on the lower surface. Scales were offered to the beetle adults in three different treatments: (1) with armor, (2) without armor, and (3) combination of scales with and without armor 50% each. Although mature scales are covered with armor, we used the scales without armor for comparison to determine the effect of armor on the adult and larval feeding of C. coeruleus. Scale armor removal was done under a stereomicroscope using a needle. One adult beetle or larva (3rd–4th instar) was exposed to ten scales from each group (with armor, without armor, and combination of with and without armor) in the experimental arena (15 × 6 cm2 Petri dish sealed with parafilm). In total, 30 adults of the beetles were exposed to 300 female scales across three treatments. For the larvae, 20 replicates were used for each of the choice or no-choice test. In total, 60 larvae were exposed to 600 female scales across three treatments. At 24, 48, and 72 h post-treatment, data were recorded for adult and larval survival in each treatment and their consumption rate of scale through observations made under a stereoscope. All experiments were conducted in an incubator (PR205745R Precision Incubator, ThermoFisher Scientific, Massachusetts, USA) set to 25°C under cool white fluorescent light with a photoperiod of 16:8 h, L:D.
Evaluation of the effect of previous diet experience on the predatory potential of the C. coeruleus
These experiments were conducted to evaluate the effect of beetle feeding experience on a particular type of diet or life stage of the scale either for a short period or complete development on its predatory potential on the next diet.
Experiment 1
Adults of C. coeruleus that fed on a diet of scales with armor, without armor, or a mix diet of the two types were tested for their predatory potential on a different type of diet either with or without armor. Those on the diet containing scales with armor were tested for their predatory potential on scales without armor, whereas those on the diets containing scales without armor or the mix of the two types were tested on scales with armor. Beetles were starved for 24 h prior to the experiments. Experimental conditions and protocols for data recording were similar to the previous experiment.
Experiment 2
The larvae were first tested on three diets (with armor, without armor, and mix of the two types) for their potential to consume scales and then reared to adulthood on the same three diets. Upon emergence from pupae, adults from the diets containing scales with or without armor were starved for 24 h before they were tested for their predatory potential on a different diet. For the larvae that were reared on the combination of scales with and without armor, the adults were starved for 24 h and tested on armored scales. The adults were introduced to Petri dishes (15 × 6 cm2) which were then sealed with parafilm to prevent the beetle from escape. In total, there were 20 replicates of each group. The Petri dishes containing beetle adults and scales were kept in the incubator under similar conditions as described in the previous experiments. Assessment of beetle's predation was performed by counting the number of scales consumed. Data were recorded at 24, 48, and 72 h after exposure.
Statistical analysis of the data
One-way analysis of variance (ANOVA) (P = 0.05) was used to test the differences in the mean number of C. aonidum consumed by adult or larvae of C. coeruleus in three treatments: (1) with armor, (2) without armor, and (3) the combination treatment with mix of the two types. The t-test (at P < 0.05) was applied to compare the significant difference in the beetles’ consumption of scales with armor or without armor after they were exposed or reared on the diets of scales with armor, without armor, or the mix of both. All statistical analyses were performed using SPSS (version 22.0 for windows).
Results
C. coeruleus consumption of C. aonidum with and without armor
There was statistically significant difference in the consumption of C. coeruleus adults (table 1, fig. 1a) across three treatments. Throughout the experiment, adults consumed significantly more C. aonidum in the treatment without armor compared to the other two treatments containing mix of armor and without armor or the armor only with no difference in the consumption between the two latter treatments. At 72 h, scale consumption averaged ten in the treatment without armor, nine in the treatment with armor only, and eight in the treatment with mix of armor and without armor.

Figure 1. Mean (±SEM) number of C. aonidum consumed by C. coeruleus adults (a) and larvae (b) in three diets at 24, 48, and 72 h post-exposure. Columns not sharing a common letter represent significant different means consumed by adult or larvae at a particular time (Tukey's test, P < 0.05).
Table 1. Results of one-way ANOVA comparing the consumption rate of adults and larvae of C. coeruleus among three diets of C. aonidum, with armor, without armor, and a mix of the two types

Larvae consumed significantly more C. aonidum in the treatment without armor followed by the treatments containing mix of armor and without armor and armor only (table 1, fig. 1b). These differences were observed at all the three time points. At 72 h, an average of 100% C. aonidum were consumed in the treatment without armor, 70% in the treatment with mix of armor and without armor, and 40% in the treatment with armor. In the mix treatment where adults and larvae had choice of C. aonidum with and without armor, both showed significant attraction to the scales without armor (table 2).
Table 2. Mean (±SEM) number of C. aonidum consumed by C. coeruleus adult and larvae in the mix diet of with and without armor
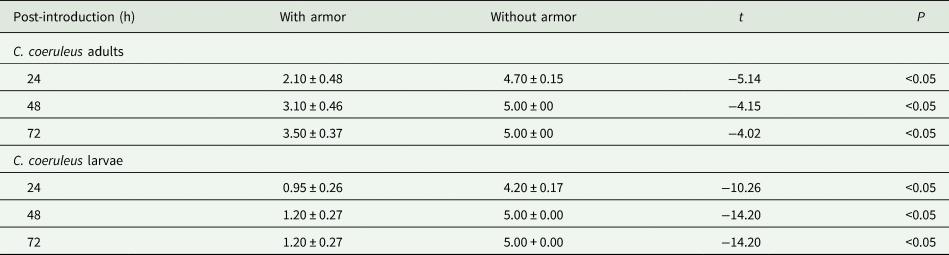
t-Test was used to compare the means of consumed scales between two groups within a mixed diet.
Effect of the previous diet experience on the predatory potential of the C. coeruleus
Experiment 1
Adults of C. coeruleus tested on the diet containing C. aonidum with armor consumed significantly more scales when tested on the diet without armor with an increase of 40% in the consumption rate at 24 h, however, this difference was not observed at 48 and 72 h (table 3). Those tested on diet without armor consumed significantly less scales when moved to diet with armor with a decrease of 31% in the consumption rate at 24 h. The same effect persisted at 48 h with a decrease to 25% in the consumption rate. This trend of decrease in the consumption rate continued averaging 18% at 72 h but was not statistically significant (table 3). Adults tested on the mix diet containing C. aonidum with armor and without armor did not show an increase or decrease in the consumption rate when tested on diet with armor only.
Table 3. Mean (±SEM) number of C. aonidum consumed by C. coeruleus adults in the treatments with armor, without armor, and the mix of armor and without armor and in follow-up treatments with or without armor

Means within a row with different alphabet indicate significant difference in consumption by adults in first and second diet treatment. Adults were starved for 24 h before experiments.
Experiment 2
More than 95% larvae of C. coeruleus survived to adulthood on all three diets of C. aonidum, with armor, without armor, and the mix of the two types. Adults developed from the larvae reared on the diet containing C. aonidum with armor consumed significantly more scales from which armor was removed compared to those with armor at 24, 48 and 72 h (table 4). However, adults developed from larvae reared on the mixed diet containing scales with and without armor or those which developed from the larvae reared on the scales without armor only did not show significant difference in the consumption rate between with and without armor scales (table 4).
Table 4. Mean (±SEM) number of C. aonidum with and without armor consumed by C. coeruleus adults which developed from larvae reared on three different diets, with armor, without armor, and the mixed diet of the two types

Means within a column with different alphabets indicate significant difference in consumption by adult C. coeruleus of C. aonidum with and without armor against a reference larval diet. Larvae and adults were starved for 24 h before experiments.
Discussion
Traditionally, ladybeetles have been used intensively in the biological control programs showing strong potential to control aphids (Frazer, Reference Frazer, Minks and Harrewijn1988; Dixon et al., Reference Dixon, Hemptinne and Kindlmann1997; Michaud, Reference Michaud, Hodek, van Emden and Honek2012; Riddick, Reference Riddick2017) as well as other pests (Uygun and Elekcioglu, Reference Uygun and Elekcioglu1998; Lopez et al., Reference Lopez, Kairo and Irish2004; Hodek and Honek, Reference Hodek and Honek2009). Ladybeetles including C. coeruleus are known to actively prey on several pests, such as Leucaena H. cubana and D. citri psyllids (Michaud and Olsen, Reference Michaud and Olsen2004; Soemargono et al., Reference Soemargono, Ibrahim, Ibrahim and Osman2008; Qureshi and Stansly, Reference Qureshi and Stansly2009). In general, coccinellid adults and larvae tend to attack softer parts of the body of the prey. Michaud and Olsen (Reference Michaud and Olsen2004) found that larvae of five coccinellid species including C. coeruleus consumed only a small portion of D. citri nymph and avoided the heavily chitinous structures such as wing buds. Khan et al. (Reference Khan, Qureshi, Afzal and Stansly2016) noticed prolonged larval development of two-spotted ladybeetle, Adalia bipunctata (L.) (Coleoptera: Coccinellidae), on the nymphs of D. citri compared to corn leaf aphid Rhopalosiphum maidis (Ashmead) (Hemiptera: Aphididae), which could be due to larva experiencing deterrence from wing pads particularly in the mature instars. However, such effects were not observed when the convergent lady beetle, Hippodamia convergens Guérin-Méneville was tested against D. citri, brown citrus aphid Toxoptera citricida Kirkaldy, and green citrus aphid Aphis spiraecola Patch (Qureshi and Stansly, Reference Qureshi and Stansly2011).
The armor on the body of the C. aonidum is a physical barrier which could interfere with the predator behavior in attacking this pest and reduce its consumption of the prey. We manually removed the armor from C. aonidum to provide the scales without armor to test against the treatment containing scales with armor to determine the effect of the later. Considering that crawler is the only life stage without armor but mobile and much smaller in size than the mature scales, their use as without armor treatment would not constitute a valid comparison. However, we did observe adults and larvae of C. coeruleus feeding on the crawlers and later instar C. aonidum (personal observations).
C. coeruleus adults were equally aggressive in feeding on the C. aonidum provided to them with or without armor. By removing the armor from C. aonidum, we provided beetles with soft prey which most predators like to attack. As expected, C. coeruleus adults consumed more scales without armor in the first 24 h (100%) compared to the other two situations (average 66%) where all or half of the scales were covered with the armor. However, predation rate improved to 83–89% in these later two treatments during the 72 h with no significant difference between the treatments. Adult ability to consume 66–89% of the C. aonidum with armor in a no-choice situation and not different from the mixed treatment indicate their potential as good candidates for suppressing C. aonidum populations. Larval feeding on the armored C. aonidum was less compared to adults as indicated through their reduced consumption and compared to other two treatments. Most consumption was observed on scales with armor removed averaging 81% within 24 h and 92–100% within 72 h. Only 25% scales with armor were consumed at 24 h and 41% within 72 h, however, consumption rate improved to 54% at 24 h and 67% at 72 h in the mix treatment indicating the influence of the presence of the scales with armor removed. The improvement in larval feeding of the armored scale over time indicate their potential to adapt and they will have the opportunity to feed on the crawlers in the field which are without armor to further support their survival, development, and impact on C. aonidum. Adults and larvae of C. coeruleus may tend to attack the C. aonidum scales from the hole in the posterior of the armor which is used to release the nymphs (crawlers). The larvae of Rhyzobius ventralis during their attack on Eriococcus coriaceus exhibited similar behavior (Richards, Reference Richards1981). Although both adults and larvae of C. coeruleus were able to consume a significant proportion of C. aonidum with or without armor, the discrepancy in their consumption of armored scale may have resulted from the attack rate, handling time, and digestion potential as reported by other researchers (Ganjisaffar and Perring, Reference Ganjisaffar and Perring2015). However, in some species such as Harmonia axyridis larvae are more voracious than adults as observed against D. citri (Huang et al., Reference Huang, Qureshi, Zhou, Pu, Chen, Jihua and Zhang2019). Hodek and Honek (Reference Hodek and Honek2009) suggested that the foraging process in the ladybeetles is always directed by several cues (e.g., chemical, visual, and olfactory) (Seagraves, Reference Seagraves2009) which could be likely factors in our experiments.
We also tested the hypothesis that predator previous experience with the prey affects its selection and consumption of prey, by altering the same adults between diets containing C. aonidum with and without armor and testing adults on diet on which they developed as larvae and a different diet. Adults showed a high propensity to feed on the scales with armor removed consuming 95% within 24 h, an increase of 40% in the consumption rate compared with the previous diet of scales with armor intact. This behavior was reversed with a decrease of 31% in the consumption rate within 24 h on the diet with armor intact compared with the previous diet with armor removed. The shift from a mixed diet of scales with and without armor to the diet with armor intact did not produce any significant effect but a trend of reduced consumption rate on the later diet. We observed similar behavior and trend in the second experiment when the adults were tested on the same diet on which they developed as larvae and a different diet. When the larval diet was C. aonidum with armor, the resulting adults tested between the treatments of with or without armor consumed more scales without armor. A similar trend was observed with adults resulting from the larvae on mixed diet or without armor. Although variation in the diet is not necessarily apparent between larvae and adult coccinellids (Ricci, Reference Ricci1986), larvae may consume different diet compared to the adults, indicating their special predatory nutritional requirements, behavior, and predation abilities (Weber and Lundgren, Reference Weber and Lundgren2009). This could also be due to the variations in the physiological and metabolic requirements between larvae and adults (Hodek and Honek, Reference Hodek and Honek2009; Weber and Lundgren, Reference Weber and Lundgren2009). In addition to these variations, the presence of the armor as a physical barrier influenced their propensity to consume more C. aonidum when armor was removed irrespective of if diets were offered alone or mix of armor and without armor. The consumption of C. aonidum with armor intact by adults and larvae of C. coeruleus suggests their effectiveness in suppressing the populations of this pest. Although, larval consumption of scales with armor was lower compared with adults, it did not stop them from developing to adulthood. Under field conditions, C. aonidum crawlers which are without armor will also be available to the larvae and adults of C. coeruleus to further support their survival, development, and impact on the pest populations.
Acknowledgements
This research was supported by the National Institute of Food and Agriculture, U. S. Department of Agriculture's Specialty Crop Research Initiative (SCRI) Citrus Disease Research and Extension Program (CDRE) (Award no. 2018-70016-27387).








