Since the mid-twentieth century, the question of whether an association exists between antidepressant use and suicide-related events has been the subject of ongoing interest. Reference Mayer-Gross, Slater and Roth1 Although the initial enquiries were about adult users of antidepressants, the focus expanded early on to include paediatric users. Reference Hammad, Laughren and Racoosin2–Reference Tollefson4 Both the US Food and Drug Administration (FDA) and the European Union European Medicines Agency (EMA) responded with warnings for all or some of these agents, leading to changes in patterns of medical care and use of antidepressants. Reference Libby, Orton and Valuck5–Reference Valuck, Libby, Orton, Morrato, Allen and Baldessarini7 Currently, specific concerns centre on the possibility of increased risk of suicide-related events with the newer-generation serotonin–noradrenaline reuptake inhibitor (SNRI) antidepressants including venlafaxine and duloxetine, particularly when used by children, adolescents or young adults. 8 However, the generalisability of previous study results to current medical practice is unclear, as many have been limited by brief trial duration, 9–Reference Gunnell, Saperia and Ashby12 varying definitions of suicide-related events, lack of statistical power for rare events such as suicide or suicide attempt Reference Simon, Savarino, Operskalski and Wang13–Reference Jick, Kaye and Jick15 and inadequate control for non-random allocation, among other issues. Reference Schneeweiss, Patrick, Solomon, Mehta, Dormuth and Miller16 The lack of a community-based estimate of the risk of suicide-related events among people diagnosed with depression, but not treated with antidepressants, has also been an important gap in the literature, as has the lack of an estimate for the baseline rate of such events in people in the community at large (i.e. the general population). This information is critically important to the debate. Without the inclusion of these comparison groups in an analysis, it is not possible to determine the extent to which the risk of suicide-related events is the result of the underlying disease process itself, for example depression, to a possible increase in risk because of the antidepressant treatment in general, or to a possible increase in risk for specific antidepressant drugs or drug classes. The present study, initiated in response to a regulatory request by the EMA, seeks to address these gaps. Specifically, we aim to contribute to the debate in three major ways. First, by relying on data on real-world depression treatment across the USA from 1997 to 2007 to allow a focus on the most commonly used classes of antidepressants (SNRIs, selective serotonin reuptake inhibitors (SSRIs) and tricyclic antidepressants (TCAs)), to establish risk estimates for patients treated with the newest group of antidepressants (SNRIs) relative to the other classes. Second, by creating two distinct populations, an incident depression cohort (both pharmacologically treated and untreated) and a general population cohort against whom suicide attempt risk is compared. Third, by using state of the art epidemiological and statistical methods to account for non-random allocation for treatment and consequent differences in the characteristics of individuals in the different cohorts, using propensity-matched new-user cohorts and multivariate adjustment for known, measured covariates.
Method
Data source
This study used data from the IMS (formerly PHARMetrics) LifeLink Database, a commercially available claims database provided by IMS Health. 17 The LifeLink database is among the largest patient-centric databases of longitudinal, integrated medical and pharmacy claims data in the USA, making it ideal for studies of rare outcomes associated with commonly prescribed drug therapies. The integrated data obtained for this study included paid claims from 85 managed care plans nationally – representing 55 million covered lives – from January 1997 to December 2007. Two different data extracts were obtained by the authors. One extract included all records for patients with any mental health diagnosis (ICD-9 code 296.xx – 300.xx or 311.xx) 18 or an antidepressant prescription dispensed or one or more medically coded suicide attempts; this resulted in approximately 6 million adult patients and was used to create the pharmacologically treated and untreated depression groups. The second extract was a 10% random sample of the entire LifeLink database, irrespective of diagnosis or treatment; this resulted in approximately 3.5 million adult patients and was used to create a community-based general population sample for comparison. Data were unidentified and anonymous, and the study protocol and data use agreement were approved by the Colorado Multiple Institutional Review Board (COMIRB, Aurora, Colorado, USA).
Study cohorts
Figure 1 presents a consort chart depicting the creation of the study cohorts. First, a prevalent cohort of participants with current depression was created by identifying patients in the LifeLink mental health extract with a claim for depression (ICD-9 code 296.2x, 296.3x, 300.4x or 311.xx) between 1 August 2004 and 30 June 2007. Patients were excluded if they were younger than 19 years of age (to match FDA age-group specifications) or had less than 6 months of continuous insurance enrolment prior to any depression claim; this selection resulted in a cohort of 1 155 356 participants age 19 and older with at least one depression claim during the study period. The first depression claim was identified as the participant's index depression diagnosis.

Fig. 1 Flow chart depicting creation of study cohorts.
SNRI, serotonin–noradrenaline reuptake inhibitor; SSRI, selective serotonin reuptake inhibitor; TCA, tricyclic antidepressant.
From the prevalent depression cohort, an incident depression cohort was created by excluding patients who had less than 12 months of continuous insurance enrolment prior to an index depression diagnosis or had any of the following during the 12 months prior to their index depression diagnosis: any previous depression diagnosis codes, receipt of any antidepressant or receipt of any psychotherapy services as a paid claim. The time period prior to a depression diagnosis was also used to assess other known risk factors for suicide attempts, such as prior attempts. The resulting incident depression cohort represents participants with ‘new’ episodes of depression; it should be noted that these episodes are not necessarily ‘first-ever’ episodes, as the time period used for defining incident episodes was 1 year, and participants may have had episode(s) at some point in the more distant past.
The incident depression cohort (n = 434 498) was divided into five mutually exclusive treatment groups: those with a dispensed prescription for one of four types of antidepressants (SNRI, SSRI, TCA or multiple antidepressants, defined below) within 30 days of their index depression diagnosis, and those who did not receive antidepressant therapy at all during the study period (untreated depression group). Participants who received an antidepressant other than an SNRI, SSRI or TCA, and participants who received an antidepressant more than 30 days after their index depression diagnosis were excluded (n = 76 147).
Measures
Treatment/no treatment groups defined by drug class
Treatment groups were defined on the basis of a dispensed prescription for one of four drug classes within 30 days following the index depression date. The no treatment group was defined as the absence of an antidepressant prescription dispensed of a given type during the study period. SNRIs included duloxetine and venlafaxine and were identified using Medi-Span Electronic Drug File™ (Med-File) v.2 Generic Product Identifier (GPI) code 5818. SSRIs included citalopram, escitalopram, fluoxetine, fluvoxamine, paroxetine and sertraline and were identified using GPI code 5816. TCAs were identified using GPI code 5820. The multiple antidepressants group was defined as at least one dispensed prescription in a drug class under study within the first 30 days after diagnosis and at least one antidepressant prescription dispensed from another drug class within the 6-month follow-up period. Non-SNRI, -SSRI or -TCA antidepressants – namely bupropion, isocarboxazid, maprotiline, mirtazapine, nefazodone, phenelzine, selegiline, tranylcypromine and trazodone – were excluded.
Index date
For the four antidepressant treatment groups (SNRI, SSRI, TCA or multiple antidepressants), the index date was defined as the date of the first dispensing of the drug in the study period. For the untreated depression group, the index date was defined as the date of their index depression diagnosis. For the general population sample, the index date was defined as the index date for their match from the matched SNRI group.
Suicide attempt
The primary study outcome was suicide attempt leading to a medical encounter. Suicide attempts leading to medical encounters were identified from all medical or facility claim records by a diagnosis code in the following range (following Centers for Disease Control and Prevention guidelines): ICD-9 codes E950-E959 19 or ICD-10 codes X60-X84 or Y87.0. 20 Suicide attempts were considered study outcomes if they occurred at any time during the follow-up period, which started on the index date and lasted for 6 months; or up until the participant had a 1-month gap in continuous insurance enrolment; or had their first suicide attempt. Completed suicides are not captured in the LifeLink database and were thus not included as an outcome measure in this study.
Covariates
Demographic covariates included gender, region (East, Midwest, South and West), Medicaid status at time of index depression diagnosis and age at index depression diagnosis. Age groups were created based on the following definitions for comparison with FDA suicidality risk communications: young adult, age 19–24 years; adult, age 25–64 years; and older adult, age 65 and older.
Other covariates included both specific and total numbers of mental health comorbidities; specific chronic and acute non-mental health comorbidities; indices of chronic comorbidity including the Chronic Disease Indicator (CDI) Reference Malone, Billups, Valuck and Carter21 score and the Charlson comorbidity score; Reference D'Hoore, Bouckaert and Tilquin22 prior medication use (drug-months of exposure to both psychotropic medications and all prescription medications); use of health services; history of suicide attempt; severity of the index depression diagnosis; physician-level covariates; and a market-level covariate reflecting rates of prescribing of each drug group by generalist v. specialist prescribers during the month that antidepressant therapy was initiated. Covariates were all assessed during the 6 months prior to the index depression diagnosis and are detailed in Tables 1 and 2.
Table 1 Demographic and clinical characteristics of the matched incident depression cohorts and the community-based general population sample

| SNRI
group (n = 8403) |
SSRI
group (n = 24 398) |
TCA
group (n = 1119) |
Multiple antidepressants group (n = 17 194) |
Untreated depression group (n = 1241) |
General
population sample (n = 75 756) |
|
|---|---|---|---|---|---|---|
| Age at index date, years: mean (median) | 41.99 (43.00) | 42.18 (42.00) | 44.92 (45.00) | 41.28 (42.00) | 44.22 (45.00) | 41.93 (43.00) |
| Range, minimum–maximum | 19–98 | 19–98 | 19–98 | 19–98 | 19–98 | 19–98 |
| Age group, n (%) | ||||||
| Young adult, 19–24 years | 787 (9.37) | 2675 (10.96) | 66 (5.90) | 1698 (9.88) | 106 (8.54) | 7341 (9.69) |
| Adult, 25–64 years | 7546 (89.80) | 21 051 (86.28) | 1016 (90.80) | 15 272 (88.82) | 1094 (88.15) | 67 731 (89.41) |
| Older adult, 65+ years | 70 (0.83) | 672 (2.75) | 37 (3.31) | 224 (1.30) | 41 (3.30) | 684 (0.90) |
| Female, n (%) | 5644 (67.17) | 16 560 (67.87) | 757 (67.65) | 11 339 (65.95) | 807 (65.03) | 49 209 (64.96) |
| Medicaid status, n (%) | 77 (0.92) | 276 (1.13) | 31 (2.77) | 236 (1.37) | 25 (2.01) | 1026 (1.35) |
| Region, n (%) | ||||||
| East | 1893 (22.53) | 5417 (22.20) | 237 (21.18) | 3545 (20.62) | 361 (29.09) | 17 982 (23.74) |
| Midwest | 3626 (43.15) | 10 390 (42.59) | 500 (44.68) | 8227 (47.85) | 407 (32.80) | 31 371 (41.41) |
| South | 2298 (27.35) | 6756 (27.69) | 261 (23.32) | 4139 (24.07) | 369 (29.73) | 20 845 (27.52) |
| West | 586 (6.97) | 1835 (7.52) | 121 (10.81) | 1283 (7.46) | 104 (8.38) | 5558 (7.34) |
| Suicide attempt history (6
months prior to index depression date) |
||||||
| Any previous attempt, n (%) | 35 (0.42) | 92 (0.38) | 2 (0.18) | 106 (0.62) | 6 (0.48) | 10 (0.01) |
| Number of previous
attempts, mean (median) |
0.00 (0.00) | 0.00 (0.00) | 0.00 (0.00) | 0.01 (0.00) | 0.00 (0.00) | 0.00 (0.00) |
| Range, minimum–maximum | 0–3 | 0–2 | 0–1 | 0–2 | 0–1 | 0–1 |
| Any attempts resulting
in admission to hospital, n (%) |
0 (0.00) | 0 (0.00) | 0 (0.00) | 2 (0.01) | 0 (0.00) | 2 (0.00) |
| Prior drug overdose history, a n (%) | 29 (0.35) | 73 (0.30) | 4 (0.36) | 80 (0.47) | 14 (1.13) | 53 (0.07) |
| Index depression severity indicators, n (%) |
||||||
| Recurrent depression | 815 (9.70) | 1964 (8.05) | 99 (8.85) | 2335 (13.58) | 160 (12.89) | N/A |
| Index depression coded
as hospital discharge diagnosis |
191 (2.27) | 496 (2.03) | 24 (2.14) | 647 (3.76) | 46 (3.71) | N/A |
SNRI, serotonin-noradrenaline reuptake inhibitor; SSRI, selective serotonin reuptake inhibitor; TCA, tricyclic antidepressant.
a. Not coded as suicide attempt in claims records.
Table 2 Comorbidities (6 months prior to index depression date) in the matched incident depression cohorts and the community-based general population sample

| SNRI
group (n = 8403) |
SSRI
group (n = 24 398) |
TCA
group (n = 1119) |
Multiple antidepressants group (n = 17 194) |
Untreated depression group (n = 1241) |
General population sample (n = 75 756) |
|
|---|---|---|---|---|---|---|
| Chronic Disease Indicator score, Reference Malone, Billups, Valuck and Carter21 mean (median) |
2.66 (2.00) | 2.82 (2.00) | 4.71 (4.00) | 3.30 (3.00) | 1.52 (0.00) | 1.56 (1.00) |
| Range, minimum–maximum | 0–21 | 1–21 | 2–18 | 0–19 | 0–13 | 0–22 |
| Charlson comorbidity score,
Reference D'Hoore, Bouckaert and Tilquin22
mean (median) |
0.71 (0.00) | 0.71 (0.00) | 0.94 (1.00) | 0.74 (0.00) | 1.17 (1.00) | 0.00 (0.00) |
| Range, minimum–maximum | 0–10 | 0–14 | 0–9 | 0–11 | 0–12 | 0–0 |
| Prior depression, n (%) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 1964 (2.59) |
| Bipolar disorder, n (%) | 34 (0.40) | 78 (0.32) | 8 (0.71) | 80 (0.47) | 24 (1.93) | 382 (0.50) |
| Schizophrenia, n (%) | 6 (0.07) | 19 (0.08) | 1 (0.09) | 5 (0.03) | 19 (1.53) | 51 (0.07) |
| Psychotic disorder, n (%) | 47 (0.56) | 147 (0.60) | 7 (0.63) | 120 (0.70) | 19 (1.53) | 174 (0.23) |
| Anxiety spectrum disorder, n (%) | 588 (7.00) | 1624 (6.66) | 61 (5.45) | 1187 (6.90) | 99 (7.98) | 1713 (2.26) |
| Attention–deficit
hyperactivity disorder, n (%) |
44 (0.52) | 130 (0.53) | 0 (0.00) | 76 (0.44) | 7 (0.56) | 325 (0.43) |
| Substance use disorder, n (%) | 320 (3.81) | 869 (3.56) | 51 (4.56) | 791 (4.60) | 70 (5.64) | 1438 (1.90) |
| Other mental health disorder, n (%) | 307 (3.65) | 864 (3.54) | 47 (4.20) | 696 (4.05) | 66 (5.32) | 1877 (2.48) |
| Number of mental health
disorders, mean (median) |
0.16 (0.00) | 0.15 (0.00) | 0.15 (0.00) | 0.17 (0.00) | 0.24 (0.00) | 0.08 (0.00) |
| Range, minimum–maximum | 0–5 | 0–5 | 0–4 | 0–5 | 0–5 | 0–5 |
| Terminal diagnosis (HIV or
malignant neoplasm), n (%) |
233 (2.77) | 688 (2.82) | 45 (4.02) | 434 (2.52) | 57 (4.59) | 1436 (1.90) |
| Smoking, n (%) | 28 (0.33) | 86 (0.35) | 5 (0.45) | 55 (0.32) | 8 (0.64) | 119 (0.16) |
| Seizure disorder, n (%) | 54 (0.64) | 157 (0.64) | 13 (1.16) | 90 (0.52) | 20 (1.61) | 265 (0.35) |
| Chronic pain, n (%) | 16 (0.19) | 30 (0.12) | 1 (0.09) | 14 (0.08) | 19 (1.53) | 41 (0.05) |
| Headaches (including migraine), n (%) | 579 (6.89) | 1533 (6.28) | 170 (15.19) | 1340 (7.79) | 98 (7.90) | 3123 (4.12) |
| Stress urinary incontinence, n (%) | 38 (0.45) | 102 (0.42) | 4 (0.36) | 76 (0.44) | 12 (0.97) | 223 (0.29) |
| Fibromyalgia, n (%) | 325 (3.87) | 837 (3.43) | 62 (5.54) | 677 (3.94) | 87 (7.01) | 1491 (1.97) |
| Diabetic neuropathy, n (%) | 206 (2.45) | 559 (2.29) | 51 (4.56) | 447 (2.60) | 62 (5.00) | 1005 (1.33) |
| Low back pain/back pain, n (%) | 959 (11.41) | 2567 (10.52) | 173 (15.46) | 2194 (12.76) | 220 (17.73) | 4616 (6.09) |
SNRI, serotonin-noradrenaline reuptake inhibitor; SSRI, selective serotonin reuptake inhibitor; TCA, tricyclic antidepressant.
Statistical analysis
To account for non-random treatment allocation, we used propensity score matching. This is among the strongest forms of adjustment, balancing measured covariates for the treatment and comparison groups before analysis. Reference Cleophas and Zwinderman23 First, the propensity for receiving an SNRI (v. SSRI, TCA, multiple antidepressants or no antidepressants) was estimated for each participant in the incident depression cohort using a multivariate unconditional logistic regression model. This model included SNRI treatment (yes/no) as the dependent variable and specified baseline patient-, physician- and market-level measures as covariates. The sets of patient-, physician- and market-level variables were based on prior research and published literature and were assessed for each participant during the 6 months prior to their index depression diagnosis.
Next, participants in the SNRI group were matched to participants from the SSRI, TCA, multiple antidepressants and no antidepressant groups, in a pairwise fashion, using the propensity scores calculated in the previous step. Matching ratios (i.e. the number of participants in the SNRI group matched to those in each comparison group) were 1:3 for each drug-group pair with the exception of TCA, which was matched to the SNRI group with a ratio of 1:1 because of the small sample of adults receiving TCAs as initial therapy. Caliper width (i.e. the allowable amount of deviation in scores among matches) was set at 0.001 for each match. For each pairwise match, if there were more than three (or one for TCA) participants that matched on propensity score within 0.001, the participants to be matched were chosen randomly from those eligible. This step in the pairwise matching on propensity score resulted in an incident cohort of 52 355 participants. In the original SNRI group, 69 participants did not have a match in any of the other drug groups and therefore the size of the matched SNRI group is slightly smaller than the original SNRI group.
The final step in creating the analytic cohort was to match participants from the matched SNRI group (n = 8403) – the primary group of interest – to participants in the 10% general population random sample. Participants were matched on age (plus or minus 1 year), gender and region, with up to 10 participants from the general population sample being matched to each participant in the matched SNRI group. If there were more than 10 eligible matches from the general population sample for any participant in the matched SNRI group, 10 general population sample participants were randomly chosen for the match. The resulting analytic cohort included a total of 128 111 participants (52 355 from the incident depression cohort and 75 756 from the general population sample).
Analyses included both the propensity-matched drug-group cohorts and the SNRI–general population sample cohort matched on age, gender and region (n = 128 111). Analyses were initially stratified by age group (young adult, adult and older adult), then combined and reported in aggregate when similar results were obtained across stratified groups. SAS version 9.1 was used for all data creation and analyses. An alpha-level of 0.05 and 95% confidence intervals were used to indicate statistical significance.
To identify periods of potentially increased risk of suicide attempt in relation to initiation of antidepressant treatment or no treatment, rates of suicide attempt were aggregated monthly, by antidepressant class, in relation to the patients' index date. Rates are reported per 100 000 participants for each month during the 6 months prior to and 6 months following index dates by treatment cohort. For patients in the four treatment groups, monthly incidence rates are anchored by the initiation of antidepressant treatment; for patients in the untreated depression group, incidence rates are anchored by the index depression diagnosis; and for patients in the general population sample, incidence rates are anchored by their SNRI-matched-participant index date. To characterise risk of suicide attempt following initiation of treatment, crude suicide attempt rates during the follow-up period were calculated per 100 000 participants for each treatment/no treatment group. Absolute (attributable) risk differences were calculated by subtracting the rate for each depression group (treated and untreated) from the rate for the general population sample.
To estimate a fully adjusted effect of treatment group (SNRI, SSRI, TCA or multiple antidepressants, untreated depression, general population sample) on risk of suicide attempt, multivariate Cox proportional hazards regression models were used, with propensity score included as a covariate, for the outcome days to first suicide attempt. Patients with multiple suicide attempts were censored at the time of first attempt, regardless of how many additional attempts they may have had. Patients with no suicide attempt during the follow-up period were by default censored at the end of follow-up. Demographic characteristics, comorbidities, drug-months of exposure to prior prescriptions (psychotropic and total) and suicide attempt history were included in the models as covariates. Models were run separately by age group and the referent group was varied to estimate the statistical effects compared with the general population sample, untreated depression group or the SNRI group, respectively.
Results
Descriptive statistics on the matched, incident depression cohort (treated and untreated) and the general population sample are presented in Tables 1 and 2 (online Tables DS1–6 provide descriptive statistics on the cohorts both prior to, and after, propensity score matching to illustrate both the need for and the effect of the propensity score matching process, based on the demographic and clinical characteristics of the study groups). As seen in Table 1, the study cohorts are comparable on factors associated with suicide risk after propensity matching. Mean age of study participants varied only from 41 to 44 years. Roughly two-thirds of each cohort were women, as would be expected in samples of adults with depression; the proportion of women was similar in the general population sample because of age and gender matching. Based on the exclusion criteria used to create the cohorts, by definition, there was no prior depression in the depression groups during the 12 months prior to the index episode; 2.6% of the general population sample had a depression diagnosis during the 6 months prior to their index date. The most common comorbidity (Table 2) seen prior to the index date was anxiety spectrum disorder (5–8% in the depression groups, 2% in the general population sample). Prevalence of prior suicide attempt was low (Table 1), ranging from 0.18% to 0.62% in the depression groups and only 0.01% in the general population sample. The index depression diagnosis indicated recurrent illness for 8–14% of patients (Table 1).
Monthly aggregated suicide attempt rates in relation to index date are presented for all ages in Fig. 2. For all the treated depression groups (SNRI, SSRI, TCA and multiple antidepressants), suicide attempt rates were highest in the month before diagnosis and treatment initiation, and steadily decreased over the next 6 months. The highest rate for the untreated depression group was seen in the second month following the depression diagnosis. Although the low rate for the general population sample was difficult to determine from the figure, it was higher 6 months prior to the index date (5.3 per 100 000) and 3 months following the index date (4.2 per 100 000) and displayed much less of a clear pattern or trend over time, as would have been expected.

Fig. 2 Crude rates of suicide attempt during the 6 months prior to and following the index antidepressant prescription date, all age groups aggregated.
Suicide attempts occurring on the index date were included in the prior month. Standard errors shown by whiskers. SNRI, serotonin–noradrenaline reuptake inhibitor; SSRI, selective serotonin reuptake inhibitor; TCA, tricyclic antidepressant.
Figure 3 displays suicide attempt rates for the follow-up period by age group. Young adult and adult groups are shown; there were no suicide attempts to display in the older adult age group. Treatment cohort crude suicide attempt rates are presented as absolute (attributable) risk differences from the general population sample community-based cohort. The multiple antidepressants group had the highest suicide attempt rate, with an absolute risk difference (ARD) of 2156 additional attempts per 100 000 and 498 additional attempts per 100 000 in the young adult and adult cohorts, respectively, compared with the general population sample. In the adult age group (ages 25–64), the untreated depression group had statistically significantly higher suicide attempt rates (ARD of 163 additional attempts per 100 000) relative to the general population sample, an ARD that was higher than those observed for the SNRI, SSRI or TCA treated groups (ARDs of 113, 81 and 79 additional attempts per 100 000 respectively). In the young adult (age 19–24) age group, no suicide attempts were observed for participants in the untreated depression or TCA groups, resulting in ARDs of 82 attempts per 100 000 lower (less) than the general population sample, although the rarity of events in these two specific groups makes the estimates less precise.
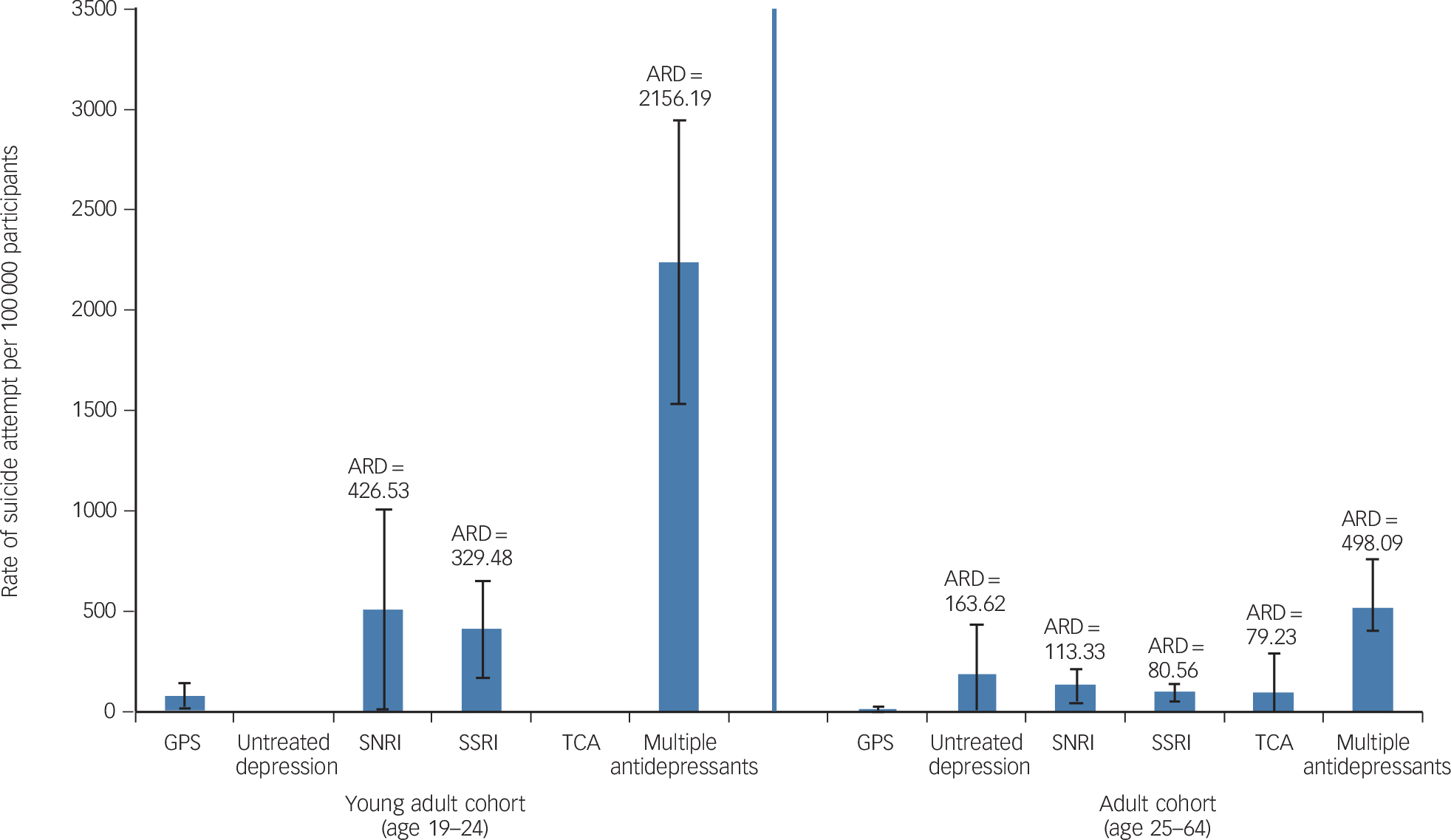
Fig. 3 Rate of suicide attempt and absolute risk difference (ARD) for depression cohorts relative to the general population sample (GPS) cohort, by age group.
No results are presented for the older adult age cohort because there were no suicide attempts in that group. Whiskers show 95% confidence intervals. SNRI, serotonin–noradrenaline reuptake inhibitor; SSRI, selective serotonin reuptake inhibitor; TCA, tricyclic antidepressant.
Table 3 presents multivariate Cox proportional hazard model results on time to suicide attempt across the study groups, stratified by age group (again, without reporting older adults because of no events). The association of treatment drug class on suicide risk was estimated fully adjusting for propensity to receive an SNRI, comorbidities, drug-months of exposure to prior prescriptions and suicide attempt history, among other relevant variables (listed in Tables 1 and 2). Three separate multivariate models were estimated for young adults and adults; each model varied the reference group (SNRI, untreated depression or general population sample) in order to more fully describe the relative associations between different classes of drug treatments and suicide risk. The top panel reports hazard ratios (HR) with the general population sample as the referent group. The depression cohorts, both treated and untreated, had significantly higher risk for suicide attempt compared with the general population, with a greater magnitude for adults over age 24 than for young adults. Indeed, comparison with the general population sample as a referent group shows the statistically significant association of depression with suicide risk present in the study cohorts.
Table 3 The effect of study cohort on suicide attempt risk, estimated using multivariate Cox proportional hazard models of time to suicide attempt, stratified by age group with varying referent groups a

| Hazard ratio (95% CI) | ||
|---|---|---|
| Group | Young adult, age 19–24 (n = 12 673) | Adult, age 25–64 (n = 113 710) |
| Referent group, general population sample | ||
| Serotonin–noradrenaline reuptake inhibitor | 7.62 (1.99–29.20) | 9.00 (3.60–22.50) |
| Selective serotonin reuptake inhibitor | 4.51 (1.50–13.59) | 6.87 (3.10–15.20) |
| Tricyclic antidepressant | NE | 5.10 (0.64–40.84) |
| Multiple antidepressants | 20.92 (7.81–56.04) | 23.87 (11.70–48.73) |
| Untreated depression | NE | 10.65 (2.11–53.80) |
| Referent group, untreated depression group | ||
| Serotonin–noradrenaline reuptake inhibitor | NE | 0.85 (0.17–4.19) |
| Selective serotonin reuptake inhibitor | NE | 0.65 (0.14–3.02) |
| Tricyclic antidepressant | NE | 0.48 (0.04–5.65) |
| Multiple antidepressants | NE | 2.24 (0.50–10.02) |
| General population sample | NE | 0.09 (0.02–0.46) |
| Referent group, serotonin–noradrenaline reuptake inhibitor | ||
| Selective serotonin reuptake inhibitor | 0.59 (0.19–1.90) | 0.76 (0.36–1.63) |
| Tricyclic antidepressant | NE | 0.57 (0.07–4.46) |
| Multiple antidepressants | 2.75 (0.97–7.82) | 2.65 (1.63–5.16) |
| Untreated depression | NE | 1.18 (0.24–5.86) |
| General population sample | 0.13 (0.03–0.50) | 0.11 (0.04–0.28) |
NE, not estimable.
a. Older adult cohort not reported because there were no suicide attempts in that age group; model s also adjusted for comorbidities, drug-months of prior medications filled and suicide attempt history. Results in bold are significant.
The middle panel reports hazard ratios using the untreated depression group as the referent group; antidepressant-treated groups were not significantly different from the untreated depression group on the relative hazard of suicide attempt. These data are consistent with the illness of depression as the major driver of suicide attempts, with rates decreasing on a population basis after antidepressant treatment is initiated. Nonetheless, population data do not fully address the risk of suicidal behaviour that has been reported in placebo-controlled randomised trials in some populations (i.e. adolescents). As such, clinical monitoring for suicide risk in at-risk populations is still prudent.
The bottom panel reports results with the SNRI group as referent group in order to examine suicide attempt risk associated with specific classes/types of antidepressants. There was no significant difference in suicide attempt risk between groups treated with SNRIs and SSRIs. There were only two SNRIs included in the analysis – duloxetine and venlafaxine – and they were examined as individual agents as well as an SNRI class (individual agent results provided in online Table DS7). When compared with patients treated with duloxetine (Table DS7), patients treated with venlafaxine (HR = 1.01, 95% CI 0.6–1.8) or SSRIs (HR = 0.89, 95% CI 0.5–1.5) showed no statistically different rate of suicide attempt. The multiple antidepressants group had significantly increased risk of suicide attempt compared with the duloxetine subgroup (HR = 3.11, 95% CI 1.9–5.0).
Discussion
Main findings
This study presents the most complete set of comparative risk estimates published to date regarding the risk of suicide attempt associated with antidepressant treatment in adults during naturalistic treatment. In addition to antidepressant drug class v. class comparisons, we included a general population cohort that permitted estimation of the association between the underlying illness (depression) and suicide attempt. In the Cox proportional hazard models, the depression cohort, whether treated or untreated, had significantly higher risk for suicide attempt compared with the general population. The comparison with an untreated depression group further supports the hypothesis that depressive illness underlies the risk of suicide attempt observed among the antidepressant-treated groups. In fact, in comparison with the untreated depression group, patients taking antidepressants of any individual class did not have a significantly higher risk of suicide attempt. Only users of multiple antidepressant classes (either concurrently or consecutively) had higher risk of suicide attempt.
It might have been hypothesised that patients in the depression cohort who were treated would have had lower rates of suicide attempt than those individuals who were not. In reality, however, the lack of pharmacological treatment in the untreated depression group may have reflected a judgement by the prescribing physician that the patient's depression was mild and/or that the perceived risk of suicide or other adverse outcomes was low, and that pharmacological treatment was therefore not required. Conversely, given that TCAs are increasingly reserved for more severely ill patients, a higher rate of suicide attempts might have been expected in this cohort, but we did not see this. The relatively low rate of suicide attempts in this group may reflect the fact that TCAs are highly toxic and at times lethal in overdose relative to SNRIs and SSRIs, Reference Whyte, Dawson and Buckley24 and as a consequence prescribers may avoid their use in patients considered to be at a risk of suicide. The risk of suicide attempt in the multiple antidepressants group was significantly higher than in other groups, perhaps reflecting a harder to treat patient population or a situation in which the use of multiple antidepressant classes might carry additional unknown risks.
SNRIs
Considering the SNRIs, there are only two individual agents in this class. They were examined both individually and combined, with no differences in results, thus providing the first results for the latest SNRI on the market, duloxetine. Contrary to some previous reports, 8,Reference Rubino, Roskell, Tennis, Mines, Weich and Andrews25 our study did not suggest the existence of a significantly higher suicide attempt risk among patients using SNRIs as a class compared with those using SSRIs. It may be that the SNRIs are reserved by prescribers for the treatment of patients with severe or chronic depression or whose depression is treatment-resistant compared with those prescribed SSRIs, so the SNRI-treated group may represent one at higher risk of suicide attempt. Reference Mines, Hill, Yu and Novelli26 Some prior studies have not controlled for baseline differences (such as prior suicide attempt history), so more suicide attempts would be expected in patients receiving SNRIs v. SSRIs. The fact that our study controlled for many baseline differences makes the study important, and may at least partially explain the lack of any observed differences in risk of suicide attempt between the SNRI and SSRI cohorts. Likewise, no differences among individual SSRI subgroups were detected (in comparison with either fluoxetine as a referent SSRI, or the TCA, multiple antidepressants or the untreated depression groups; results not shown, because of lack of observed differences between groups).
Strengths and limitations
The cohort creation and multivariate methods used in this study represent the state of the art in accounting for non-random allocation to treatment and the associated confounding that might be observed in a treatment-outcome relationship. Reference Velentgas, Dreyer, Nourjah, Smith and Torchia27 We used the standard approach of creating an incident depression cohort with the associated clean sweep period. We then were able to utilise the large sample size in our database to create propensity-matched cohorts. Finally, we used further multivariate adjustment using measures of known, measurable risk factors in claims data for later suicide attempt (such as history of suicide attempt, prior depression episode) to further account for residual confounding. Our results are consistent with prior work showing an elevation in risk of suicide attempt prior to initiation of antidepressant therapy, and a gradual reduction in risk over time after initiation. Reference Simon, Savarino, Operskalski and Wang13,Reference Valuck, Libby, Sills, Giese and Allen28
Limitations to this study should be taken into account when interpreting the findings. Data for the study were drawn from integrated health insurance claims records, which are subject to underreporting of diagnosis because of stigma or financial disincentives, to underreporting of antidepressant prescriptions because of the use of samples and to underreporting of suicide attempts that may not reach the level of seriousness to result in a medical claim, but may nevertheless be real. These limitations may potentially bias our estimates in a downward direction, leaving the results as a low estimate of the statistical parameter. There is no reason, however, to expect that any of the possible underreporting would bias any one study group or finding in a particular direction, or bias across-group (relative risk) comparisons. Second, our study does not capture completed suicides, aborted attempts or suicidal thoughts or ideation. Thus, only one dimension of suicide-related behaviour is addressed, and our findings do not generalise to other dimensions of this complex phenomenon. Further, the use of propensity score matching, as intended, ‘levelled the playing field’ by making the study drug groups more comparable in terms of many demographic and clinical variables, but there were still some residual imbalances between the groups (such as rates of recurrent depression were higher in the untreated depression group and multiple antidepressants group than the single antidepressant groups). In addition, propensity score matching can only match on the basis of identified, measured confounders and there may be other confounders that are either unknown or unmeasured, that contribute to residual differences between groups. Finally, we did not study child or adolescent populations, having addressed them in prior work, Reference Valuck, Libby, Sills, Giese and Allen28 so our findings are generalisable only to adult populations.
In summary, although focusing on the risk of suicide attempt among users of SNRI antidepressants, this study adds risk estimates to the literature on key, previously unmeasured cohorts: the general population, with depression but pharmacologically untreated and the SSRI, SNRI and multiple antidepressants groups. We also contribute new data in regard to the most recent SNRI introduced to the market. Large, retrospective new-user cohorts are the ideal study design for such work because of the need to detect not only the rare outcome of suicide attempt, but also to create statistically matched cohorts. These estimates are readily interpretable for such metrics as number needed to harm and likelihood of help and harm because they were derived from real-world effectiveness samples from a broad-based US managed care population. Future work will follow up on this analysis with further investigation into the complexity of describing drug regimens and measuring suicide-related behaviours and events among users of multiple antidepressants, and the study of multiple suicide attempts over time.









eLetters
No eLetters have been published for this article.