Globally, approximately 2 billion individuals have an insufficient iodine intake and sub-Saharan Africa (SSA) is the most affected continent(Reference Zimmermann, Jooste and Pandav1). Iodine is an essential micronutrient required in small amounts for healthy brain development and normal thyroid functioning(Reference Ahad and Ganie2–Reference Roy, Chaturvedi and Agrawal4). Iodine deficiency, defined as an insufficient amount of iodine in the human body, is one of the leading causes of preventable mental impairment around the globe(Reference Zimmermann, Jooste and Pandav1,Reference Ahad and Ganie2,5) . Iodine-deficiency disorders (IDD) are a major contributor to the burden of diseases of public health significance in Africa, where many people do not consume sufficient iodine in their diet. Up to 65 % of pregnant women suffer from iodine deficiency in developing countries and this deficiency is associated with an increased risk of poor birth outcomes such as low birth weight, spontaneous abortions and congenital malformations. Children born to mothers with iodine deficiency are at risk of developing neurological deficits, cretinism and death(Reference de Benoist, McLean and Andersson6–Reference Majumder, Jaiswal and Chatterjee10). In addition, iodine deficiency in women is responsible for goitre, a condition associated with stigmatization and poor quality of life(Reference Zimmermann, Jooste and Pandav1,Reference Ahad and Ganie2,5,Reference Patrick11,Reference Verheesen and Schweitzer12) . Goitre is present in about 28·3 % of the African population(Reference Okosieme13–Reference Gitau16). The mainstay of treatment and prevention of IDD is salt iodization, the fortification of salt for human consumption with iodine, which is an effective and inexpensive approach to increase iodine intake at the population level(Reference Zimmermann14,Reference Darcan and Goksen17) . Sources of iodine that are recommended include plants grown in iodine-rich soils, eggs, saltwater fish, yoghurt, nuts and dairy products(Reference Zimmermann14,Reference Bouga, Lean and Combet18) . However, people in developing countries like those in SSA may not have access to those sources due to poverty. The iodization of salt is the most cost-effective and simplest method of preventing IDD with a cost of only $US 0·02–0·05 per individual per year(Reference Zimmermann, Jooste and Pandav1). The WHO/UNICEF/International Council for the Control of IDD recommends a national rate of iodized salt of at least 90 %(19), but low socio-economic households in SSA are less likely to buy iodized salt(Reference Knowles, Garrett and Gorstein20,Reference Mtumwa, Ntwenya and Paul21) . While the rates of non-iodized salt consumption in SSA countries have decreased considerably since the introduction of universal salt iodization (USI) in 1994 by the WHO and UNICEF, information about the independent factors that contribute to the consumption of non-iodized salt among women of reproductive age in SSA countries is limited and little is known about the use of non-iodized salt among women during pregnancy.
Although a study by Knowles et al. in 2018 looked at factors associated with household salt iodine content, such as salt brand, grain type, and whether the respondent had heard of iodized salt and had looked for iodized salt when purchasing salt, their study included only two countries from SSA(Reference Knowles, Kupka and Dumble22). In addition, Knowles et al.’s study did not focus specifically on women of reproductive age. During the earliest months of pregnancy, the production of maternal thyroxine increases by more than 50 % to meet fetal requirements for healthy brain development(23,Reference Zimmermann24) , which requires crucial iodine intake by the mother. Because IDD during pregnancy has potentially serious consequences for the fetus as mentioned above, the current study examined factors related to use of non-iodized salt among women of reproductive age, using a large sample of more than 100 000 women across multiple SSA countries.
Methods
Data source
The Demographic and Health Survey (DHS) programme collects, analyses and disseminates high-quality data through more than 300 surveys in over ninety developing countries around the world. The DHS data are a nationally representative survey that uses a multistage, stratified design to collect information on population demographics and health in each country. These projects are funded by the US Agency for International Development and are implemented by ICF International. We conducted a multi-country analysis using the most recent data available from the DHS, starting from 2015 to 2018. Countries that had the most recent DHS data and had conducted testing of salt for iodine were (n 11): Benin, Angola, Malawi, Rwanda, Uganda, Burundi, Senegal, Ethiopia, Tanzania, Zimbabwe and South Africa, and were included for analysis. First, from each country’s household data we extracted salt iodization information which we linked to each woman’s data using DHS merging guidelines. We did not use the sampling weights for the current study because we did not use standard errors for any descriptive tables. According to the DHS, weighting matters only when the statistical analysis involves standard errors. The files from the eleven countries were then combined into a single file. The year of survey extracted for each country is presented in Table 1.
Table 1 Characteristics of the surveys and respondents in the current study: women of reproductive age (15–49 years; n 108 318) from eleven sub-Saharan African countries that participated in the Demographic and Health Survey programme since 2015 and measured the presence of iodine in household salt
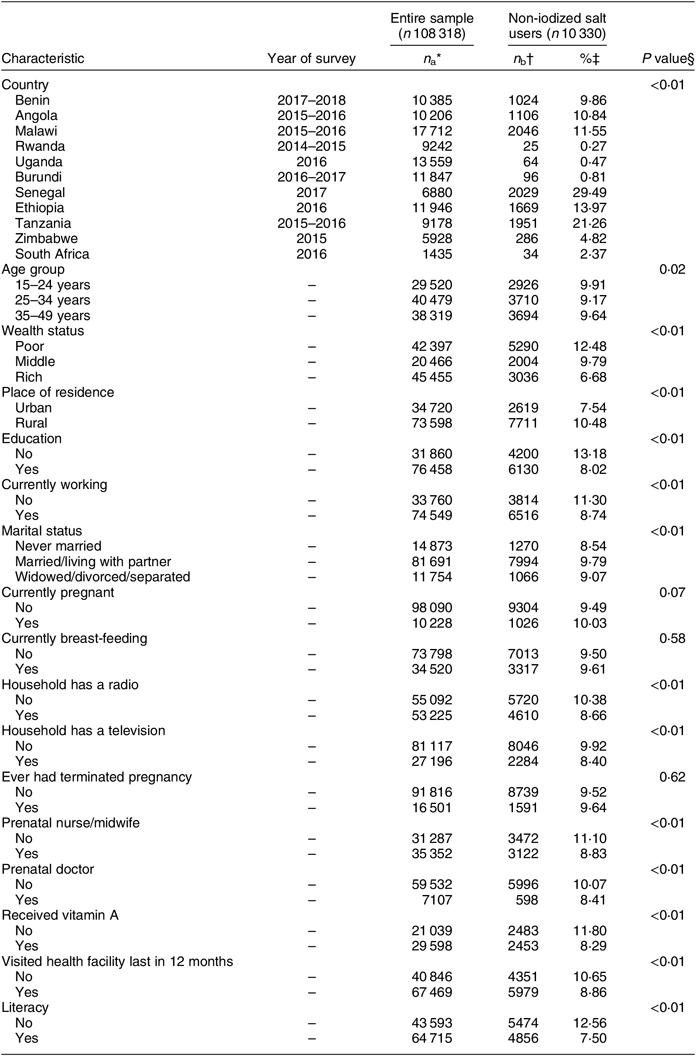
* Entire sample for each category (the total number for each variable may not sum to the total unweighted sample size of 108 318 due to missing values).
† Number of non-iodized salt users (the number of non-iodized salt users for each variable may not sum to 10 330 due to missing values).
‡ Rate of non-iodized salt calculated as n b/n a × 100 for each category.
§ P value reflects statistically significant association in the χ 2 test between non-iodized salt v. iodized salt users (latter not shown in the table).
Study population
The analysis included 108 318 women of reproductive age (15–49 years) from eleven SSA countries where household salt was tested for iodine content (Fig. 1). To avoid repeated measures, we used data from only one woman per household: the first woman listed who was aged 15–49 years.
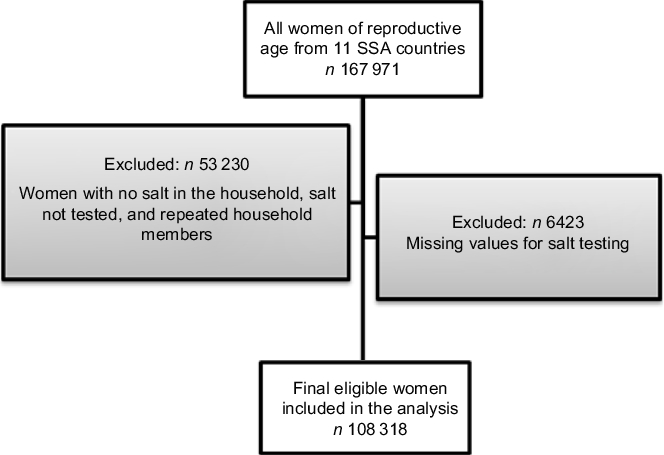
Fig. 1 Flow diagram of participants in the present study of non-iodized salt consumption among women of reproductive age (15–49 years) in sub-Saharan Africa (SSA)
Salt testing for iodine
The question in the DHS for salt testing includes the instruction to ‘ask respondent for a teaspoonful of cooking salt’. The salt specimen is then tested using a rapid test kit to determine whether iodine is present or not. The DHS rapid test kit contains a starch-based solution that generates a chemical reaction, resulting in a colour change when iodine is present(Reference Roy, Chaturvedi and Agrawal4). The data are reported as ‘1 – iodine present’, ‘0 – no iodine present’, ‘3 – no salt in household’ and ‘6 – salt not tested’. We excluded from the current analysis participants with ‘no salt in household’, ‘salt not tested’ and missing values for the salt test.
Outcome variable
The main outcome variable of interest for the present study was the consumption of non-iodized salt.
Explanatory variables
The independent variables included individual-level and household-level sociodemographic characteristics, including: current pregnancy status, current breast-feeding status, age, education status, country, history of terminated pregnancy, having a prenatal nurse or doctor, television and radio in the home, marital status, wealth index, employment status, rurality of residence (urban/rural), visited a health-care facility in the last 12 months and literacy. Based on previous literature, we recategorized the wealth index from five quintiles into three categories (‘poor’, ‘middle’ and ‘rich’) by grouping ‘poorest’ and ‘poorer’ into one category as ‘poor’, retaining ‘middle’, and grouping ‘richer’ and ‘richest’ into another category as ‘rich’(Reference Lunani, Abaasa and Omosa-Manyonyi25,Reference Titilayo, Palamuleni and Olaoye-Oyesola26) . Educational status comprised two categories: ‘yes’ (ever attended school for education) or ‘no’ (never had any school-based education).
Statistical analysis
The descriptive results were presented as rates of consuming non-iodized salt among reproductive age women in each country. Bivariate analyses were conducted using Pearson’s χ 2 tests to investigate factors associated with the consumption of non-iodized v. iodized salt among women of reproductive age. Multivariable logistic regression with stepwise selection was used (entry/removal P value = 0·05) to determine independent factors associated with the consumption of non-iodized salt. Variance inflation factors were used to assess multicollinearity, leaving variables in the model only if they had a variance inflation factor of 3 or less. The multivariable logistic regression results were presented as adjusted odds ratios (aOR) with their 95 % confidence intervals and P values. The statistical tests were reported as significant if the level of significance was α ≤ 0·05 (two-sided). The total number for each variable may not sum to the total sample size of 108 318 due to missing values. Furthermore, the number of non-iodized salt users for some categories may not sum to 10 330 owing to missing values for some participants (Table 1). In Table 2 we report only the number of non-iodized salt users for each variable by country. The numbers are not meant to sum up. We used the total number for each country as the denominator when calculating the non-iodized salt rates. All statistical analyses were conducted using the statistical software package SAS version 9.4 and figures were generated using R version 3.4.3.
Table 2 Rates of non-iodized salt use by country, stratified according to respondents’ sociodemographic characteristics, among women of reproductive age (15–49 years; n 108 318) from eleven sub-Saharan African countries that participated in the Demographic and Health Survey programme since 2015 and measured the presence of iodine in household salt
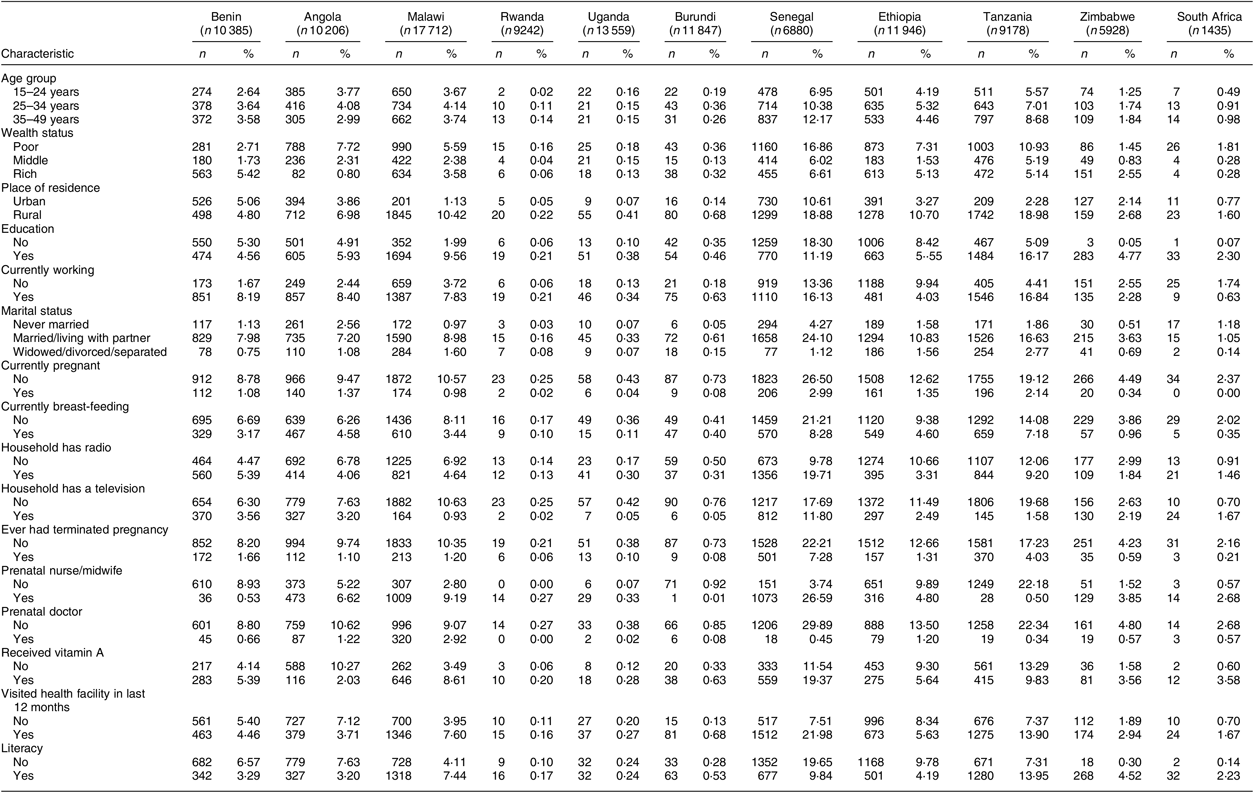
Table 2 reports only the number of non-iodized salt users for each variable by country. The numbers are not meant to sum up. The total number for each country was used as the denominator when calculating non-iodized salt rate.
Results
Country profile of non-iodized salt rates
Countries with the highest rate of non-iodized salt were Senegal (29·5 %) followed by Tanzania (21·3 %), Ethiopia (14·0 %), Malawi (11·6 %), Angola (10·8 %), Benin (9·9 %), Zimbabwe (4·8 %) and South Africa (2·4 %). The rate of non-iodized salt was less than 1 % in Rwanda (0·3 %), Uganda (0·5 %) and Burundi (0·8 %; Fig. 2). There was a statistically significant difference between countries on the presence of non-iodized salt in the household (P < 0·01).
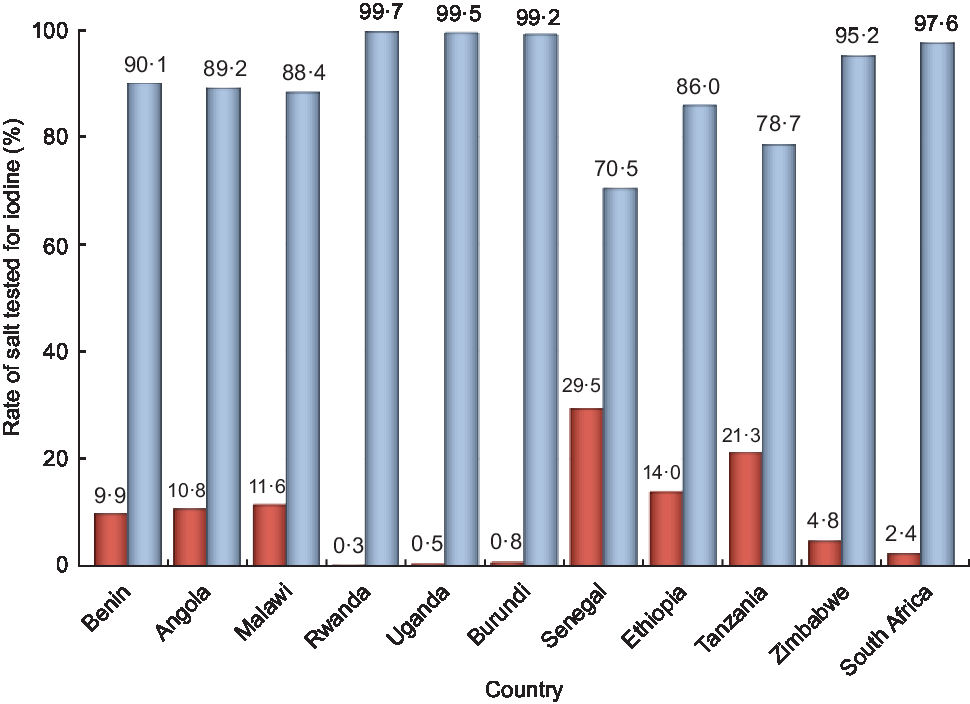
Fig. 2 Rates of salt testing positive (i.e. iodized; ![]() ) and negative (i.e. non-iodized;
) and negative (i.e. non-iodized; ![]() ) for iodine, by country, among eleven sub-Saharan African countries that participated in the Demographic and Health Survey programme since 2015 and measured the presence of iodine in household salt. Countries with the highest rate of non-iodized salt were Senegal (29·5 %) and Tanzania (21·3 %)
) for iodine, by country, among eleven sub-Saharan African countries that participated in the Demographic and Health Survey programme since 2015 and measured the presence of iodine in household salt. Countries with the highest rate of non-iodized salt were Senegal (29·5 %) and Tanzania (21·3 %)
Figure 3 shows how countries in the present study meet the WHO’s USI recommendation of at least 90 % of salt iodized.
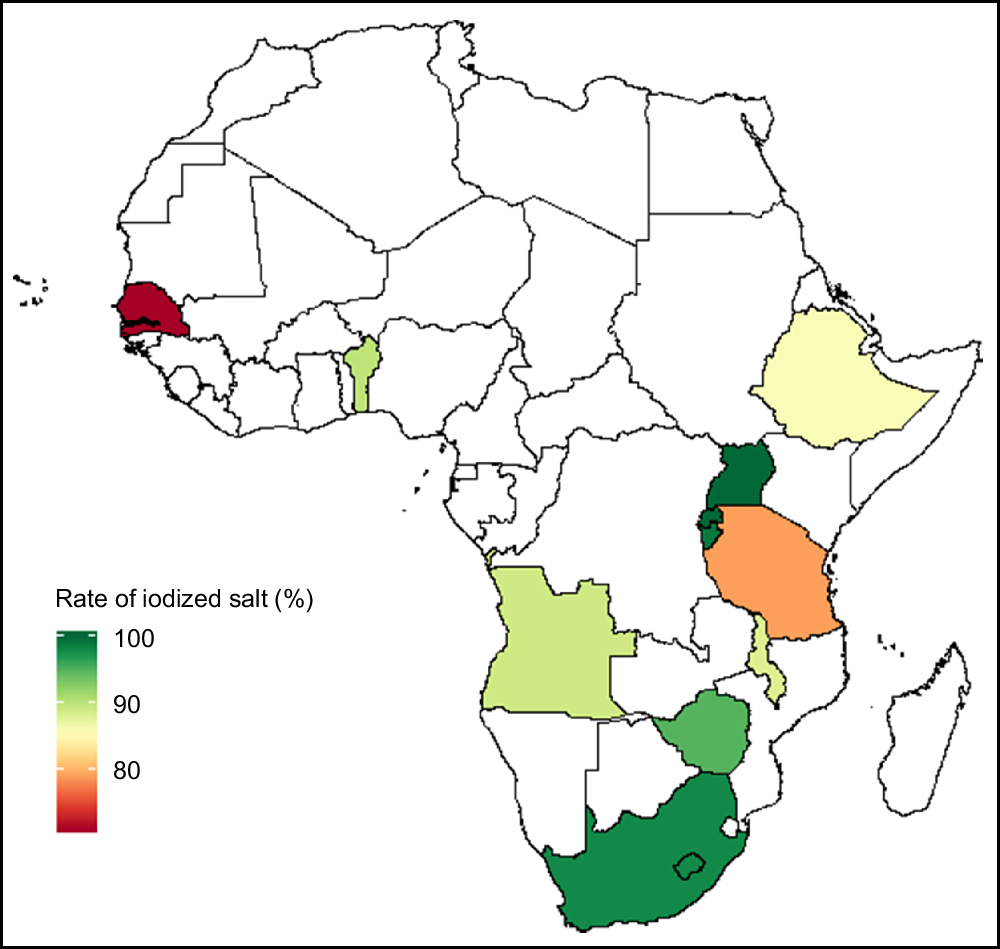
Fig. 3 Map of Africa showing the distribution of the rate of salt iodization among eleven sub-Saharan African countries that participated in the Demographic and Health Survey programme since 2015 and measured the presence of iodine in household salt. Benin, Rwanda, Uganda, Burundi, Zimbabwe and South Africa reached the goal of at least 90 % universal salt iodization
Sociodemographic factors associated with non-iodized salt consumption
The sociodemographic characteristics of the sample are presented in Table 1. Younger women aged 15–24 years had the highest rate of non-iodized salt consumption (9·9 %) compared with older women aged 35–49 years. Similarly, poor women were more likely to consume non-iodized salt (12·5 %) compared with women from the highest-wealth-index households. There was a significant association between content of iodine in salt and education status (P < 0·01). Women with no education were more likely to use non-iodized salt (13·2 %) compared with those who were educated (8·0 %). In the unadjusted analyses pregnant women had a slightly higher rate of consuming non-iodized salt (10·0 %) compared with non-pregnant (9·5 %), although this association was marginally significant (P = 0·07). The rate of non-iodized salt use was higher among women who did not have a radio in the household (10·4 %) compared with those who did have a radio (8·7 %). Furthermore, women with no prenatal care nurse or midwife were most likely to use non-iodized salt (11·1 %) compared with those having a prenatal nurse or midwife (8·4 %). In addition, statistically significant differences were observed regarding literacy status (P < 0·01) where illiterate women were most likely to use non-iodized salt (12·6 %) compared with those who were literate (7·5 %). Non-iodized salt rates also varied widely between countries in relation to different factors such as wealth index, pregnancy status, marital status, place of residence and literacy status (Table 2). Senegal and Tanzania had higher rates of non-iodized salt among poor women (16·9 and 10·9 %, respectively) compared with other countries. The rates of non-iodized salt consumption were consistently higher among women living in the rural area in all countries (except for Benin) with Tanzania and Senegal having the highest rate (19·0 %), followed by Ethiopia (10·7 %), Malawi (10·4 %), Angola (7·0 %), Zimbabwe (2·7 %), South Africa (1·6 %), Burundi (0·7 %), Uganda (0·4 %) and Rwanda (0·2 %). The rates of non-iodized salt consumption among pregnant women were slightly higher in Senegal (3·0 %), Tanzania (2·1 %), Angola and Ethiopia (1·4 %) compared with the remaining countries. The rates of non-iodized salt consumption in relation to marital status were significantly higher among women who were married or those living with partners in Senegal (24·1 %), Tanzania (16·6 %) and Ethiopia (10·8 %). Finally, the rates of non-iodized salt were higher among women who were illiterate in Senegal (19·7 %), Ethiopia (9·8 %) and Angola (7·6 %) compared with other countries.
Independent factors associated with consuming non-iodized salt among women
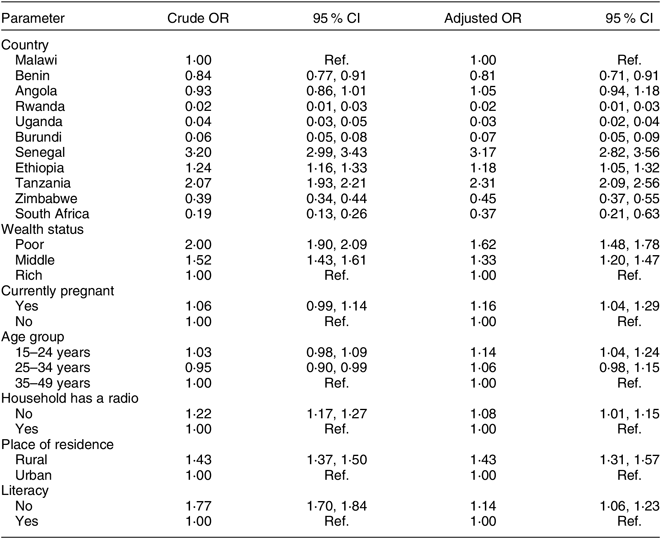
Table 3 and Fig. 4 show independent factors associated with consuming non-iodized salt. The country of residence was the factor most strongly associated with the consumption of non-iodized salt. Compared with Malawi, the odds of consuming non-iodized salt was significantly higher in Senegal (aOR = 3·17; 95 % CI 2·82, 3·56), Tanzania (aOR = 2·31; 95 % CI 2·09, 2·56) and Ethiopia (aOR =1·18; 95 % CI 1·05, 1·32). After controlling for country and other factors, poor women were 62 % more likely to use non-iodized salt (aOR = 1·62; 95 % CI 1·48, 1·78) compared with women in the highest socio-economic class. In addition, compared with non-pregnant women, pregnant women were 16 % more likely to be consuming non-iodized salt (aOR = 1·16; 95 % CI 1·04, 1·29). Furthermore, women living in rural areas were 43 % more likely to consume non-iodized salt (aOR = 1·43; 95 % CI 1·31, 1·57) compared with those living in urban areas. Illiterate women were 14 % more likely to use non-iodized salt (aOR = 1·14; 95 % CI 1·06, 1·23) compared with literate women. Lastly, compared with older women aged 35–49 years, younger women aged 15–24 years were 14 % more likely to use non-iodized salt (aOR = 1·14; 95 % CI 1·04, 1·24).
Table 3 Multivariable logistic regression showing independent predictors of non-iodized salt use among women of reproductive age (15–49 years; n 108 318) from eleven sub-Saharan African countries that participated in the Demographic and Health Survey programme since 2015 and measured the presence of iodine in household salt
Ref., reference category.
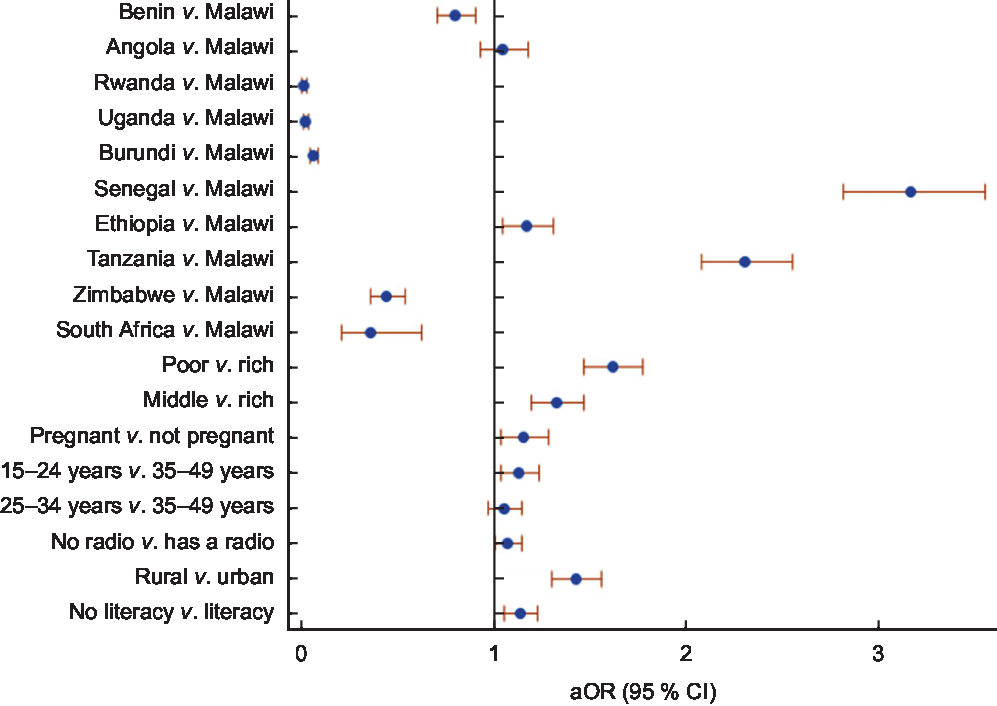
Fig. 4 Adjusted odds ratios (aOR), with 95 % confidence intervals represented by horizontal bars, for the independent factors associated with the use of non-iodized salt among women of reproductive age (15–49 years; n 108 318) from eleven sub-Saharan African countries that participated in the Demographic and Health Survey programme since 2015 and measured the presence of iodine in household salt
Discussion
Findings from our study show significant differences in the consumption of non-iodized salt across SSA countries. Our results indicated that non-iodized salt consumption in women varied significantly between and within countries. According to the WHO, an estimated 42 % of the population of Eastern Africa was iodine deficient in 2003(Reference de Benoist, Andersson and Egli27). Following the World Summit for Children in 1990, over 120 countries committed to eliminating IDD by implementing USI as an integral part of their national nutrition strategies(Reference Zimmermann, Jooste and Pandav1). However, almost three decades later, the consumption of iodized salt is still a major challenge in SSA, a region that carries a disproportionally higher burden of poverty and illiteracy. Our results show that of the eleven countries included in our study, five countries still had not achieved USI (≥90 % household coverage with adequately iodized salt) and only 70·5 % of women in their reproductive years in Senegal had iodized salt in their household, followed by Tanzania (78·7 %) and Ethiopia (86·0 %). Between-country differences remained large even after adjusting for other factors that may be associated with the use of non-iodized salt. In 2010 the national iodine survey in Senegal indicated that household coverage of iodized salt was only about 56 %(Reference Spohrer, Knowles and Jallier28). In addition, a previous study showed that 41 % of the population living in different geographic areas in Tanzania were subject to IDD(Reference Kavishe, Maletnlema, van de Haar and Kavishe29). In order to achieve USI, the Tanzania Salt Producers Association was formed in 1994, which provides technical support and monitoring of the USI(Reference Carlsson, Ismail and Jitta30). For instance, a study by Knowles et al. reported that the national household coverage of adequately iodized salt was 37·2 % in Senegal and 97·0 % in Uganda(Reference Knowles, Garrett and Gorstein20). These findings are consistent with our findings which showed that Senegal has the lowest rate of iodized salt compared with other countries in the present study. The evidence from our study indicates that gains have been made across different countries regarding the rate of iodized salt, from a rate of 97·0 % of iodized salt in Uganda to 99·5 %, from 67·9 to 78·7 % in Tanzania, and from 37·2 to 70·5 % in Senegal(Reference Knowles, Garrett and Gorstein20). Furthermore, the higher rate of non-iodized consumption among women with low socio-economic status and those living in the rural areas in Tanzania and Senegal from the present study agrees with the previous findings(Reference Knowles, Garrett and Gorstein20,Reference Assey, Peterson and Kimboka31) .
Other factors associated with non-iodized salt consumption in women
Independent factors associated with a higher rate of non-iodized salt consumption from the multivariable-adjusted analysis were pregnancy, poverty, younger age, not having a radio, living in a rural area and lack of literacy. These observations are in agreement with previous reports which indicated that households in the richest wealth quintile are more likely to access iodized salt than those in the poorest wealth quintile(Reference Knowles, Garrett and Gorstein20). This could be explained by the higher cost of purchasing iodized salt compared with non-iodized salt(Reference Sen, Das and Biswas32–Reference Agarwal, Sethi and Sharma34). However, it should be noted that between-country wealth index differences regarding non-iodized salt consumption are mostly driven by two countries (Senegal and Tanzania), compared with other countries where wealth index plays a very limited role. In addition, lack of consumption of iodized salt among women who were not literate could be due to lack of awareness or misinformation about the benefit of iodized salt for prevention of IDD(Reference Sen, Das and Biswas32). Our findings showed that pregnant women had higher odds of consuming non-iodized salt, rendering them susceptible to poor birth outcomes associated with iodine deficiency. These include increased risk of spontaneous abortion, stillbirth and low birth weight. Furthermore, iodine deficiency is a risk factor for impaired fetal neurodevelopment which possesses an increased risk of infant mortality(Reference Zimmermann, Jooste and Pandav1,Reference Zimmermann14,Reference Glinoer35–Reference Yarrington and Pearce37) . It is well established that iodine deficiency during the second trimester to the second year of life is associated with devastating consequences such as permanent damage to the brain(Reference Ahad and Ganie2). In addition, children from 2 months to 15 years old born in regions with IDD have the highest risk of having low intelligence quotient scores(Reference Qian, Wang and Watkins38,Reference Bath and Rayman39) . IDD in pregnant and lactating women can be avoided by consuming iodized salt. One teaspoon of iodized salt contains approximately 400 μg iodine, which fulfils the RDA for iodine of 150 μg/d (for adult men and women) and 250 μg/d (for pregnant and lactating women)(Reference Li and Eastman40). Therefore, at a population level, the easiest and most cost-effective strategy to prevent IDD is to vigilantly monitor USI(Reference Zimmermann14).
Policy implications
There are two potential options available at the policy level for achieving the WHO recommendation of at least 90 % USI, including salt industry consolidation and support for small-scale producers. Each of the options has pros and cons.
First, the pros of salt industry consolidation include: improved internal and external monitoring; improved quality control of laboratories; and effective enforcement such as strengthening surveillance at points of entry in salt-importing countries. The option of consolidation has been used successfully in Africa. For example, in Uganda, Rwanda and Burundi, the low prevalence of non-iodized salt is in part due to the higher level of industrial consolidation and mechanization of the salt supply in addition to the mandatory USI(Reference Knowles, Garrett and Gorstein20). On the other hand, countries such as Ethiopia, Tanzania and Senegal that have extensive small-scale salt producers also had high prevalence of non-iodized salt. Therefore, poor performers could learn from countries such as Uganda by consolidating their salt supply. However, the downside of consolidation is putting small-scale salt producers out of business, and yet these producers create employment for more than thousands of workers in isolated areas(Reference Assey, Tylleskär and Momburi41).
Second, the support of small-scale producers, although an option, is more likely not to be an effective strategy to eliminate the circulation of non-iodized salt on the market. Small-scale producers are less likely to adhere to government regulations(42). As a result, unmonitored small-scale businesses will still produce salt of which the majority will not be iodized due to poor technical capacity and lack of quality assurance(Reference Kupka, Ndiaye and Sall43,Reference Assey, Peterson and Greiner44) . Support of small-scale producers is critical for achieving USI(Reference Assey, Tylleskär and Momburi41,Reference Haxton and Mannar45) . Nevertheless, the major pro of supporting small-scale salt producers is creating employment for citizens in isolated regions. Although small-scale salt producers have limited financial means to stay in business, by collaborating with the government, they can develop a business plan, financing mechanism and follow-up mechanisms to ensure sustainable programme implementation(Reference Assey, Tylleskär and Momburi41). There are technological advances available in salt iodization that are applicable at small-scale salt producers. Some of the options available for policy makers to support small-scale salt producers include: procuring and installing salt iodization machines to replace the current method of hand-and-knapsack sprayers that produce highly variable iodine content(Reference Assey, Tylleskär and Momburi41); replacing the widely used qualitative method using rapid test kits by the quantitative titration method for determining salt iodate content; and subsidizing the cost of potassium iodate. The major disadvantage of supporting small-scale salt producers is the need for continuous financial support from the government and international partners, which may not be sustainable.
Furthermore, countries like Rwanda, with zero tolerance for corruption, may be more likely to enforce the importation of adequately iodized salt. However, some SSA countries, particularly those from West Africa, have failed to enforce these policies in part due to corruption, where cheaper inadequately iodized salt crosses over the border to enter the general market. Finally, South Africa has the strongest economy among the SSA countries(Reference Naudé and Fosu46) and therefore its citizens are able to afford the more expensive adequately iodized salt. Continuous monitoring for quality assurance, control and enforcement of the addition of iodine to salt among small-scale salt producers and salt industry consolidation could be an effective approach to achieve USI and eliminate IDD in low-performing countries.
Study strengths and limitations
The strength of our study is the analysis using a nationally representative sample with a large number of women aged 15–49 years from each of eleven countries. As far as we are aware, this was the biggest sample ever analysed using DHS data of salt testing for iodine. However, the study has several limitations. First, it is a cross-sectional study. The cross-sectional nature of the survey does not allow for the determination of a temporal or causal relationship between the explored variables and IDD among women of reproductive age. The household salt was tested for iodine (non-iodized or iodized) using a rapid test kit, which measured the presence of iodine in salt but could not quantify the amount of iodine. The rapid test kit is easy and cost-effective; however, it cannot be used for estimating the salt iodine concentration or whether the salt is adequately iodized or not. Lastly, the gold standard test for determining IDD – urinary iodine concentration – was not done by DHS for each country, hence these data are not available for the present study. Notwithstanding these limitations, our study provides important information concerning the use of non-iodized salt in SSA and associated factors among women of reproductive age.
Conclusion
In the present study, we found that poverty and lack of literacy were significantly associated with using non-iodized salt among women of reproductive age in SSA. In addition, the positive association between non-iodized salt and pregnancy calls for action to implement and enforce USI so as to prevent IDD among women of reproductive age, as well as potentially devastating outcomes in their offspring. Awareness campaigns about the benefits of iodized salt need to be implemented, particularly for the most vulnerable and economically disadvantaged members of the population. Lastly, countries not performing well could learn practical solutions of legislation and industry consolidation from those that have attained the USI recommendation.
Acknowledgements
Acknowledgements: The authors thank the DHS program for its support and for free access to the original data (available at https://dhsprogram.com/data/available-datasets.cfm). Financial support: This research received no specific grant from any funding agency in the public, commercial or not-for-profit sector. Conflict of interest: None. Authorship: P.S., D.L., P.D., K.H.K. and D.B. participated in the conception and design of the study, data cleaning, analysis and interpretation of results, and drafting and revision of the manuscript. All authors participated in the review of statistical methods, results interpretation and revision of the manuscript. All authors read and approved the final manuscript. Ethics of human subject participation: Procedures and questionnaires for standard DHS surveys have been reviewed and approved by the Institutional Review Board (IRB) of ICF International and the IRB of the host countries. The privacy of the study participants was protected. Written and oral informed consent was obtained from the study participants before the beginning of each survey question and biomarker tests. Individuals in the host countries were not coerced into participation. All data are protected by the Health Insurance Portability and Accountability Act (HIPAA) and de-identified. All ethical issues were handled by the IRB of ICF International and the IRB of the host countries who conducted the primary surveys, not the current authors of this manuscript.









