Management Implications
Gibasis pellucida (Tahitian bridal veil) is an emerging invasive plant species of concern in Florida and Texas, but there are no previous reports of effective herbicide management options. Nine herbicides and combinations were evaluated for postemergence control of G. pellucida grown in pots in a shaded greenhouse. Results showed that glufosinate and triclopyr (acid and amine formulations evaluated separately) provided 100% control, followed by triclopyr + 2,4-D with 70% control and fluroxypyr with 50% control, as evidenced by visual control ratings and dry shoot weight. Aminopyralid, 2,4-D, and metsulfuron-methyl provided low or no control in comparison with a nontreated control group. While further work is needed to evaluate these and other herbicides under field conditions, data reported here are noteworthy, as this is the first report on herbicide selection for G. pellucida control. Results also suggest that based on previous reports, the same herbicides that have shown high efficacy for Tradescantia fluminensis (small leaf spiderwort) would also be effective for G. pellucida management.
Introduction
Tahitian bridal veil [Gibasis pellucida (M. Martens & Galeotti) D.R. Hunt; Commelinaceae] is native to Mexico and parts of Central and South America. Originally introduced to the United States as an ornamental plant (Fantz and Nelson Reference Fantz and Nelson1995), it soon naturalized and has become invasive, specifically in Texas (Rosen and Faden Reference Rosen and Faden2005) and Florida, where it has been vouchered in 12 counties across the state (Wunderlin et al. Reference Wunderlin, Hansen, Franck and Essig2022). While not classified as a category 1 or 2 invasive by the Florida Invasive Species Council (FISC), it is a species of increasing concern with the Florida Fish and Wildlife Conservation Commission (S Yuan, personal communication) and other land management groups (FISC 2019) and has shown the propensity for becoming highly invasive in previous examinations of the Commelinaceae family (Moriuchi Reference Moriuchi2006).
Gibasis pellucida spreads primarily vegetatively by rooting at the nodes along decumbently growing stems. Seeds have been found on plants, but previous studies have noted that it is usually self-incompatible (Hunt Reference Hunt1986). Leaves are asymmetrical, 1- to 5-cm long and up to 3-cm wide and typically dark green in color on the surface to reddish-purple underneath, and stems are 1 mm in diameter (Fantz and Nelson Reference Fantz and Nelson1995). Observations of spread and invasive potential in Florida are similar to those reported in Texas, where G. pellucida establishes (escapes from cultivation) and spreads rapidly in riparian or disturbed areas under broken to closed-canopy forests (Rosen and Faden Reference Rosen and Faden2005). Although G. pellucida has not been documented altering native plant communities on a large scale, there is reason for concern due to its similarity in biology and growth with another invasive Commelinaceae member, small leaf spiderwort (Tradescantia fluminensis Vell.) (Figure 1). Tradescantia fluminensis is currently classified as a Category I invasive by the FISC based on documented ecological damage and displacement of native species (FISC 2019). While G. pellucida is not officially classified as invasive, in studies of invasive Commelinaceae genera potential, it was estimated to have a higher rate of spread compared with T. fluminensis (Moriuchi Reference Moriuchi2006). Superficially, both plants are very similar in appearance and growth habit, especially when not in flower, possibly leading to misidentification in field. While detailed differences between the two species have been summarized previously (Fantz and Nelson Reference Fantz and Nelson1995; Seitz and Clark Reference Seitz and Clark2016), the most easily observable difference in the field are stipitate cymes with long peduncles (12- to 17-mm long) and pedicels (7- to 14-mm long) of G. pellucida (Fantz and Nelson 2005) compared with sessile cymes of T. fluminensis (Fantz and Nelson Reference Fantz and Nelson1995; Seitz and Clark Reference Seitz and Clark2016).

Figure 1. The flower (left) and whole plant (middle) of Gibasis pellucida compared with Tradescantia fluminensis (right).
Due to the widespread impacts of T. fluminensis in New Zealand, Brazil, Florida, and other subtropical regions, many studies have evaluated management options for it, including herbicide and cultural options such as shading and manual removal. Overall, herbicides have been reported to be the most effective and only feasible option on a large scale (Standish Reference Standish2002), with triclopyr reported as the most consistently effective herbicide for T. fluminensis. Triclopyr ester, amine, and choline have all been found to consistently provide 90% control or greater in multiple experiments (Brown and Brown Reference Brown and Brown2015; Hurrell et al. Reference Hurrell, James, Lusk and Trolove2008, Reference Hurrell, James, Lamoureaux, Lusk and Trolove2009; Lusk et al. Reference Lusk, Hurrell and Lamoureaux2012; Marble and Chandler Reference Marble and Chandler2021) when applied at labeled rates. Glyphosate has also been evaluated extensively in multiple studies, with results ranging from ∼30% to 60% control depending on rate and location (Brown and Brown Reference Brown and Brown2015; Marble and Chandler Reference Marble and Chandler2021), but multiple applications have typically been needed to provide optimal control (McCluggage Reference McCluggage1998). Glufosinate and fluroxypyr have been successful in limited testing (Marble and Chandler Reference Marble and Chandler2021), while metsulfuron-methyl, sulfentrazone, clopyralid, aminopyralid, and 2,4-D have generally been found to be ineffective on mature populations (Kelly and Skipworth Reference Kelly and Skipworth1984; Marble and Chandler Reference Marble and Chandler2021; McCluggage Reference McCluggage1998).
While there are numerous reports of herbicide efficacy on T. fluminensis, there are no documented herbicide efficacy data for G. pellucida. As these species can be easily misidentified due to morphological similarities and tend to invade similar habitats, management recommendations are now needed to help prevent further spread and ecological impact. Therefore, the objective of this research was to evaluate efficacy of postemergence herbicides for control of G. pellucida in small-scale pot studies using herbicides previously evaluated for T. fluminensis. The overall objective of this work was to identify herbicide active ingredients that show promise as control options to support future field testing in areas where this species is becoming increasingly more common.
Materials and Methods
Studies were conducted in a shade house (60% reduction of ambient light) located at the Mid-Florida Research and Education Center, University of Florida, in Apopka, FL, USA. Gibasis pellucida cuttings were collected from a local park (Big Tree Park, Longwood, FL, USA, 28.7214°N, 81.3306°W) and rooted into nursery pots (top diameter: 16.4 cm, bottom diameter: 12.5 cm; depth: 17.5 cm; volume: 2.84 L) filled with a soilless substrate composed of pine bark (Southeast Soils, Okahumpka, FL, USA; pine bark:Florida peat:sand = 9:1:1 v:v:v) with four terminal stems per pot on October 1, 2021. At 2 wk after sticking, each container was top-dressed with 11 g of control release fertilizer [17 N-2.2 P-9.1 K, Osmocote® Blend 17-5-11, 8 to 9 month; ICL Specialty Fertilizers, Dublin, OH, USA), which represented the manufacturer’s recommended low rate.
On December 6, 2021 (clear skies, 23 C, 76% relative humidity, calm winds), all plants were removed from the shade house and placed onto a gravel area outdoors, where selected herbicides (Table 1) were applied using a CO2 backpack sprayer calibrated to deliver 234 L ha−1 using a TeeJet® 8004 flat-fan nozzle (TeeJet Technologies, Wheaton, IL, USA) at 241 kPa. A non-ionic surfactant (AirCover, Winfield Solutions, St Paul, MN, USA) was added at a 0.5% v:v rate to treatments including 2,4-D, triclopyr (acid and amine), metsulfuron-methyl, and fluroxypyr based on manufacturers’ recommendations. After 24 h of herbicide treatment, plants were moved back inside the shade house, where they remained for the duration of the experiment. At the time of treatment, each pot contained four individual fully rooted plants that were approximately 40- to 50-cm in length. Plants were irrigated 1.3 cm daily with overhead irrigation (Xcel-Wobbler™, Senninger Irrigation, Clermont, FL, USA) via two irrigation cycles (7:00 AM and 2:45 PM) throughout the experiment. The study was repeated following the same methodology and timeline on December 15, 2021 (overcast, 24 C, 78% relative humidity, calm winds).
Table 1. Herbicides evaluated for postemergence control of Gibasis pellucida.
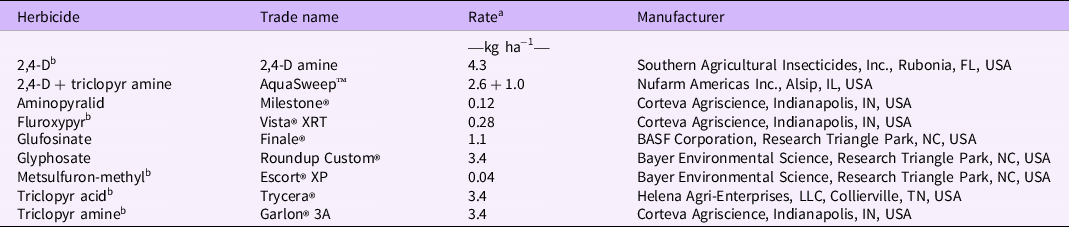
a Rates are given in kg ae ha−1, with the exception of aminopyralid, metsulfuron-methyl, and glufosinate, which are presented in kg ai ha−1.
b Herbicides were applied with the addition of a non-ionic surfactant (AirCover, Winfield Solutions, St Paul, MN, USA) at a 0.5% v/v rate based on the manufacturers’ recommendations.
Table 2. Mean visual control rating and shoot biomass of Gibasis pellucida following application of selected postemergence herbicides.
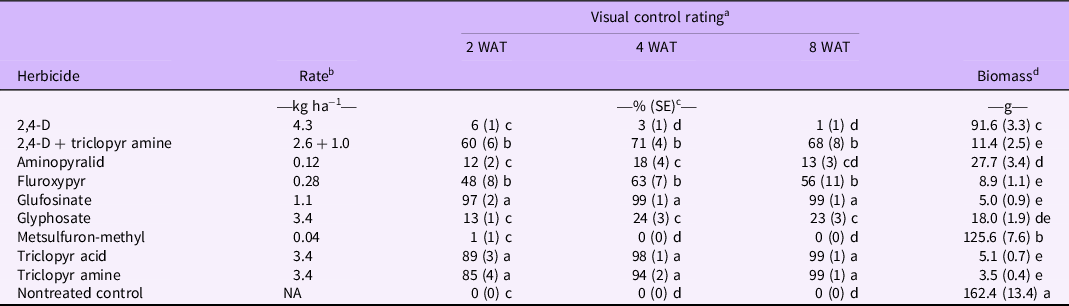
a Control was estimated visually using a 0 to 100 scale, where 0 = healthy plant and 100 = dead plant or no visible green tissue. WAT, weeks after treatments were applied.
b Rates are given in kg ae ha−1, with the exception of aminopyralid, metsulfuron-methyl, and glufosinate, which are presented in kg ai ha−1. Triclopyr acid, triclopyr amine, 2,4-D, metsulfuron-methyl, and fluroxypyr were applied with the addition of a non-ionic surfactant (AirCover, Winfield Solutions, St Paul, MN, USA) at a 0.5% v/v rate based on the manufacturers’ recommendations.
c SEs are reported parenthetically (n = 16). Means within a column followed by the same letter are not significantly different according to the Holm-Bonferroni method (P = 0.05).
d Shoot biomass dry weight was assessed by clipping plants at the soil line and drying shoots until reaching constant weight. Shoots were harvest at 8 WAT (n = 16).
At 2 wk after herbicide treatment (2 WAT), plants were rated visually on a 0 to 100 rating scale (Figure 2), with 0 indicating no control (no damage) and 100 representing complete control (100% damage). Subsequent visual ratings were taken at 4 WAT and 8 WAT. At the conclusion of the experiment at 8 WAT, plant shoots were clipped at the soil line, and shoot dry weight was determined after placing shoots in a forced-air oven at 60 C for 7 d. The experiment was arranged as a randomized complete block design with eight replications for each treatment. The non–herbicide treated plants served as the control group.

Figure 2. Example for visual control rating scale of Gibasis pellucida used in this study (0 to 100), where 0 indicates no control and condition similar to the nontreated check, and 100 indicates complete control and no living/green tissue observed.
Statistical Analysis
The data from the two experimental runs were combined, as there were no experimental run by treatment interactions. The significance of treatment effects was determined by ANOVA using R program software v. 3.5.1 (R Core Team 2018). Post hoc multiple comparisons were made using the Holm-Bonferroni method to adjust P-values for multiple comparisons. In all cases differences were considered significant at P ≤ 0.05.
Results and Discussion
Glufosinate, triclopyr acid, and triclopyr amine provided the highest level of control over the course of the experiment (Table 2). All three herbicides provided increased visual control values on subsequent evaluations, reaching 100% by 8 WAT. A high control rating for glufosinate was not surprising, as it tends to result in more rapid symptomology compared with triclopyr (Shaner Reference Shaner2014). In contrast, 2,4-D and metsulfuron-methyl had rating values similar to those of the nontreated control, which remained very low (ranging from 0 to 10) throughout the trial. Aminopyralid and glyphosate provided some level of control, with ratings ranging from 12% to 24%. Conversely, fluroxypyr and triclopyr + 2,4-D produced a marginal effect on G. pellucida, resulting in rating values ranging from 48% to 71%. Moreover, visually estimated control resulting from fluroxypyr and triclopyr + 2,4-D increased from 2 WAT to 4 WAT but decreased by 8 WAT, indicating recovery in these treatments.
When biomass was evaluated, 2,4-D (91.64 g) and metsulfuron-methyl (125.63 g) resulted in significantly greater biomass than other herbicide treatments, but still provided a minor reduction in biomass in comparison with the nontreated control group. No statistically significant differences were shown between the other treatments, with the exception of plants treated with aminopyralid (27.66 g) having greater biomass than those treated with triclopyr amine (3.53 g), glufosinate (4.96 g), and triclopyr acid (5.06 g), fluroxypyr (8.93 g), and 2,4-D + triclopyr (11.38 g).
Results from visual ratings and biomass measures indicated that triclopyr (both amine and acid formulations) and glufosinate would likely be effective options for G. pellucida control, as 100% control was achieved in these shade-house evaluations. However, research under field conditions would be needed for confirmation (Riemens et al. Reference Riemens, Dueck and Kempenaar2008). Fluroxypyr, glyphosate, and 2,4-D + triclopyr provided only marginal visual control, but based on biomass data could potentially be options at higher doses, when used as a sequential application to another herbicide, or when infestations are not severe. As G. pellucida has a spreading growth habit, it is likely that, similar to T. fluminensis, multiple applications will be needed, as complete control is often not achieved (Marble and Chandler Reference Marble and Chandler2021). Thus, having different herbicide options available, even if efficacy is not ideal, would be important to prevent exceeding maximum annual doses. No dose–response curves have been established for G. pellucida, as this is the first report of efficacy to our knowledge. Based on our results with the two triclopyr treatments alone (3.4 kg ae ha−1) compared with the lower control observed with the 2,4-D + triclopyr treatment (1 kg ae ha−1 triclopyr), it is evident that a triclopyr rate of greater than 1 kg ae ha−1 would likely be needed for effective control. Metsulfuron-methyl and 2,4-D provided poor control, and based on this study, use at the rates evaluated would not be recommended.
When comparing the herbicides used in this study and herbicides previously evaluated for T. fluminensis control, there are both similarities and differences. Like T. fluminensis, triclopyr presented as the most effective herbicide to control G. pellucida. Marble and Chandler (Reference Marble and Chandler2021) reported a >80% reduction in T. fluminensis biomass when treated with triclopyr (amine, ester, or choline) at rates as low as 1.7 kg ae ha−1; thus, further testing is needed to determine the optimal rate for G. pellucida. Also, 2,4-D and metsulfuron-methyl performed poorly in controlling both T. fluminensis and G. pellucida. Both Hurrell et al. (Reference Hurrell, James, Lusk and Trolove2008) and Marble and Chandler (Reference Marble and Chandler2021) showed satisfactory control of T. fluminensis with fluroxypyr, and this is in agreement with results for G. pellucida in our study. Glufosinate also provided effective control of both species, at least in greenhouse evaluations (Marble and Chandler Reference Marble and Chandler2021). Glyphosate, on the other hand, showed some marginal effects on G. pellucida, and mixed results have been reported for T. fluminensis. McCluggage (Reference McCluggage1998) and Brown and Brown (Reference Brown and Brown2015) showed high levels of T. fluminensis control with glyphosate, but studies conducted by Kelly and Skipworth (Reference Kelly and Skipworth1984) and Marble and Chandler (Reference Marble and Chandler2021) showed only marginal control and the need for multiple applications.
Overall, data presented here indicate that triclopyr (amine or acid) and glufosinate have a high degree of efficacy on G. pellucida, at least under greenhouse-like conditions. Based on this work, the same general treatment approach that is recommended for T. fluminensis will potentially be effective for G. pellucida, but further research is needed to evaluate both these herbicides for G. pellucida control under field conditions on mature, densely growing populations. Work is also needed to evaluate the efficacy of selected herbicides on T. fluminensis and G. pellucida in a side-by-side comparison, as results have been variable with some herbicides based on time of year, environment, and growth stage.
Acknowledgments
The authors wish to thank Samantha Yuan and the Florida Fish and Wildlife Conservation Commission for supporting and sponsoring this research and bringing our attention to this emerging invasive plant species of concern in Florida. No conflicts of interest have been declared.







