Gangliosides, amphiphilic glycosphingolipids containing sialic acid (N-acetyl neuraminic acid; NANA), are found in plasma membranes of mammalian cells and are biologically important molecules involved in cell differentiation, proliferation, growth, adhesion, migration, signalling and apoptosis(Reference Hakomori1–Reference Merrill, Schmelz, Dillehay, Spiegel, Shayman, Schroeder, Riley, Voss and Wang3). The ganglioside composition of the brush-border membrane of the developing intestine influences patterns of bacterial colonisation and susceptibility to pathogen attachment and invasion(Reference Mukai, Kaneko, Matsumoto and Ohori4, Reference Fantini, Maresca, Hammache, Yahi and Delezay5). Sialylated compounds have growth-promoting effects on bifidobacteria and lactobacilli, but further research is warranted to determine whether a specific ganglioside mediates the proliferative effect(Reference Nakano, Sugawara and Kawakami6).
In normal physiological processes such as embryogenesis and lactation(Reference Yamashita, Wada, Sasaki, Deng, Bierfreund, Sandhoff and Proia7) and in pathological conditions including tumour onset and progression(Reference Hakomori8), changes in ganglioside composition occur and have been shown to play significant regulatory roles. For example, melanoma cells, embryonic stem cells and human colostrum show an increase in ganglioside content and express more (N-acetylneuraminyl) 2-galactosylglucosyl ceramide (GD3) than normal adult cells and mature human milk(Reference Draper, Pigott, Thomson and Andrews9, Reference Ravindranath, Tsuchida, Morton and Irie10). Moreover, the N-acetylneuraminylgalactosylglucosylceramide (GM3):GD3 ratio increases during development and lactation and decreases during cancer cell differentiation from highly metastatic (poorly differentiated) cancer cells to benign (highly differentiated) cells(Reference Draper, Pigott, Thomson and Andrews9, Reference Ravindranath, Tsuchida, Morton and Irie10).
Ganglioside GM3 is an enterocyte receptor analogue for specific microbes(Reference Kyogashima, Ginsburg and Krivan11, Reference Rolsma, Kuhlenschmidt, Gelberg and Kuhlenschmidt12) and promotes cell proliferation, migration, tumorigenesis and cancer cell resistance to anti-cancer drug therapy(Reference Noguchi, Kabayama, Uemura, Kang, Saito, Igarashi and Inokuchi13–Reference Wang, Huang, Wu, Chen, Jin, Huang and Wang15). Depending on the concentration, ganglioside GD3 exhibits a diversity of effects, such as inhibiting cell growth, inducing apoptosis, enhancing radiation and anti-cancer drug therapy efficacy and exerting anti-inflammatory effects(Reference Malisan and Testi16). Change in ganglioside composition relies on the balance between activities of enzymes in ganglioside biosynthetic and degradative pathways(Reference Draper, Pigott, Thomson and Andrews9, Reference Kakugawa, Wada, Yamaguchi, Yamanami, Ouchi, Sato and Miyagi17, Reference Takamizawa, Iwamori, Mutai and Nagai18). A simplified diagram of ganglioside biosynthesis and degradation is illustrated in Fig. 1.
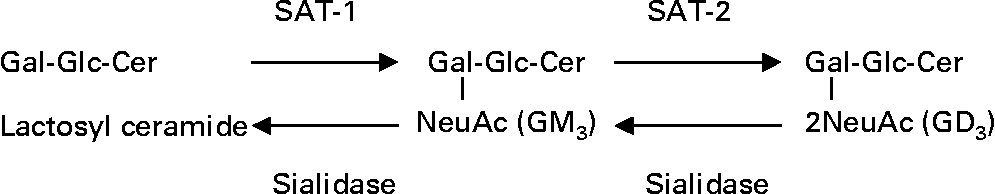
Fig. 1 Simplified schematic of ganglioside biosynthesis and degradation. SAT-1, sialyltransferase 1 or N-acetylneuraminylgalactosylglucosylceramide (GM3) synthase; SAT-2, sialyltransferase 2 or (N-acetylneuraminyl) 2-galactosylglucosyl ceramide (GD3) synthase; Gal, galactose; Glc, glucose; Cer, ceramide; NeuAc, N-acetyl neuraminic acid or sialic acid.
Sialyltransferase (SAT)-1 drives lactosylceramide towards GM3 synthesis while SAT-2 catalyses the biosynthesis of GD3 (Fig. 1). GM3 and GD3 are desialylated by a group of enzymes called the sialidases which are classified based on their location in the cell (1 = lysosome, 2 = cytosol, 3 = plasma membrane and 4 = mitochondria)(Reference Yamanami, Shiozaki, Wada, Yamaguchi, Uemura, Kakugawa, Hujiya and Miyagi19). In a recent study, the expression of SAT-1 and SAT-2 mRNA was found to be lower in tumour tissues from patients with colorectal cancer than in corresponding healthy tissues(Reference Gornati, Chini, Rimoldi, Meregalli, Schiaffino and Bernardini20). In contrast, the expression of human plasma membrane sialidase, an enzyme involved in removing terminal sialic acid from gangliosides, was found to be up-regulated in colon cancer tissue and fetal colon(Reference Kakugawa, Wada, Yamaguchi, Yamanami, Ouchi, Sato and Miyagi17). Furthermore, differentiation of colon cancer cell lines (metastatic to poorly metastatic phenotype) and susceptibility to apoptosis were found to be associated with a decrease in human plasma membrane sialidase expression and activity(Reference Kakugawa, Wada, Yamaguchi, Yamanami, Ouchi, Sato and Miyagi17). Thus, it would be expected that a differentiating colon cancer cell would have low amounts of mitotic GM3 and high amounts of apoptotic GD3 and complex gangliosides, a ganglioside profile similar to that of human colostrum and neonatal rat intestine(Reference Takamizawa, Iwamori, Mutai and Nagai18, Reference Bouhours, Bouhours and Hansson21, Reference Rueda, Maldonado, Narbona and Gil22). Despite evidence for similar changes in expression of enzymes involved in ganglioside synthesis and degradation during development, lactation and oncogenic transformation, it has not been investigated whether ganglioside compositional changes in differentiating colon cancer cells reflect changes that occur during intestine development.
Caco-2 cells are a human colon cancer cell line isolated from a 72-year-old Caucasian male presenting with an adenocarcinoma of the colon(Reference Fogh, Fogh and Orfeo23). In a study of twenty human colon tumour cell lines, Caco-2 alone showed the ability to undergo spontaneous differentiation to develop a number of characteristics more commonly associated with small-intestinal enterocytes(Reference Chantret, Barbat, Dussaulx, Brattain and Zweibaum24). The development of the enterocyte-like phenotype is only evident when the cells reach confluence. During the 7–20 d post-confluence differentiation time the cell monolayer gradually develops brush-border microvilli, tight junctions, cell polarity and expression of typical small-intestinal microvillus hydrolases and nutrient transporters(Reference Chantret, Rodolosse, Barbat, Dussaulx, Brot-Laroche, Zweibaum and Rousset25–Reference Peterson, Bement and Mooseker27). Moreover, bifidobacteria and lactobacilli species are able to adhere to Caco-2 cells and competitively exclude enterotoxigenic Escherichia coli and Salmonella typhimurium, indicating that Caco-2 cells are a beneficial cell line for studying bacterial colonisation of the gut(Reference Bernet, Brassart, Neeser and Servin28, Reference Duffy29). Caco-2 cells exhibit differences in polarity and expression of proteins depending on the time frame for differentiation, suggesting the potential use of Caco-2 cells as a model for different stages of intestinal development. Obtaining sufficient amounts of infant intestine is ethically difficult and isolated primary cultures of enterocytes have a limited survival time in culture, necessitating an alternative model for neonatal intestine.
The present study was designed to determine whether differentiating Caco-2 cells acquire a ganglioside composition profile similar to human colostrum and neonatal rat intestine, thereby resulting in a decrease in the GM3:GD3 ratio. The ganglioside composition of undifferentiated and differentiated Caco-2 cells has not been assessed. Considering that the Caco-2 cell line is the most widely used cell line for studying physiological and pathophysiological processes in the small intestine and colon, understanding the change in ganglioside composition during differentiation is important to assess the potential use of differentiating Caco-2 cells as a model for studying intestine development and paediatric intestinal disorders.
Experimental methods and materials
Cell culture
Human colon cancer Caco-2 cells (passage 44–54) were obtained from the American Type Culture Collection (ATCC, Rockville, MD, USA) and cultured in Earle's minimum essential medium (EMEM) containing 10 % (v/v) fetal bovine serum, 1 % (v/v) antibiotic/antimycotic, 26 mm-sodium bicarbonate, 10 mm-HEPES and 1 mm-pyruvic acid. Cells were grown as adherent monolayers in 75 cm2 T-flasks under standard incubator conditions (humidified atmosphere, 5 % CO2, 37°C) with medium replaced every 2–3 d. Monolayers were subcultured on reaching 80–90 % confluence at a split ratio of 1:3 (one T75 flask and two T150 flasks) using 0·25 % trypsin–0·03 % EDTA.
For each ganglioside composition experiment, sixteen confluent T150 flasks of undifferentiated cells and four 20 d post-confluent T150 flasks of differentiated cells were collected in cold 2-amino-2-hydroxymethyl-propane-1,3-diol (Tris) buffer–EDTA wash using a cell scraper and were pooled together into an undifferentiated group and a differentiated group of Caco-2 cells. Cell suspensions were centrifuged for 10 min at 1000 rpm and the resulting cell pellet was lysed in 0·5 ml of 2 mm-Tris HCl–40 mm-mannitol lysis buffer and sonicated for 30 s on ice. Cell homogenates were centrifuged for 10 min at 12 000 rpm and cell supernatant fractions were saved for protein and ganglioside analysis.
Determination of cell protein
The amount of protein in cell supernatant fractions was determined using the bicinchoninic acid assay. Cell supernatant fractions were diluted 1 in 5 with double-distilled water. Bovine serum albumin standards and diluted cell supernatant fractions (10 μl) were each mixed with 190 μl of a 50:1 mixture of bicinchoninic acid solution and 4 % (w/v) CuSO4·5H2O for 30 min at 37°C. The absorbance at 562 nm was measured with a microplate reader (Molecular Devices Corp., Sunnyvale, CA, USA).
Assessment of cell differentiation markers
Caco-2 cells were seeded on inserts in twelve-well transwell plates at a density of 400 000 cells per well in 0·5 ml EMEM-10. The bottom compartment received 1·5 ml EMEM-10. Transepithelial resistance was measured after equilibration to room temperature before media change every 2–3 d for 30 d with a voltmeter to access monolayer polarity as a marker for cell differentiation. As cell polarity increases, tight junctions begin to form and the intestinal barrier becomes intact (less permeable)(Reference Peterson, Bement and Mooseker27).
Alkaline phosphatase activity of undifferentiated and differentiated Caco-2 cells was measured as a marker of crypt–villus differentiation. Intestinal alkaline phosphatase is a brush-border enzyme expressed exclusively in villus-associated enterocytes and expression indicates the development of digestive and absorptive function(Reference Goldberg, Austen and Zhang30).
For each alkaline phosphatase activity experiment, one T150 flask each of undifferentiated (confluence) and differentiated (10 d and 20 d post-confluence) Caco-2 cells was collected and cell homogenates were prepared in Tris-mannitol buffer with sonication as described previously in the cell-culture methods section. Cell homogenates and p-nitrophenol standards (10 μl) were added to wells in a ninety-six-well plate and mixed with 190 μl alkaline phosphatase reagent for 30 min at 37°C. The reaction was stopped with 2 m-NaOH and the absorbance at 405 nm was measured with a microplate reader.
Ganglioside extraction
Total lipid was extracted from cell supernatant fractions using the Folch method(Reference Folch, Lees and Sloane Stanley31). In short, 0·9 ml samples of cell supernatant fraction were mixed with 18 ml chloroform–methanol (2:1, v/v) and incubated overnight on a shaker. Distilled water was added to give a final ratio of 5:1 chloroform–methanol (2:1, v/v)–water. The upper aqueous phase containing gangliosides was collected. To increase the yield of gangliosides, the lower organic phase was washed twice with Folch upper phase solution (chloroform–methanol–water, 3:48:47, by vol.). The upper aqueous phases containing gangliosides were pooled together and purified by passage through Sep-Pak C18 cartridges (Waters Corporation, Milford, MA, USA) pre-washed with 10 ml methanol, 20 ml chloroform–methanol (2:1, v/v) and 10 ml methanol as described by Williams & McCluer(Reference Williams and McCluer32). The upper phase extract was loaded onto C18 cartridges. Cartridges were washed with 20 ml distilled water to remove salts and water-soluble contaminants. Gangliosides were eluted with 5 ml methanol and 20 ml chloroform–methanol (2:1, v/v), dried under N2 gas and redissolved in 500 μl chloroform–methanol (2:1, v/v). Gangliosides were stored at − 20°C until analysis.
Analysis of total and individual ganglioside content
Total gangliosides were measured as ganglioside-bound NANA as described by Suzuki(Reference Suzuki, Suzuki, Michi and Matsumoto33). A 100 μl sample of purified ganglioside sample was dried under N2 gas and dissolved with 0·5 ml distilled water and 0·5 ml resorcinol-HCl in screw-capped Teflon-lined tubes. The purple blue colour developed by heating the sample for 8 min at 150–160°C was extracted into butylacetate–butanol (85:15, v/v) solvent. Optical density was read by a spectrophotometer (model 8452A, Hewlett Packard, Palo Alto, CA, USA) at 580 nm. For quantitative analysis, NANA (Sigma, St Louis, MO, USA) was used as a standard.
The remaining 400 μl of sample ganglioside was dried under N2 and redissolved in 100 μl chloroform–methanol (2:1, v/v). Individual gangliosides were separated by silica gel high-performance TLC (Whatman Inc., Clifton, NJ, USA) along with ganglioside standards GM3, GD3 and bovine brain ganglioside mixture (Alexis, San Diego, CA, USA) in a solvent system of chloroform–methanol–0·2 % (w/v) CaCl2.2H2O (55:45:10, by vol.). Individual gangliosides were visualised under UV light by spraying high-performance TLC plates with 0·1 % (w/v) 8-anilino-1-naphthalene-sulfonic acid. Each ganglioside band was scraped into a glass tube. Gangliosides were eluted from silica by vortex, sonication and shaking overnight in 10 ml chloroform–methanol (2:1, v/v). Tubes were centrifuged for 10 min at 1000 rpm to spin down the silica. The chloroform–methanol (2:1, v/v) phase was collected and combined with a 10 ml chloroform–methanol (2:1, v/v) wash and a 5 ml methanol wash of the silica. Individual gangliosides were measured as ganglioside-bound NANA as described above. To determine the percentage of individual gangliosides in the mixture, gangliosides separated on high-performance TLC plates were sprayed with resorcinol-HCl and heated for 7–10 min at 150–160°C to visualise purple ganglioside bands. Each ganglioside band was quantified as a percentage of the total gangliosides by densitometry (Beckman CDS-200; Beckman Coulter, Mississauga, ON, Canada) using Quantity One software (Biorad Laboratories Inc., Hercules, CA, USA).
Statistical analysis
Sample size determination for a one-tailed comparison at 80 % power to detect a 2-fold increase in liver gangliosides (44·3 nmol/g wet weight to 79·1 nmol/g wet weight; P < 0·01) requires a sample size of three(Reference Majer, Trnka, Vítek, Jirkovská, Marecek and Smíd34). All values are displayed as mean values with their standard errors for a sample size of six (six different passages of cells) for individual gangliosides measured by the colorimetric, NANA assay, a sample size of five (five different passages of cells) for total gangliosides and a sample size of four (four different passages of cells) for individual gangliosides measured by densitometry and differentiation markers. Significant differences in amount and composition of gangliosides between undifferentiated and differentiated Caco-2 cells were determined by a one-way ANOVA and a Tukey test with SAS statistical software (version 9.1; SAS Institute Inc., Cary, NC, USA). A P value of less than 0·05 was considered statistically significant.
Results
Transepithelial resistance and alkaline phosphatase activity of undifferentiated and differentiated Caco-2 cells
Transepithelial resistance was measured when Caco-2 cells reached confluence and every 3 d post-confluence up to 30 d to monitor cell polarity as a marker of cell differentiation and development of an intact intestinal barrier. As indicated in Fig. 2 (a), transepithelial resistance displayed a linear increase over time as Caco-2 cells differentiated. Alkaline phosphatase activity was measured when Caco-2 cells reached confluence and 10 and 20 d post-confluence as a marker of crypt–villus differentiation and development of digestive and absorptive function. As indicated in Fig. 2 (b), alkaline phosphatase activity was significantly higher 10 and 20 d post-confluence compared with undifferentiated, confluent Caco-2 cells.
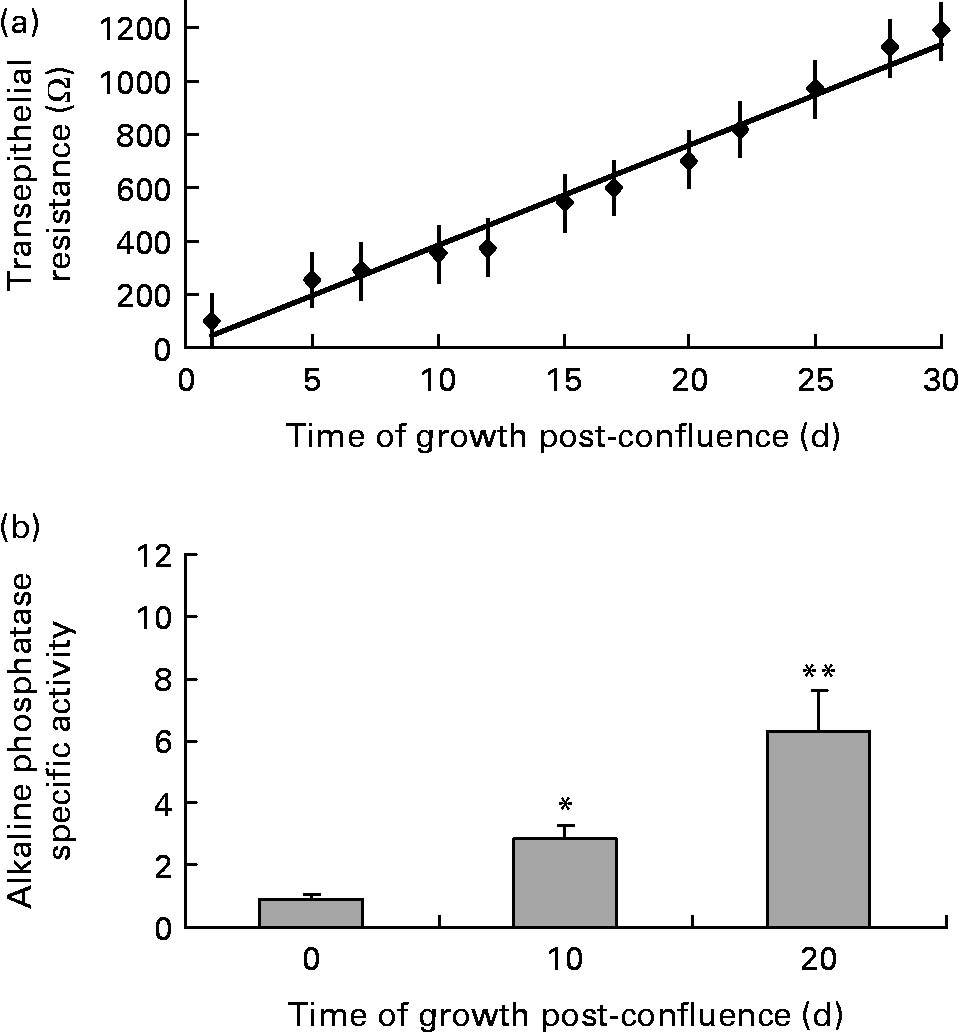
Fig. 2 Differentiation markers (a) transepithelial resistance and (b) alkaline phosphatase specific activity for Caco-2 cells measured at confluence (undifferentiated cells) and post-confluence (differentiated cells). Values are means for a sample size of four (four different cell passages), with standard errors represented by vertical bars. Mean value was significantly different from that at confluence: * P < 0·001, ** P ≤ 0·0001.
Total ganglioside content of undifferentiated and differentiated Caco-2 cells
The influence of differentiation on total ganglioside content of human colon cancer Caco-2 cells is shown in Fig. 3. Caco-2 cells differentiated for 20 d had 2·5 times higher ganglioside content compared with undifferentiated Caco-2 cells.
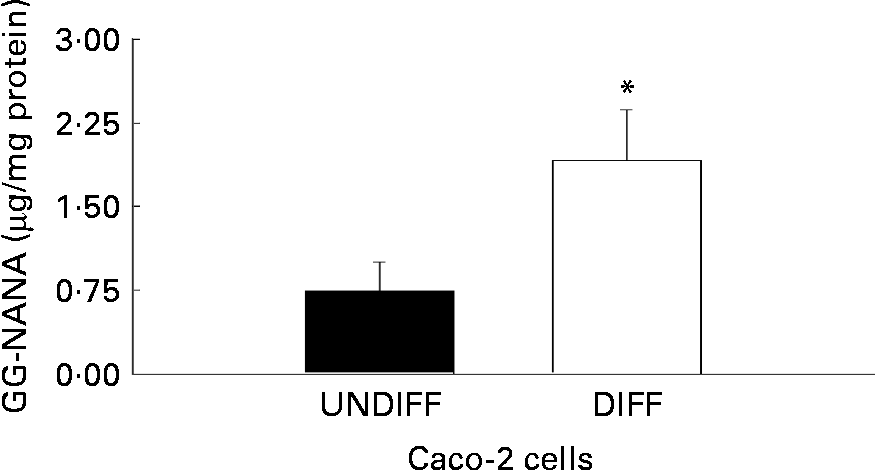
Fig. 3 Total content of ganglioside-bound N-acetyl neuraminic acid (GG-NANA) in undifferentiated (UNDIFF) and differentiated (DIFF) human colon cancer Caco-2 cells grown to confluence or differentiated 20 d post-confluence, respectively. Values are means for a sample size of five (five different cell passages), with standard errors represented by vertical bars. * Mean value was significantly different from that of the undifferentiated cells (P < 0·05).
Quantification of individual ganglioside composition in undifferentiated and differentiated human colon cancer Caco-2 cells
The amounts and percentages of individual gangliosides as well as changes in the GM3:GD3 ratio of undifferentiated and differentiated Caco-2 cells measured using a colorimetric NANA assay and scanning densitometry are illustrated (Fig. 4). Differentiated Caco-2 cells had a significantly higher amount of GD3 and polar gangliosides and a trend towards lower amounts of GM3 compared with undifferentiated cells (Fig. 4 (a)). Moreover, the percentage GD3 and polar gangliosides was also significantly higher in differentiated cells (Fig. 4 (b)). Undifferentiated cells had a higher percentage of GM3 and N-acetylgalactosaminyl-galactosyl-(n-acetylneuraminyl)-glucosylceramide (GM2) (Fig. 4 (b)). Independent of the method used to quantify individual gangliosides, the GM3:GD3 ratio decreased when Caco-2 cells were differentiated and the drop in the ratio was significant when gangliosides were quantified using the NANA assay (Fig. 4 (a) and (b)).

Fig. 4 Ganglioside composition of undifferentiated (■) and differentiated (□) Caco-2 cells measured by a colorimetric N-acetyl neuraminic acid assay (a) and scanning densitometry (b). GM3, N-acetylneuraminylgalactosylglucosylceramide; GD3, (N-acetylneuraminyl) 2-galactosylglucosyl ceramide; GM2, N-acetylgalactosaminyl-galactosyl-(n-acetylneuraminyl)-glucosylceramide; GM1, galactosyl-n-acetylgalactosaminyl-(n-acetylneuraminyl)-galactosyl-glucosylceramide. Values are means for a sample size of six (six different cell passages), with standard errors represented by vertical bars. Mean value was significantly different from that of the undifferentiated cells: * P < 0·01, ** P ≤ 0·005, *** P ≤ 0·0001.
Discussion
The discovery that changes in glycosphingolipid metabolism are similar during development and oncogenic transformation prompted research on the characterisation of ganglioside content and composition during different stages of cell differentiation. Differentiating nerve and leukaemia cell lines have been investigated thoroughly; however, knowledge on the ganglioside content and composition of differentiating intestinal cells is limited(Reference Noguchi, Kabayama, Uemura, Kang, Saito, Igarashi and Inokuchi13, Reference Varner35). With increasing evidence that parallel changes in ganglioside content and composition occur during lactation and intestinal development(Reference Draper, Pigott, Thomson and Andrews9, Reference Takamizawa, Iwamori, Mutai and Nagai18, Reference Rueda, Maldonado, Narbona and Gil22), it became of interest to investigate whether ganglioside alterations during intestinal cancer cell differentiation resemble the compositional changes during lactation and intestinal development. A comparable shift in ganglioside content and composition would demonstrate the potential use of differentiating Caco-2 cells as a model for studying the physiology and pathophysiology of the neonatal gut.
The present study investigated the ganglioside content and composition of undifferentiated and differentiated human colon cancer Caco-2 cells. The study demonstrated that differentiated human colon cancer Caco-2 cells have 2·5 times more total gangliosides than poorly differentiated Caco-2 cells. The trend for enhanced ganglioside content following differentiation is supported by the observation that ganglioside sugar-chain elongation is enhanced in differentiated human colon cancer HT-29 cells(Reference Houri, Falbo, Vignali, Codogno and Ghidoni36). Moreover, lipid-bound sialic acid levels are significantly elevated in hepatoma tissues during proliferation and differentiation(Reference Lu, Lu, Wang and Cui37). Similar to differentiated colon cancer cells, human colostrum has twice as many gangliosides as mature milk and a larger fraction of complex gangliosides with branched sugar chains(Reference Takamizawa, Iwamori, Mutai and Nagai18). Although the ganglioside content of the infant bowel has not been investigated, it is known that neonatal rat intestine contains more gangliosides than adult rat intestine and that the ganglioside composition varies along the crypt–villus axis(Reference Bouhours, Bouhours and Hansson21, Reference Glickman and Bouhours38). Recently, acidic milk oligosaccharides, some of which are precursors for the synthesis of gangliosides, were demonstrated to inhibit Caco-2 cell growth and induce differentiation at concentrations 100-fold higher than the concentration of gangliosides present in human milk, and known to be effective in down-regulating the inflammatory response(Reference Kuntz, Rudloff and Kunz39). The accumulation of individual gangliosides in colostrum, immature intestine and differentiated intestinal cells suggests a unique physiological role for specific gangliosides in cell development and differentiation.
Individual ganglioside composition of undifferentiated and differentiated Caco-2 cells was determined and compared with human colostrum and immature rat intestine to elucidate possible functions for specific gangliosides in development and differentiation. The effect of gangliosides on proliferation and migration are differential and depend on cell type and ganglioside-associated molecules in the individual cell types(Reference Kamimura, Furukawa, Kittaka, Nishio, Hamamura, Fukumoto and Furukawa40). In the present study, ganglioside accumulation in differentiated Caco-2 cells was accompanied by changes in the individual ganglioside composition. Differentiated Caco-2 cells contained more GD3 and polar, complex gangliosides than undifferentiated Caco-2 cells. Human colostrum and differentiated human embryonic stem cells also contain high amounts of GD3 and polar, complex gangliosides(Reference Rueda, Maldonado, Narbona and Gil22, Reference Kwak, Yu and Kim41). GD3 and polar gangliosides may play a role in tight-junction formation (EJ Park, ABR Thomson and MT Clandinin, unpublished results), intestinal barrier integrity and the expression of proteins involved in digestion and absorption(Reference Drozdowski, Suh, Park, Clandinin and Thomson42, Reference Birecki, Drozdowski, Suh, Park, Clandinin and Thomson43). The accumulation of GD3 and polar gangliosides in colostrum, differentiated stem cells and differentiated colon cancer cells may support a role for GD3 and polar gangliosides in promoting intestinal development and enterocyte differentiation. In Caco-2 cells, Ca-sensing receptors that regulate cell proliferation and are modulated by gangliosides are most concentrated in crypt cells and differentiated colon cancer lesions(Reference Kállay, Bajna, Wrba, Kriwanek, Peterlik and Cross44). Accumulation of GM3, GD3 and polar gangliosides in cells with receptors that regulate cell proliferation and growth suggests a role for GM3, GD3 and polar gangliosides in regulating cell proliferation during development, intestinal reconstitution and tumour progression. Perhaps the relative amount of each molecular species of ganglioside determines the balance between proliferation and apoptosis.
Differentiated Caco-2 cells also contained a relatively lower percentage of GM3 when compared with undifferentiated Caco-2 cells. This ganglioside compositional change was accompanied by a small decrease in the GM3:GD3 ratio. Nojiri demonstrated that differentiated HCT-116 colon cancer cells lose tumorigenic activity and become susceptible to apoptosis by artificially increasing GM3 content(Reference Nojiri, Yamana, Shirouzu, Suzuki and Isono45). Differentiated leukaemia cells and macrophages have been shown to have elevated levels of GM3(Reference Chung, Choi, Lee and Kim46, Reference Nojiri, Takaku, Terui, Miura and Saito47). Unlike some other differentiated cell lines, the differentiated Caco-2 cell did not have a significantly different GM3 content from that of the undifferentiated form. The intestinal barrier has a unique role in protecting the host from the external environment from birth through to adult life and undergoes frequent cell turnover. The presence of GM3 in undifferentiated Caco-2 cells may be important for cell proliferation and renewal while GM3 enrichment in the villi as observed in neonatal rat intestine may be important for regulating bacteria colonisation and protection against microbe invasion(Reference Glickman and Bouhours38). The differentiated Caco-2 cell also had a lower percentage of GM3 and GM2. Thus, the decrease in the GM3:GD3 ratio is attributed to an increase in GD3 content with a small decline in GM3. Perhaps differentiating Caco-2 cells up-regulate SAT-2 and convert GM3 into GD3 with hydrolysis of GM2 replenishing some of the lost GM3. Several studies have observed an elevation in the GD3 synthesis level of apoptotic cells(Reference Bennaceur, Popa, Portoukalian, Berthier-Vergnes and Peguet-Navarro48–Reference Omran, Saqr and Yates50). Saha demonstrated that the level of GM2 and GM3 correlates with metastatic potential(Reference Saha and Mohanty51). Furthermore, differentiation of colon cancer cell lines (metastatic to poorly metastatic phenotype) and susceptibility to apoptosis was found to be associated with a decrease in human plasma membrane sialidase expression and activity(Reference Kakugawa, Wada, Yamaguchi, Yamanami, Ouchi, Sato and Miyagi17). Taken together with previous studies, the susceptibility of differentiated Caco-2 cells to apoptosis may be explained by elevated apoptotic GD3 while malignancy may be promoted by suppressing apoptosis through hydrolysis of GD3.
The difference in the GM3:GD3 ratio between undifferentiated and differentiated Caco-2 cells was small in comparison with the large changes reported in the literature during lactation. Depending on the ganglioside analysis method, undifferentiated Caco-2 cells had a GM3:GD3 ratio that was 2·4–3·5 times higher than the GM3:GD3 ratio of differentiated Caco-2 cells. In a study that followed the change in ganglioside composition of human milk over time, the GM3:GD3 ratio was reported to be seventy times greater in mature milk when compared with colostrum (0·05 colostrum v. 3·5 mature milk)(Reference Pan and Izumi52). The shift in the ratio during lactation was associated with a large increase in GM3 levels along with a large decrease in GD3 levels(Reference Takamizawa, Iwamori, Mutai and Nagai18). The GM3:GD3 ratio of mature rat small intestine is 26, similar to the ratio of 19·8 observed in mature milk(Reference Takamizawa, Iwamori, Mutai and Nagai18, Reference Park, Suh, Ramanujam, Steiner, Begg and Clandinin53). For neonatal rat small intestine, the GM3:GD3 ratio has not been determined; however, it is known that neonatal tissues decrease in GD3 content and increase in GM3 content during development(Reference Bouhours, Bouhours and Hansson21, Reference Rueda, Maldonado, Narbona and Gil22, Reference Kwak, Yu and Kim41). Although the ganglioside composition changes are similar during rat intestine development, lactation and oncogenic transformation of colon cells, further ganglioside analysis work should be completed with infant bowel to determine changes in the GM3:GD3 ratio during development and look at the degree of change in GM3 and GD3 levels. The changes in ganglioside content and composition of Caco-2 cells should also be measured in partially differentiated Caco-2 cells and confirmed with a more sensitive ganglioside analysis method such as liquid chromatography–tandem MS to more accurately access individual ganglioside composition(Reference Sorensen54). Adapting a more sensitive ganglioside analysis method would decrease tissue requirements, eliminate cell pooling and help clarify the changes that occur in individual species of polar, complex gangliosides that are often present in small amounts.
In summary, differentiated Caco-2 cells have a much higher ganglioside content than undifferentiated cells, including significantly greater GD3 and polar gangliosides and a trend towards lower levels of GM3. An accumulation of GD3 and polar gangliosides in colostrum, differentiated stem cells and differentiated colon cancer cells may support a role for GD3 and polar gangliosides in promoting intestinal development and enterocyte differentiation. Differences in the GM3:GD3 ratio between undifferentiated and differentiated Caco-2 cells were small in comparison with the large changes observed by others during lactation; therefore future studies should employ more sensitive techniques of ganglioside analysis to confirm these findings. These preliminary results suggest that differentiated Caco-2 cells may be an alternative model for studying physiological and pathological processes in the small intestine and colon, and may help to elucidate possible functions for specific gangliosides in development and differentiation.
Acknowledgements
The present study was supported by a Canadian Institute of Health research grant and a National Sciences and Engineering Research Council scholarship.
The present study was K. L. S.'s research thesis; C. F. contributed to the study's methodology and interpretation; M. T. C. was the principal investigator and supervisor.
There are no conflicts of interest in respect of this paper.






