Internalising disorders in preadolescents
Internalising disorders represent the largest group of psychiatric disorders and encompass affective and mood disorders that share the primary characteristic of excessive anxious distress. These disorders represent the most prevalent mental health problems in adolescence and their prevalence has doubled during the COVID-19 pandemic, exacerbating critical mental health issues.Reference Racine, Mcarthur, Cooke, Eirich, Zhu and Madigan1 However, a growing number of studies revealed heterogeneity in internalising disorders, indicating clinical and biological significant subtypes, which critically impede personalised interventions.Reference Goldberg2
Impulsivity in internalising disorders
Impulsivity – classically considered a key feature of externalising disorders – may play a role in the manifestation and treatment outcomes of internalising disorders.Reference Cosi, Hernandez-Martinez, Canals and Vigil-Colet3 Both anxiety and impulsivity are major dimensions of psychopathology and have been associated with neurobiological dysregulations across diagnoses.Reference Owens, Hyatt, Gray, Miller, Lynam and Hahn4,Reference Newman, Thompson, Bartsch, Hagler, Chen and Brown5 However, although interactions between impulsivity and externalising symptoms have been studied, the contribution of impulsivity to variations in symptomatology, brain alterations and clinical trajectories in internalising disorders has not been systematically examined.
Different lines of research have conceptualised the interaction between anxiety and impulsivity, yet perspectives and results with respect to internalising disorders remained highly controversial. Traditional conceptualisations propose an inverse relationship between anxiety and impulsivity and suggest that anxiety may serve as a protective factor against impulsive and potentially harmful behaviours.Reference Taylor, Hirshfeld-Becker, Ostacher, Chow, Lebeau and Pollack6–Reference Kashdan and Hofmann9 However, this conceptualisation has been increasingly challenged by a growing number of recent studies reporting a positive relationship,Reference Cosi, Hernandez-Martinez, Canals and Vigil-Colet3,Reference Moustafa, Tindle, Frydecka and Misiak10 suggesting that impulsive behaviours may serve to provide an immediate and reinforcing reduction of excessive anxious arousal (e.g. use of drugs to alleviate stress and anxiety).Reference Tice, Bratslavsky and Baumeister11 This inconsistent relationship has also been reflected in a genetic study employing genome-wide association (GWAS) methods that reported that internalising psychopathology was positively related with urgency, but negatively related with sensation seeking.Reference Gustavson, Friedman, Fontanillas, Elson, Palmer and Sanchez-Roige8 Given these divergent findings, we hypothesise the existence of two impulsivity-dependent subtypes with opposite anxiety–impulsivity relationships in internalising disorders that are characterised by different reaction tendencies (impulsive or not) to anxiety and may exhibit distinct neurobiological, cognitive, and clinical manifestations, and divergent neurodevelopmental trajectories accelerated by ‘developmental cascades’ that characterise adolescence.Reference Masten and Cicchetti12
Emotional/behavioural dysregulations and maturational mismatch during adolescence
Most internalising disorders manifest during preadolescence. This period is characterised by fundamental processes of brain development and maturation, particularly myelination and pruning.Reference Natu, Gomez, Barnett, Jeska, Kirilina and Jaeger13 The neuromaturational changes are mirrored in the macroscale architecture of the brain particularly cortical thickness, which increases during childhood, decreases during adolescence and stabilises during early adulthood.Reference Shaw, Kabani, Lerch, Eckstrand, Lenroot and Gogtay14 Dysregulations in cortical thickness maturation have been closely linked with various psychiatric disorders and cognitive impairments.Reference Shaw, Greenstein, Lerch, Clasen, Lenroot and Gogtay15 A previous study identified a subtype within internalising disorders that was characterised by reduced cortical thickness and cognitive performance but more behavioural and emotional dysregulations.Reference Kaczkurkin, Sotiras, Baller, Barzilay, Calkins and Chand16 This aligns with the ‘maturational mismatch’ theory that proposes that regulatory prefrontal cortical regions undergo prolonged maturational changes whereas subcortical regions involved in reward and emotion processing mature earlier.Reference Casey, Getz and Galvan17 This mismatch in turn promotes emotional and behavioural regulation deficits (e.g., anxiety, depression and impulsivity) during adolescence. Previous studies also found altered glutamatergic and GABAergic transmission within prefrontal systems in individuals with internalising disorders.Reference Hare and Duman18 Genetic markers of internalising disorders were related to a number of neurotransmitter and immune systems dysregulations.Reference Lacerda-Pinheiro, Junior, de Lima, Da Silva, Dos Santos and Júnior19 Therefore, conceptualising impulsivity-dependent subtypes of internalising disorders in the perspective of adolescent neuromaturational trajectories and exploring underlying genetic and neurotransmitter systems may allow the determination of broad subtypes of neuropathological dysregulations at the core of psychiatric disorders in later life.
Aims
In this study, we aimed at determining impulsivity-dependent subtypes in preadolescent internalising disorders. Specifically, we first clustered a large sample of preadolescents with ‘pure’ internalising disorders (with internalising disorders but without comorbid externalising disorders) according to their levels of impulsivity using a data-driven approach. Next, we determined whether the identified subtypes are characterised by distinct anxiety–impulsivity relationships and brain morphological signatures. Moreover, we investigated the potential role of motivational systems in the subtypes given their fundamental associations with impulsivity, i.e., a hyperactive activation system may promote impulsive behaviour.Reference Corr20 Further, we conducted a GWAS, gene set enrichment analysis, cell type specificity analysis and spatial association analysis with neurotransmitter systems to determine the underlying neurobiological pathways that may mediate the brain morphological alterations. Finally, we examined the differences of neurocognitive and educational performance between groups, and differences at the clinical predictive level in terms of pathological trajectories during follow-up between the subtypes.
Given the clinical challenge of predicting behavioural dysregulation in adolescence and previous findings suggesting that adolescents who are impulsive–anxious show a higher inclination to suicidality and depression,Reference Askenazy, Sorci, Benoit, Lestideau, Myquel and Lecrubier21 we specifically focused on the development of suicidality, depression and behavioural dysregulation in terms of externalising symptomatology.
Method
Participants
A sample of 11 878 preadolescents aged 9–10 years was obtained from Data Release 3.0 of the Adolescent Brain Cognitive Development (ABCD) study. The ABCD study group obtained written and oral informed consent from parents and children, respectively. The Institutional Review Board at the University of California, San Diego approved the study protocol and is responsible for the ethical review.Reference Auchter, Mejia, Heyser, Shilling, Jernigan and Brown22 Details on the protocols and assessments are provided by the ABCD study group.Reference Barch, Albaugh, Avenevoli, Chang, Clark and Glantz23–Reference Casey, Cannonier, Conley, Cohen, Barch and Heitzeg25
Clinical measures
Levels of psychopathology, i.e. anxiety, depression, and behavioural problems (externalising problems, rule-breaking behaviour, aggressive behaviour, attention–deficit hyperactivity disorder (ADHD) problems, oppositional defiant problems and conduct problems), were derived from the Child Behavior Checklist (CBCL).Reference Barch, Albaugh, Avenevoli, Chang, Clark and Glantz23 Details see Supplementary Methods (available at https://doi.org/10.1192/bjp.2023.107). Psychiatric diagnoses were obtained via parent report using the structured computerised Kiddie Schedule for Affective Disorders and Schizophrenia for DSM-5.Reference Barch, Albaugh, Avenevoli, Chang, Clark and Glantz23 For the present analysis, lifetime diagnoses for the respective specific or unspecific disorders were employed. In line with overarching dimensions of psychopathology,Reference Lees, Squeglia, Mcteague, Forbes, Krueger and Sunderland26 two broad diagnostic families were determined as internalising disorders (depressive disorder, agoraphobia, panic disorder, specific phobia, separation anxiety disorder, social anxiety disorder, generalised anxiety disorder and post-traumatic stress disorder) and externalising disorders (ADHD, oppositional defiant disorder and conduct disorder).
Our initial analyses focused on individuals with ‘pure’ internalising disorders while excluding participants with comorbid externalising disorders to control for the potential influence of comorbid externalising conditions on impulsivity (Fig. 1(a)) and confounding effects of impaired cognitive functioning. Individuals classified into the ‘pure’ internalising disorders group were required to (a) fulfil the criteria for at least one internalising disorders, while (b) comorbid externalising disorders, disruptive mood dysregulation disorder, bipolar disorder, psychotic disorder, alcohol use disorder or substance use disorder led to exclusion.
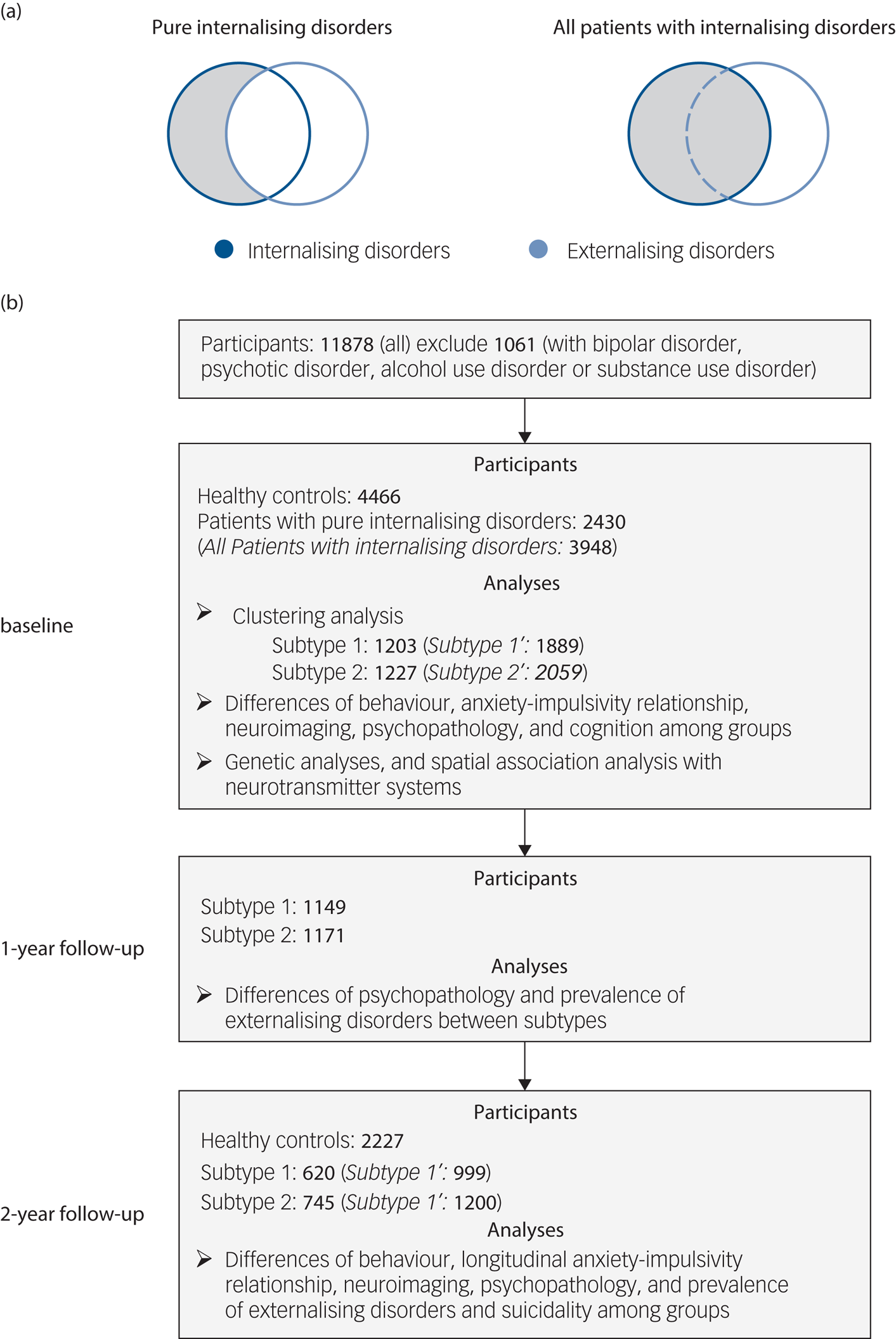
Fig. 1 Selection of participants and analyses at baseline and follow-up. (a) Selection of patients with ‘pure’ internalising disorders (with internalising disorders but without comorbid externalising disorders) and all patients with internalising disorders. (b) Flowchart showing selection of participants with timescale, and number of participants and analyses at each of baseline (10 years old), 1-year follow-up (11 years old) and 2-year follow-up (12 years old). Our initial analyses focused on individuals with ‘pure’ internalising disorders while excluding participants with comorbid externalising disorders to control for the potential influence of comorbid externalising conditions. To test the robustness of our findings we repeated the analyses in all patients with internalising disorders (italic in brackets) including those with comorbid externalising conditions.
To test the robustness of our findings we repeated the analyses in all patients with internalising disorders (including those with comorbid externalising conditions) (Fig. 1(a)). Individuals without any lifetime diagnosis were classified as healthy controls and served as a reference group. Numbers of participants and section procedures are shown in Fig. 1(b).
Measures of impulsivity, motivational systems and cognitive function
Trait impulsivity and its subfacets were assessed by the UPPS-P Impulsive Behavior Scale encompassing five subfacets: negative urgency, positive urgency, sensation seeking, lack of premeditation and lack of perseverance.Reference Barch, Albaugh, Avenevoli, Chang, Clark and Glantz23 Motivational systems were assessed by the Behavioral Inhibition Scale and Behavioral Activation Scale (BIS/BAS) including behavioural inhibition, drive, fun-seeking and reward responsiveness. Neurocognitive functioning in the domains of language vocabulary knowledge, attention, cognitive control, executive function, episodic memory, working memory, processing speed and flexible thinking as well as composite scores for crystallised intelligence, fluid intelligence and total intelligence were derived from the validated National Institutes of Health toolbox (for details see Supplementary Methods).
Structural neuroimaging
T 1-weighted structural MRI (sMRI) data processed by the validated ABCD protocols served as brain morphological data and included post-processed structural data of cortical thickness, surface area, cortical volume, and sulcus depth from cortical regions (n = 68) and subcortical volumes (left/right thalamus, caudate, putamen, pallidum, hippocampus, amygdala and accumbens area).Reference Hagler, Hatton, Cornejo, Makowski, Fair and Dick24 Participant data were excluded from the neuroimaging analysis if the T 1 images failed to pass visual inspection and FreeSurfer quality control (imgincl_t1w_include = 1). Further details can be found in Supplementary Methods.
Statistical analyses
Clustering analysis
We capitalised on data-driven clustering techniques to determine subtypes of patients with internalising disorders based on the five impulsivity dimensions of the UPPS-P model. In line with established guidelines for cluster analyses a combination of hierarchical and non-hierarchical procedures was employedReference Hair, Black, Babin and Anderson27 (for advantages of this approach see Supplementary Methods).
First, we conducted a hierarchical agglomerative clustering analysis. All variables of UPPS-P were standardised (Z-score), so that each variable contributes equally to the cluster formation. The hierarchical cluster analysis employed Ward's method with the squared Euclidian distance and was conducted using the pheatmap function in the pheatmap package with ‘ward.D2’ method in R. This method can minimise the variance within each cluster, thus finding compact clusters. The largest average silhouette width was used to determine the optimal number of clusters.Reference Rousseeuw28 Second, means of every variable assigned to each cluster were calculated to initialise K-means clustering. Finally, cluster assignment was fine-tuned by a non-hierarchical K-means cluster analysis conducted using the kmeans function in R. To validate the robustness of the composition in each cluster we chose a subset of 50% of randomly selected patients with internalising disorders and repeated the same clustering method. This procedure was repeated 1000 times and Cohen's kappa coefficient was used to measure the agreement of the case memberships with the original clustering for each permutation.Reference Fereshtehnejad, Zeighami, Dagher and Postuma29
Cross-sectional and longitudinal associations between anxiety and impulsivity
We used cross-lagged panel models (CLPM) implemented by the lavaan package in R (https://cran.r-project.org/web/packages/lavaan/index.html) to explore the anxiety–impulsivity relationship. Anxiety and impulsivity scores were assessed at baseline (10 years) and 2-year follow-up (12 years). The model was estimated by using maximum-likelihood estimation. Cross-sectional correlation coefficients and standardised longitudinal regression coefficients and P-values were reported. Age, gender and ethnicity were regressed out as nuisance covariates. To account for multiple comparisons, false discovery rate (FDR) correction at q < 0.05 was performed for the 30 measures (There are five subfacets of impulsivity, with each subfacet corresponding to a CLPM model. In each model, there are six cross-sectional/longitudinal correlations between anxiety or impulsivity, thus 5 × 6 = 30 measures totally).
Characterisation of cognition, behaviour and clinical trajectory of subtypes
To determine phenotypical differences in the domains of anxiety, impulsivity and motivational systems between groups, we initially conducted one-way ANOVA models. Results were corrected for multiple comparisons using FDR at q < 0.05 for the ten measures (one anxiety measure, five impulsivity measures and four motivational systems measures). Post hoc t-tests were performed to further disentangle the ANOVA level effects for which Bonferroni correction was used for multiple comparisons (P < 0.05/3, to control for three pair-wise comparisons between the three groups).
Direct comparisons were implemented to compare depressive problems and externalising psychopathology (measured by the symptom scores on the CBCL) between subtype 1 and 2 at baseline and follow-up. We also compared the transition rate to externalising disorders and the prevalence of suicidality (including suicidal ideation, suicide attempt and non-suicidal self-injury) at follow-up of the two subtypes using the chi-square test. Results were all corrected for multiple comparisons using FDR at q < 0.05 separately for the seven measures of CBCL, three measures of diagnosis at 1-year follow-up, and six measures of diagnosis at 2-year follow-up.
As an index of cognitive performance and functioning in daily life we examined differences in academic performance (grades) and cognition between groups at baseline using one-way ANOVA models. Results were corrected for multiple comparisons using FDR at q < 0.05 for the 11 measures of academic performance and cognition. Post hoc t-tests were performed between the three groups on measures significant in ANOVA, in which Bonferroni correction was used for multiple comparisons (P < 0.05/3) for three pair-wise comparisons between the three groups.
Covariates were controlled in these analyses with age, gender and ethnicity as fix-effects and family identifier (ID) and acquisition site ID as random-effects using linear mixed-effects models (LMMs) in lme4 package (https://cran.r-project.org/web/packages/lme4/index.html) in R.
Characterisation of brain morphological alterations of subtypes
To characterise the neurobiological basis of the phenotype differences, i.e., the brain morphological alterations of the two subtypes, we performed one-way ANOVA models for the three groups (two subtypes of patients and controls) using LMMs that included random-effects for family ID and acquisition site ID and fixed-effects for a categorical variable representing groups (subtype 1 group, subtype 2 group and healthy control group), age, gender, ethnicity, family income, parental years of education, puberty score, body mass index and intracranial volume. To account for multiple comparisons, FDR at q < 0.05 was performed separately for the 68 cortical thickness measures, 68 surface area measures, 68 cortical volume measures, 68 sulcus depth measures and 14 subcortical volume measures. Post hoc pair-wise contrasts were employed on measures significant in ANOVA also by the LMM including random-effects for family ID and acquisition site ID and fixed-effects for a dichotomous variable representing the groups (two of subtype 1, subtype 2 and health controls were coded as 0 or 1 in each pair-wise contrasts), age, gender, ethnicity, family income, parental years of education, puberty score, body mass index and intracranial volume. Bonferroni correction was used for multiple comparisons (P < 0.05/3) for three pair-wise comparisons between three groups.
In addition to the categorical approach, we also employed a dimensional approach examining associations between anxiety, impulsivity and cortical thickness in regions exhibiting significant between-group differences in the entire sample (n = 11 878) (for a similar approach see elsewhereReference Xu, Dai, Chen, Liu, Xin and Zhou30). LMMs were used to regress out the following covariates for neuroimaging variables: family ID and acquisition site ID for random-effects and age, gender, ethnicity, family income, parental years of education, puberty score, body mass index and intracranial volume for fixed-effects. Results were corrected for multiple comparisons using FDR at q < 0.05 for the six measures of significantly altered brain regions.
Genetic analyses and spatial association analysis with neurotransmitter systems for the brain alteration
We used GWAS to determine the genetic underpinnings of cortical thickness of the identified regions. We first performed genetic ancestry inference, genotype imputation and strict quality control on genotype data and filtered 4468 genetically unrelated preadolescents with European ancestry who passed structural image quality control. We then performed a GWAS using PLINK v2.0 to examine the genetic variants associated with cortical thickness of the identified regions.Reference Chang, Chow, Tellier, Vattikuti, Purcell and Lee31 On the premise of additive genetic effects, general linear regression models were fitted to determine the association between cortical thickness and allele dosages of single nucleotide polymorphisms (SNPs) in genetically unrelated preadolescents with European ancestry. To correct for population stratification, the first 10 genetic principal components were derived using genetic principal component analysis performed on these individuals. Sex, age, mean cortical thickness, top 10 principal components and study sites were included as covariates. Then SNPs were annotated and mapped to genes by FUMA online platform (version v1.3.7),Reference Watanabe, Taskesen, Van Bochoven and Posthuma32 which is an integrative tool for functional mapping and annotation of genetic associations. For further details see Supplementary Methods.
To identify biological functions and pathways of candidate genes, gene set enrichment analysis was performed to test if these genes are overrepresented in predefined gene sets obtained from the Molecular Signatures Database and GWAS catalogue.Reference Liberzon, Subramanian, Pinchback, Thorvaldsdóttir, Tamayo and Mesirov33,Reference Welter, Macarthur, Morales, Burdett, Hall and Junkins34 Protein-coding genes were set as background genes. Bonferroni correction was performed per gene set by FUMA. Other parameters in these analyses were set as default.
To examine the association between regional cortical thickness and specific cell types through gene expression, we performed cell type specificity analysis within FUMA,Reference Watanabe, Umićević Mirkov, de Leeuw, van den Heuvel and Posthuma35 using seven single-cell RNA sequencing data-sets from human brain tissue (Supplementary Table 1) and associations between genes and regional cortical thickness. We used Bonferroni correction for multiple testing within each dataset to identify significantly associated cell types.
To determine which neurotransmitter systems are associated with brain morphological alterations in each subtype relative to healthy individuals, JuSpace toolbox (v1.3, https://github.com/juryxy/JuSpace) was used to calculate the spatial correlation between the t-map of each subtype versus healthy controls and 28 positron emission tomography (PET)-based neurotransmitter receptor/transporter density maps.Reference Dukart, Holiga, Rullmann, Lanzenberger, Hawkins and Mehta36 Spearman correlation and permutation test were used to test whether the correlation between t-map and PET-maps is significant (based on the Neuromorphometrics atlas; exact P-values, n = 1000 permutations; adjusted for spatial autocorrelation). FDR was used for multiple comparisons correction at q < 0.05 for the 28 measures of neurotransmitter receptor/transporter density maps. For details see Supplementary Methods.
Sensitivity analyses
To test the robustness of associations between anxiety and impulsivity, we considered additional covariates including age, gender and ethnicity as fix-effects and family ID and acquisition site ID as random-effects using LMMs.
To test the robustness of the cognitive, behavioural and clinical characterisation of subtypes at baseline, we included covariates such age, gender, ethnicity, family income, parental years of education as fix-effects and family ID and acquisition site ID as random-effects using LMMs.
To test the robustness of group differences when including patients with internalising disorders and who also had comorbid externalising conditions (i.e. all patients with internalising disorders), we included these patients and repeated the analyses of differences between groups in the domains of anxiety, impulsivity, motivational systems, anxiety–impulsivity relationship and brain morphology.
Results
Participant characteristics
A total n = 4066 healthy controls and n = 2430 patients with ‘pure’ internalising disorder at baseline were included in all primary analyses. Demographic information is presented in Table 1.
Table 1 Participant characteristics at baseline
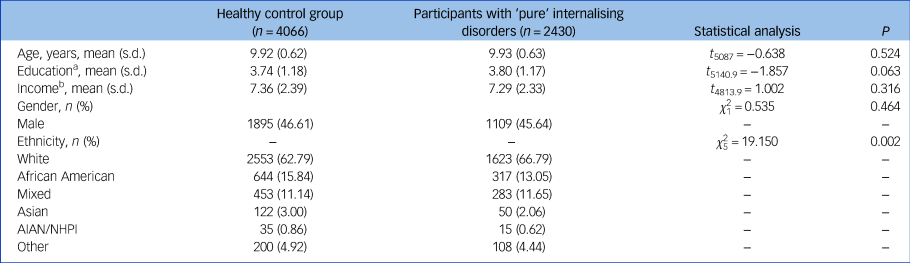
AIAN, American Indian/Alaska Native; NHPI, Native Hawaiian and other Pacific Islander.
a. Education of parents was measured by the years of education of the parent with the highest education, categorised as an ordinal variable across five bins (1: < high school diploma; 2: high school diploma/passing General Educational Development tests; 3: Some college; 4: bachelor; 5: post graduate degree).
b. Income was the sum of the annual incomes of both parents, categorised as an ordinal variable across ten bins (1: < $5000; 2: $5 000–11 999; 3: $12 000–15 999; 4: $16 000–24 999; 5: $25 000–34 999; 6: $35 000–49 999; 7: $50 000–74 999; 8: $75 000–99 999; 9: $100 000–199 999; 10: > $200 000).
Identifying subtypes of internalising disorders
In line with our hypothesis of two impulsivity-dependent subtypes of internalising disorders, the curve of average silhouette width for the clustering indicated a two-cluster solution (Supplementary Figure 1(a)). Data-driven clustering of the patients with internalising disorders, based on the impulsivity measured by five UPPS-P dimensions (negative urgency, positive urgency, lack of perseverance, lack of planning, sensation seeking) revealed two subtypes characterised by high or low impulsivity, respectively (Fig. 2(a)).
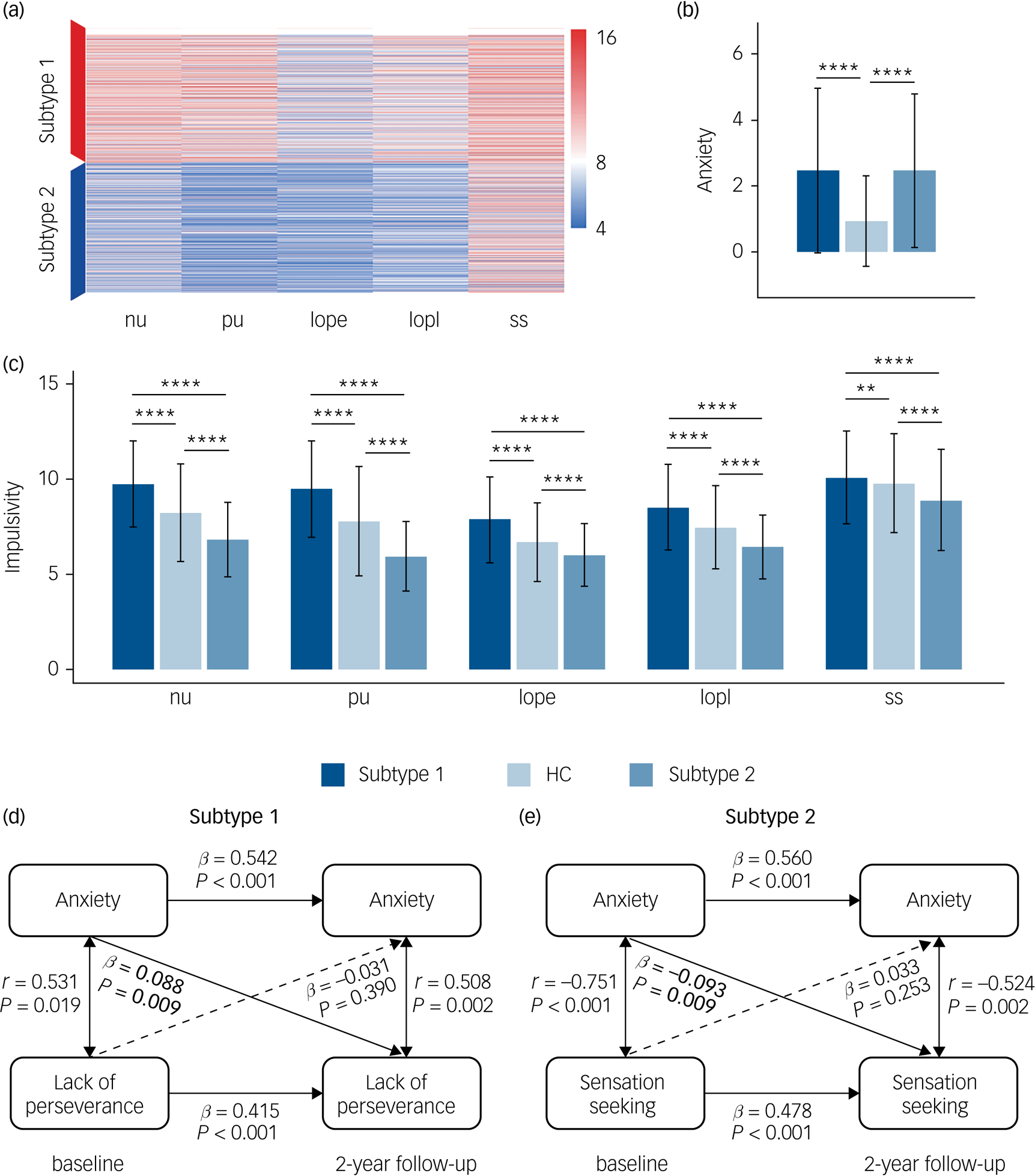
Fig. 2 Differences of anxiety, impulsivity and anxiety–impulsivity relationship between two subtypes of patients with ‘pure’ internalising disorders (with internalising disorders but without comorbid externalising disorders) and the controls groups. (a) Clustering results using five dimensions of impulsivity. (b) Comparisons of anxiety (Child Behavior Checklist-Anxiety Problems) between all groups. (c) Comparisons of impulsivity (five dimensions of UPPS-P) between all groups. (d) Cross-sectional and longitudinal association between anxiety and lack of perseverance in subtype 1. (e) Cross-sectional and longitudinal association between anxiety and sensation seeking in subtype 2. nu, negative urgency; pu, positive urgency; lope, lack of perseverance; lopl, lack of planning; ss, sensation seeking. HC, healthy control. In (b) and (c), ANOVA models revealed significant differences for each of six measures of anxiety and impulsivity, which passed false discovery rate (FDR) correction at q < 0.05 for the ten measures (one anxiety measure, five impulsivity measures and four motivational systems measures). * P < 0.5, ** P < 0.01, *** P < 0.001, **** P < 0.0001, which in (b) and (c) were Bonferroni corrected at P < 0.05/3 in post hoc tests for three pair-wise comparisons between three groups. In (d) and (e), associations labelled by solid line were significant after FDR correction at q < 0.05 for the 30 measures (There are five subfacets of impulsivity, with each subfacet corresponding to a cross-lagged panel model. In each model, there are six cross-sectional/longitudinal correlations between anxiety or impulsivity, thus 5 × 6 = 30 measures in total).
The five UPPS-P dimensions were not all positively related with each other thus our clustering procedure did not just dichotomise the continuous dimension of impulsivity (Supplementary Figure 1(b)). Cohen's kappa coefficients were all in the substantial range (0.88–1, see Supplementary Figure 1(c)) confirming the clustering robustness. The subtypes did not differ with ethnic distribution, however, participants in the subtype 2 group were slightly older and included slightly more females and patients with higher social economic status (significant differences, but with small effect sizes, see Supplementary Tables 2 and 3).
Characteristics of the subtypes in anxiety, impulsivity and motivational systems
We next compared the levels of anxiety and impulsivity between participants in the subtype 1, subtype 2 and the healthy control groups at baseline (Figs. 2(b), 2(c) and Supplementary Table 4). ANOVA models revealed significant differences for each of these six measures (anxiety level and five impulsivity dimensions) between groups. Post hoc pair-wise comparisons revealed that whereas both internalising groups exhibited comparably high levels of excessive anxiety relative to the healthy control group, the subtype 1 group exhibited increased whereas the subtype 2 group exhibited decreased impulsivity relative to the healthy control group.
With respect to the motivational systems the subtype 1 group exhibited increased whereas the subtype 2 group exhibited decreased levels of BAS compared with the healthy control group (Supplementary Figure 2). A further longitudinal analysis capitalising on the 2-year follow-up data revealed that differences in the anxiety and impulsivity domain remained stable over the follow-up period (Supplementary Figure 3 and Supplementary Table 5). For all analyses appropriate control of multiple comparisons were employed (for details see the caption of respective figures).
Characteristics of the subtypes in the association between anxiety and impulsivity
We further explored the cross-sectional and longitudinal association between anxiety and impulsivity for each subtype. We not only found two distinct and opposing associations both at baseline and follow-up, but also found that anxiety at baseline can predict different trends of impulsivity at follow-up for the identified two subtypes, which further verifies our hypothesis of different reaction tendencies towards increased anxiety in different subtypes. Specifically, for the cross-sectional association, higher anxiety was related with increased lack of perseverance in subtype 1 both at baseline (r = 0.531, P = 0.019) and 2-year follow-up (r = 0.508, P = 0.002) (Fig. 2(d)), but with decreased sensation seeking in the subtype 2 group both at baseline (r = −0.751, P < 0.001) and 2-year follow-up (r = −0.524, P = 0.002) (Fig. 2(e)). For the longitudinal association, in the subtype 1 group higher anxiety at baseline was longitudinally associated with increased lack of perseverance (β = 0.088, P = 0.009) at 2-year follow-up (Fig. 2(d)), whereas in the subtype 2 group higher anxiety at baseline preceded a stronger decrease in sensation seeking (β = −0.093, P = 0.009) at 2-year follow-up (Fig. 2(e)). These correlations were all significant after FDR correction at q < 0.05. Associations between anxiety and five subfacets of impulsivity are listed in Supplementary Table 6.
Characteristics of the subtypes in neurodevelopment
To determine the neurodevelopmental basis of the phenotypic differences we compared baseline brain morphological metrics including cortical thickness, surface area, cortical volume, sulcus depth and subcortical volume for the subtype 1, subtype 2 and healthy control groups. ANOVA models revealed that only cortical thickness of six brain regions differed between the groups (Fig. 3(a)), and that surface area, cortical volume, sulcus depth and subcortical volume were non-significant at corrected levels (Supplementary Tables 7–11).
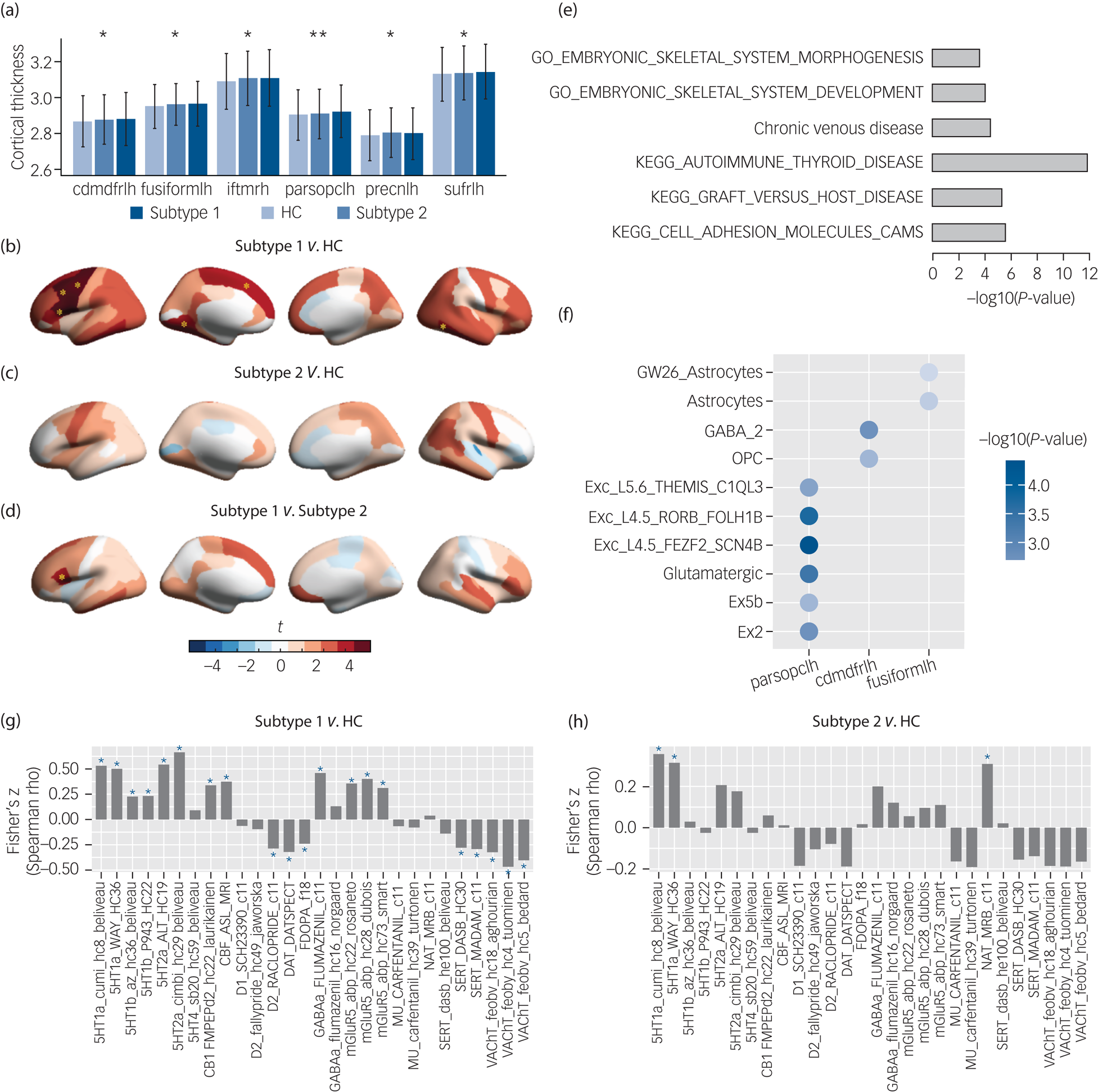
Fig. 3 Neurobiological characterisation of the subtypes of the patients with ‘pure’ internalising disorders (with internalising disorders but without comorbid externalising disorders) at baseline, and genetic analyses and spatial association analysis with neurotransmitter systems for the brain alteration. (a) Thickness of brain regions with significant differences between the two identified subtypes and healthy controls in ANOVA. (b) Thickness alterations in the subtype 1 group compared with the healthy control group. (c) Thickness alterations in the subtype 2 group compared with the healthy control group. (d) Thickness alterations in the subtype 1 group compared with the subtype 2 group. (e) Gene set enrichment analysis for the altered thickness. (f) Cell type specificity analysis for the altered thickness. (g) Spatial association between neurotransmitter receptor/transporter density maps and altered thickness (t-map) of the subtype 1 group.Reference Dukart, Holiga, Rullmann, Lanzenberger, Hawkins and Mehta36 (h) Spatial association between neurotransmitter receptor/transporter density maps and altered thickness (t-map) of the subtype 2 group.Reference Dukart, Holiga, Rullmann, Lanzenberger, Hawkins and Mehta36 Cdmdfrlh, left caudal middle frontal gyrus; fusiformlh, left fusiform gyrus; iftmrh, right inferior temporal gyrus; parsopclh, left pars opercularis; precnlh, left precentral gyrus; sufrlh, left superior frontal gyrus; GO, gene ontology; KEGG, Kyoto Encyclopedia of Genes and Genomes; OPC, oligodendrocyte progenitor cell; CAMS, cell adhesion molecules; HC, healthy control group. In (a), * q < 0.05, ** q < 0.01, which were false discovery rate (FDR) corrected for the 68 cortical thickness measures in ANOVA. In (b), (c) and (d), yellow asterisks indicate P-values passed Bonferroni correction (P < 0.05/3) in post hoc tests for three pair-wise comparisons between three groups. In (g) and (h), blue asterisks indicate P-values that passed FDR correction at q < 0.05 for the 28 measures of neurotransmitter receptor/transporter density maps.
Post hoc pair-wise comparisons (Supplementary Table 7) showed that the subtype 1 group had significantly thicker cortices than the healthy control group in left pars opercularis (t = 4.15, PBonferroni = 1.0 × 10−4), left caudal middle frontal gyrus (t = 3.61, PBonferroni = 9.4 × 10−4), left superior frontal gyrus (t = 3.23, PBonferroni = 3.7 × 10−3), left precentral gyrus (t = 3.81, PBonferroni = 4.3 × 10−4), right inferior temporal gyrus (t = 3.31, PBonferroni = 2.8 × 10−3) and left fusiform gyrus (t = 3.34, PBonferroni = 2.5 × 10−3) (Fig. 3(b)) and thicker cortices than the subtype 2 group in left pars opercularis (t = 3.00, PBonferroni = 8.1 × 10−3) (Fig. 3(d)), whereas the subtype 2 group did not display significant differences from the healthy control group (Fig. 3(c)).
We additionally employed the conventional approach of pooling all patients in the ‘pure’ internalising group (i.e. subtype 1 + subtype 2) and compared these patients with the healthy control group, which revealed similar significant differences compared with those between the subtype 1 group and healthy control group (Supplementary Figure 4 and Supplementary Table 12).
To investigate the stability of neurodevelopmental alterations between groups, we further compared 2-year follow-up cortical thickness between the two identified subtype groups and the healthy control group. We found similar differences as those at baseline (Supplementary Figure 5 and Supplementary Table 13) such that the left pars opercularis remained thicker in the subtype 1 group compared with both the healthy control group and subtype 2 group (t = 3.14, PBonferroni = 5.1 × 10−3 and t = 3.82, PBonferroni = 4.2 × 10−4, respectively; determined in ANOVA models followed by post hoc tests), whereas the left caudal middle frontal gyrus (t = 2.08, P = 0.037) and right inferior temporal gyrus (t = 2.83, P = 0.005) remained thicker in the subtype 1 group compared with the healthy control group (although this failed to pass correction in ANOVA). The subtype 2 group still did not exhibit any significant differences from the healthy control group. In contrast to the baseline data, the two subtype groups differed more strongly at follow-up, specifically, in addition to the left pars opercularis, the subtype 1 group additionally exhibited thicker right middle temporal gyrus (t = 3.60, PBonferroni = 1.0 × 10−3) and right superior temporal gyrus (t = 3.58, PBonferroni = 1.1 × 10−3) compared with the subtype 2 group.
Associations between cortical thickness alterations and anxiety and impulsivity
Employing dimensional analyses in the entire sample focusing on regions with significant differences between the subtype 1 and healthy control groups with both anxiety and impulsivity scores revealed that thicker left pars opercularis and left superior frontal gyrus were associated both with increased positive urgency (t = 2.83, q = 0.014, and t = 2.97, q = 0.014, respectively) and sensation seeking (t = 2.46, q = 0.042, and t = 2.49, q = 0.042, respectively), and a thicker left precentral gyrus was also associated with increased positive urgency (t = 2.26, q = 0.047) (all FDR corrected, Supplementary Figure 6 and Supplementary Table 14).
Genetic underpinnings of cortical thickness alterations
To understand the genetic basis underlying thickness alterations in the subtype 1 group, we performed GWAS analysis, and identified 39 significant SNPs (after clumping, linkage disequilibrium R2 = 0.1, 250 kb, P < 5 × 10−8) across three of the identified regions, including left pars opercularis, left caudal middle frontal gyrus and left precentral gyrus (Supplementary Table 15). Manhattan plots and Q–Q plots can be found in Supplementary Figures 7–18. In total, 257 genes were mapped onto these regions by FUMA (Supplementary Table 16). Further, gene set enrichment analysis identified phenotypes (Fig. 3(e) and Supplementary Table 17) related to autoimmune diseases, such as autoimmune thyroid disease (PBonferroni = 7.17 × 10−3), graft versus host disease (PBonferroni = 1.82 × 10−2) and cell adhesion molecules (CAM) (PBonferroni = 4.71 × 10−2).
For the cell types associated with brain regions, we found that the altered prefrontal and temporal cortical regions were genetically related with excitatory and inhibitory neurons as well as glial cells (Fig. 3(f) and Supplementary Table 18). Specifically, cortical thickness of left pars opercularis was related with glutamatergic neurons (β = 0.088, PBonferroni = 1.37 × 10−3). Cortical thickness of left caudal middle frontal gyrus was related with GABAergic neurons (β = 0.063, PBonferroni = 2.18 × 10−2). Cortical thickness of left caudal middle frontal gyrus and left fusiform gyrus was related with oligodendrocyte progenitor cells (OPCs) (β = 0.036, PBonferroni = 7.70 × 10−3) and astrocytes (β = 0.066, PBonferroni = 9.42 × 10−3), respectively.
Spatial association between cortical thickness alterations and neurotransmitter systems
We further investigated if thickness alterations in each subtype are associated with distinct neurotransmitter systems through spatial association analysis. We found that cortical thickness alterations (t-map) in the subtype 1 group were related with the serotonin, dopamine, cannabinoid, acetylcholine, γ-Aminobutyric acid (GABA) and glutamate systems (Fig. 3(g)), whereas cortical thickness alterations (t-map) in the subtype 2 group were only related with the serotonin and noradrenaline systems (Fig. 3(h)). Significance of each correlation was tested by permutation test (n = 1000) and was adjusted for spatial autocorrelation. Results were corrected for multiple comparisons using FDR at q < 0.05 for the 28 measures of neurotransmitter receptor/transporter density maps.
Characteristics of the subtypes in psychopathological trajectory and cognition
Finally, to determine distinct neurocognitive and longitudinal psychopathological profiles for the two subtypes, we compared the respective indices between the subtype 1 and subtype 2 groups. We found that the subtype 1 group had higher scores for externalising problems (e.g. scores for ADHD, oppositional defiant disorder and conduct disorder in CBCL) compared with those in the subtype 2 group, both at baseline and follow-up, and that those in the subtype 1 group had a higher depressive symptom load than those in the subtype 2 group both at baseline and 2-year follow-up (Fig. 4(a)).
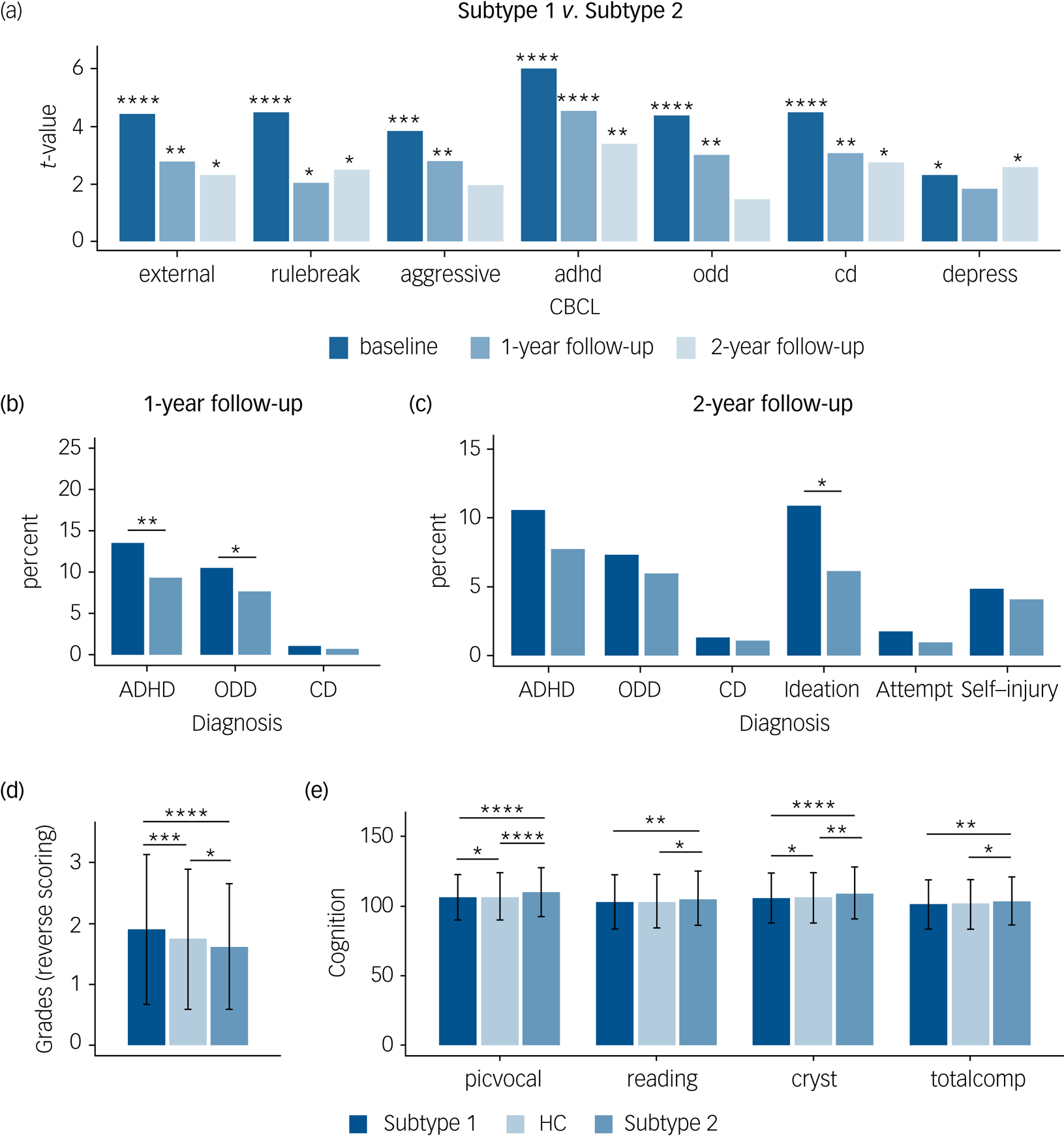
Fig. 4 Distinct neurocognitive and longitudinal psychopathological profiles of the two subtypes in the patients with ‘pure’ internalising disorders (with internalising disorders but without comorbid externalising disorders). (a) Differences of psychopathology at baseline and follow-up between subtypes. (b) Differences of transition rate to externalising disorders at 1-year follow-up between subtypes. (c) Differences of transition rate to externalising disorders and prevalence of suicidality at 2-year follow-up between subtypes. (d) Differences of grades at baseline between groups (grades were scored reversely and 1 = excellent, 2 = good, 3 = average, 4 = below average, 5 = struggling a lot, and 6 = ungraded). (e) Differences of cognition at baseline between groups. external, externalising problems; rulebreak, rule-breaking behaviour; aggressive, aggressive behaviour; adhd, attention–deficit hyperactivity disorder problems; odd, oppositional defiant problems; cd, conduct problems; depress, depressive problems; ADHD, attention–deficit hyperactivity disorder; ODD, oppositional defiant disorder; CD, conduct disorder; Ideation, suicidal ideation; Attempt, suicide attempt; Self-injury, non-suicidal self-injury; picvoc, picture vocabulary; Reading, oral Reading recognition; cryst, crystallised intelligence; totalcomp, total intelligence; HC, healthy control group. In (a–c), *q < 0.05, **q < 0.01, ***q < 0.001, ****q < 0.0001, false discovery rate (FDR)-corrected separately for the seven measures of Child Behavior Checklist (CBCL), three measures of diagnosis at 1-year follow-up, and six measures of diagnosis at 2-year follow-up. In (d) and (e), ANOVA models revealed significant differences in grades as well as picvoc, Reading, cryst and totalcomp of cognition, which passed FDR correction at q < 0.05 for the 11 measures of academic performance and cognition. In (d) and (e), *P < 0.5; **P < 0.01; ***P < 0.001; ****P < 0.0001, which were Bonferroni corrected at P < 0.05/3 in post hoc pair-wise comparisons between three groups.
With respect to the clinical predictive utility of the subtypes we found that more patients in the subtype 1 group developed externalising disorders (ADHD and oppositional defiant disorder) during the 1-year follow-up (Fig. 4(b)) and reported higher prevalence of suicidality, which is mainly driven by suicidal ideation, during the 2-year follow-up compared with those in the subtype 2 group (Fig. 4(c)).
With respect to cognitive performance, ANOVA models revealed significant differences in grades as well as picture vocabulary (picvoc), oral reading recognition (reading), crystallised intelligence (cryst) and total intelligence (totalcomp) of cognition. In post hoc pair-wise comparisons between the three groups, we found that in terms of daily functioning the subtype 1 group had a poorer whereas the subtype 2 group had a better academic performance in terms of grades at baseline compared with the healthy control group (Fig. 4(d)), and that the subtype 2 group had a better cognitive performance, especially in crystallised intelligence (Fig. 4(e)). Results passed Bonferroni correction and statistical details are provided in Supplementary Table 19.
Sensitivity analyses
For sensitivity analyses, when additionally controlled for family ID and acquisition site ID, the significance of the association between anxiety and impulsivity decreased a little, but there were still opposite relationships in the two subtype groups (Supplementary Figure 19 and Supplementary Table 20). When additionally controlled for family income and parental years of education, the relevant results of the cognitive, behavioural and clinical characterisation of subtypes at baseline generally remained the same (Supplementary Figure 20 and Supplementary Table 21). After including patients with comorbid externalising conditions, the results of the clustering analysis and between-group differences generally remained stable (including levels of anxiety, impulsivity and motivational systems, associations between anxiety and impulsivity and cortical thickness, shown in Supplementary Figures 21, 22 and Supplementary Tables 22–24).
Discussion
Main findings
The present study identified two distinct subtypes of patients with internalising disorders who had comparably elevated levels of anxiety but different levels of impulsivity, i.e. enhanced (subtype 1) or decreased (subtype 2) compared with controls in a large preadolescent sample using data-driven clustering. This differentiation was mirrored by opposite relationships between anxiety and impulsivity, different neuroanatomical, neurocognitive and academic profiles, and different clinical trajectories with respect to the conversion to externalising disorders, prevalence of suicidality, severity of externalising and depressive problems.
Individuals with subtype 1 exhibited a distinct neuroanatomical profile characterised by thicker prefrontal and temporal cortices that remained stable or even became more pronounced during the follow-up. Decoding on the genetic and molecular level revealed associations with genetic markers for immune-associated diseases and glia cells as well as inhibitory and excitatory signalling via glutamatergic and GABAergic systems. Findings indicate that impulsivity represents a key defining factor for subtypes of preadolescent internalising disorders, with distinct neurodevelopmental, anatomical and clinical trajectory signatures.
Interpretation of our findings
The subtypes may account for the inconsistent findings about the relationship between anxiety and impulsivity. In those with subtype 1 higher anxiety preceded increasing impulsivity (lack of planning) over the subsequent 2 years, whereas baseline anxiety in those with subtype 2 preceded a decrease in impulsivity (sensation seeking) over this period. These subtypes may harmonise previous controversial findings. On the one hand, anxiety might serve as a defensive function for avoiding potential danger and thus show an inverse relation with impulsivity as observed in individuals with subtype 2.Reference Taylor, Hirshfeld-Becker, Ostacher, Chow, Lebeau and Pollack6–Reference Kashdan and Hofmann9 On the other hand, recent evidence suggests that anxiety is positively associated with impulsivity,Reference Cosi, Hernandez-Martinez, Canals and Vigil-Colet3,Reference Moustafa, Tindle, Frydecka and Misiak10 possibly as a consequence of anxiety-associated greater salience of immediacy, overestimation of the value of immediate rewards and greater motivation to respond to immediacy.Reference Xia, Gu, Zhang and Luo37 This corresponds to the characteristics we identified in subtype 1. The distinct associations may reflect different coping strategies. Excessive impulsivity and behavioural activation have been associated with increased sensitivity to immediate rewards and thus subtype 1 may adopt dysfunctional coping such as pursuing immediate gratification or avoiding behaviours, whereas the lower impulsivity and BAS in individuals with subtype 2 reflect low sensitivity to distractions by rewards, thus those with subtype 2 may adopt rather functional coping strategies to resolve anxiety-associated problems.Reference Portillo-Reyes, Capps, Loya-Mèndez, Reyes-Leal and Quiñones-Soto38 The different strategies may in turn contribute to opposite clinical trajectories and cognitive developments during ‘developmental cascades’.Reference Masten and Cicchetti12
At the neural level, we found a cortical thickness-specific subtype profile. A similar pattern for those with subtype 1 was found when all patients with (pure) internalising disorders (subtype 1 + subtype 2) were examined, whereas those with subtype 2 displayed normal morphology indicating thickness alterations in patients with internalising disorders were driven by subtype 1. Specifically, higher impulsivity in subtype 1 is related to increased thickness in cognitive control networks involving the ventrolateral PFC (VLPFC) and the dorsolateral PFC (DLPFC). These findings align with previous work,Reference Newman, Jernigan, Lisdahl, Tamm, Tapert and Potkin39,Reference Zhu, Wang, Cao, Zhang and Qiu40 and a previous study examining impulsivity–brain structural associations in the ABCD study.Reference Owens, Hyatt, Gray, Miller, Lynam and Hahn4 Thickness variations in the VLPFC have been associated with high impulsivity,Reference Lim, Kim, Seo, Kang, Kim and Kang41 and the VLPFC and DLPFC play an essential role in emotion and behavioural regulation, cognitive control and gratification delay.Reference Miller and Cohen42 Our previous work has shown VLPFC and DLPFC alterations in externalising disorders implying a shared neurological basis for behavioural dysregulation in both externalising disorders and internalising disorders with high impulsivity.Reference Yu, Wu, Liu, Becker, Kuang and Kang43 Morphological alterations in these regions may underlie low academic performance and maladaptive and dysfunctional stress-coping strategies.Reference Portillo-Reyes, Capps, Loya-Mèndez, Reyes-Leal and Quiñones-Soto38 Furthermore, the intact brain morphology in individuals with subtype 2 and the lack of cortical thickness–anxiety associations confirm that the alterations observed in those with subtype 1 are driven by impulsivity rather than anxiety. However, impulsivity is not a unitary construct and different facets have been related with brain variations (see also previous work on the ABCD data-set).Reference Owens, Hyatt, Gray, Miller, Lynam and Hahn4
Our findings resonate with the ‘maturational mismatch’ theory proposing that the PFC attains functional maturity later than the limbic system and this developmental mismatch results in poor behaviour and emotion regulation during adolescence.Reference Casey, Getz and Galvan17 Thickness of PFC increases during childhood, followed by a decrease in adolescence.Reference Shaw, Kabani, Lerch, Eckstrand, Lenroot and Gogtay14 Thicker PFC in individuals with subtype 1 may thus indicate delayed development, which results in ineffective control over behavioural impulses in response to reward and punishment mediated by the limbic system that in turn may promote engagement in dysfunctional coping strategies under anxiety. Increased PFC thickness differences between subtypes over the 2-year follow-up may reflect distinct neurodevelopmental trajectories over the follow-up period. Given the association with longitudinal development trajectories these regions may represent particular promising targets for e.g. transcranial magnetic stimulation or neurofeedback interventions.Reference Zhao, Yao, Li, Sindermann, Zhou and Zhao44
Those with subtype 1 additionally exhibited thicker cortices in temporal regions, which have been associated with perception and emotion,Reference Fusar-Poli, Placentino, Carletti, Landi, Allen and Surguladze45 and play a central role in anxiety disorders.Reference Frick, Howner, Fischer, Kristiansson and Furmark46 Greater thickness of these regions has been observed in individuals with paediatric generalised anxiety disorder and has been associated with fear learning, fear extinction and deficits in the regulation of the amygdala,Reference Strawn, Wegman, Dominick, Swartz, Wehry and Patino47 suggesting that developmental delay within these regions may promote a lack of adaptive responses during stress.
With heritability measures for preadolescent cortical thickness ranging from 60% to 80% maturation-related cortical thickness changes are under strong genetic control and increase their influence on behaviour and cognition during this period.Reference Teeuw, Brouwer, Koenis, Swagerman, Boomsma and Hulshoff Pol48 The GWAS identified genetic factors that have been associated with immune system dysregulations and may underpin the alterations in those with subtype 1. The identified genes were enriched in individuals with autoimmune diseases, including e.g. autoimmune diseases of the thyroid system that plays a crucial role in brain development and inflammation-induced cortical thickness alterations.Reference Rovet49 These genes were also enriched in central immune system molecules like CAM, and cells including OPC. CAM can promote the migration and survival of OPC, which critically regulates myelination that is reduced in ADHD,Reference Dark, Homman-Ludiye and Bryson-Richardson50 and was related with cortical thickness changes during neuromaturation.Reference Natu, Gomez, Barnett, Jeska, Kirilina and Jaeger13 Results suggest that immune-related processes may underly the cortical maturation alterations see in those with subtype 1. In addition, genes linked with glutamatergic and GABAergic signalling were enriched in the identified regions and transporter density analysis further confirmed subtype 1 specific alterations in these neurotransmitter systems. Both, levels of frontostriatal glutamate and GABA have been associated with impulsive behaviour and disinhibition across disorders.Reference Murley, Rouse, Jones, Ye, Hezemans and O'Callaghan51 Taken together, findings suggest that alterations in individuals with subtype 1 are related to the immune system and dysbalanced excitatory–inhibitory signalling.
Suicidal ideation, depressive and externalising symptoms at baseline and follow-up were increased in individuals with subtype 1. Previous studies reported an association between increased impulsivity and suicidality in patients with internalising disorders.Reference Askenazy, Sorci, Benoit, Lestideau, Myquel and Lecrubier21 Another work from the ABCD study distinguished a suicide group from a clinical control group based on factors including impulsivity and depression.Reference van Velzen, Toenders, Avila-Parcet, Dinga, Rabinowitz and Campos52 Given that suicidal ideation has been associated with actual suicide attempts it is of particular clinical relevance Reference Hubers, Moaddine, Peersmann, Stijnen, Van Duijn and Van der Mast53 and suicide rates have increased strongly during the pandemic years.Reference Charpignon, Ontiveros, Sundaresan, Puri, Chandra and Mandl54 Together anxiety, depression and impulsivity represent critical suicide risk factors that are generally modifiable via early interventions.Reference Fawcett55 Therefore, individuals with subtype 1 who are characterised by high anxiety and high impulsivity represent a risk group and may benefit from early interventions. Individuals with subtype 1 exhibit cognitive problems and lower academic grades whereas those with subtype 2 exhibit higher crystallised intelligence and academic performance, potentially reflecting a neurocognitive compensation allowing adaptation despite elevated levels of anxiety. Individuals with subtype 1 may represent a group with a strongly increased psychopathology requiring early interventions for anxiety and impulsivity.Reference Subotic-Kerry, Baillie, Stapinski, Teesson, Sannibale and Haber56
Strengths and limitations
Although we employed multimethodal and longitudinal characterisation in a large sample the results have to be considered in the context of limitations. We found a positive relationship between cortical thickness and impulsivity but previous studies have also reported an inverse relationship,Reference Lim, Kim, Seo, Kang, Kim and Kang41 or no association,Reference Boedhoe, van Rooij, Hoogman, Twisk, Schmaal and Abe57 which might be because of the different impulsivity measures used in the study or different age ranges across studies. We focused on brain structure, future studies should examine alterations of functional MRI markers.
Implications
Impulsivity plays an important role in preadolescent internalising disorders. Accounting for impulsivity can determine distinct subtypes in symptomatology, clinical trajectories and genetically controlled neurobiology suggesting differential pathological bases and treatment indications. We identified two distinct subtypes with opposite anxiety–impulsivity associations, which unifies past controversies about the relationship between anxiety and impulsivity. Clinically, the subtype with high impulsivity exhibits a detrimental trajectory over the following 2 years including higher suicide risks thus early interventions targeting emotional and behavioural dysregulations are warranted.
Supplementary material
Supplementary material is available online at https://doi.org/10.1192/bjp.2023.107
Data availability
The data that support the findings of this study can be accessed from ABCD 3.0 Data Release at https://nda.nih.gov/study.html?id=901.
Author contributions
H.F., X.W., T.J., K.Z., B.B. and J.Z. conceived and designed the study. H.F., X.W., G.Y., N.K., Y.L. and X.G. did the statistical analyses. H.F. wrote the first draft of the manuscript. H.F., Z.L., X.W., G.Y., X.G., T.J., B.J.S., T.W.R., G.S., B.B. and J.Z. contributed to critical revision of the report for important intellectual content. W.C. and J.F. contributed to data curation. W.C., J.F., B.B. and J.Z. were the project manager. All authors have read and approved the final version of the manuscript. B.B. and J.Z. were responsible for the final decision to submit for publication.
Funding
J.Z. was supported by STI2030-Major Projects 2021ZD0200204, Shanghai Municipal Science and Technology Major Project (No.2018SHZDZX01), ZJLab, NSFC 61973086, and Key Laboratory of Computational Neuroscience and Brain-Inspired Intelligence (Fudan University), Ministry of Education, China. W.C. was supported by grants from the National Natural Sciences Foundation of China (No. 82071997) and the Shanghai Rising Star Program (No. 21QA1408700). J.F. was supported by the 111 Project (No. B18015), and Shanghai Center for Brain Science and Brain-Inspired Technology. B.B. was supported by the National Key Research and Development Program of China (2018YFA0701400), and National Natural Science Foundation of China (No. 82271583, No. 32250610208).
Declaration of interest
B.J.S. consults for Cambridge Cognition, Greenfield BioVentures and Cassava Sciences. T.W.R. consults for Cambridge Cognition, Shionogi, Heptares, Takeda, Arcadia, and Greenfield BioVentures; and receives royalties from Cambridge Cognition and research grants from Shionogi and GlaxoSmithKline. All other authors report no biomedical financial interests or potential conflicts of interest.









eLetters
No eLetters have been published for this article.