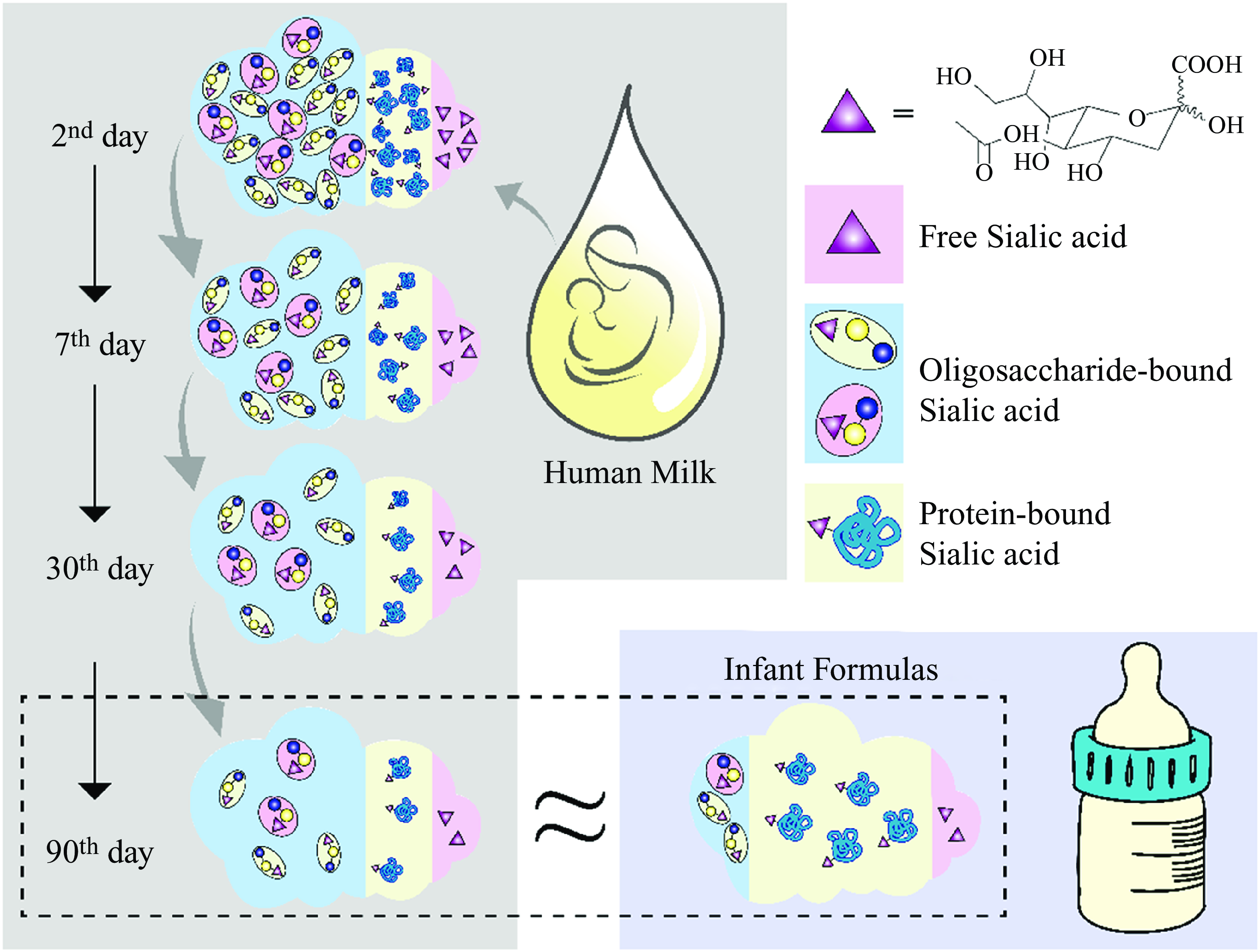
Sialic acids (SA) are a family of derivatives of neuraminic acid with a nine-carbon backbone typically found at the outermost ends of glycan chains in the deuterostome lineage of animals(Reference Blix, Gottschalk and Klenk1). Based on the group attached to carbon C-5, SA can be divided into four categories: 5-N-acetylneuraminic acid (Neu5Ac), 5-N-glycolylneuraminic acid (Neu5Gc), 2-keto-deoxynonulosonic acid and neuraminic acid. Neu5Ac is the most widespread form of SA and almost the only form found in humans(Reference Wang and Brand-Miller2),which is usually a component of the oligosaccharide chains of mucins, glycoproteins and gangliosides. Meanwhile, Neu5Gc cannot be produced in humans throughout evolution.
Human milk is a rich source of sialylated structures, such as human milk oligosaccharides (HMO), and glycoconjugates, such as glycoproteins, and contains SA as a free molecule. Consequently, a vital part of the newborn and infants’ diet is SA delivered with the mother’s breast milk. The lactose residues in HMO can be extended into straight or branched chains and then into sialylated oligosaccharides by sialyltransferases(Reference Ruhaak and Lebrilla3). The restricted hydrolysis of HMO by gastric acid allows Neu5Ac to be retained relatively intact in the intestinal digestive phase, interfering with the interaction of harmful substances with the host by modelling(Reference Quinn, Joshi and Hickey4) or adhering to intestinal epithelial cell-binding ligands(Reference Newburg, Ruiz-Palacios and Morrow5) and regulating the immune response(Reference Eiwegger, Stahl and Schmitt6), thus enhancing the health status of the newborn. For infant formulas, galactooligosaccharide and fructooligosaccharides are the two main ingredients used to mimic HMO, while the recent addition of both is considerably below the effective doses that can improve the gastrointestinal microbiome(Reference Bakker-Zierikzee, Alles and Jan7,Reference Euler, Mitchell and Kline8) .
Additionally, it was observed that neither galactooligosaccharide nor fructooligosaccharides has the basic structure (galactose residue or N-acetylglucosamine) for subsequent linkage of Neu5Ac(Reference Tungland and Meyer9). Moreover, various components of human milk, including SA, are dynamically related to the phase of lactation, which is consistent with the physical growth demands of infants. This makes SA in human milk a unique nutrient both statically and dynamically, and infant formula may not be able to achieve comprehensive simulation.
The stimulating effect of SA on the neurological system occurs mainly during the early postnatal period. Neu5Ac enhances neurite outgrowth by stimulating electrolyte enzymes and regenerating neurons in the central nervous system through several mechanisms, thus improving learning and cognitive capacities(Reference Oliveros10,Reference Schnaar11) . The mean brain weight of 2-year-old children is 80 % of that of adults, indicating that age 0–2 is a critical period for brain development and functional establishment(Reference Hüppi12). However, the critical enzyme in the liver, UDP-N-acetylglucosamine-2-isomerase(responsible for catalysing the first step in the biosynthesis of SA), is less active because the infant liver may not have the full capacity for synthesis during the early postnatal period(Reference Gal, Ruano and Puente13). Thus, SA in human milk, a critical part of newborns’ and infants’ diets, may supplement the limited capacity for endogenous synthesis of SA in human infants.
Studies comparing the SA content of Chinese breast milk with infant formula have not yet been reported. This study aimed to determine differences in the content, type or distribution of SA in human milk at different stages of lactation and in infant formulas. The primary aim is to provide a reference for the choices of dairy products for lactating infants, and the secondary aim is to contribute possibilities for achieving a full-scale simulation of human milk by infant formula.
Materials and methods
Human milk
The study recruited 246 mothers (aged 23–38 years) from the Maternal and Child Care Service Centre in Xiamen, China, using a non-probability sampling method. They are healthy, full-term, exclusively breastfed, basically Han Chinese, almost Xiamen resident, with 46·3 % of first birth and 45·9 % of second birth. Their eligibility criteria were assessed based on relevant medical reports. This study was conducted according to the guidelines laid down in the Declaration of Helsinki, and all procedures involving human subjects were approved by the hospital ethics committee (No. XDYX2020008), and all mothers provided written informed consent before inclusion in the study. The sample size calculation was performed according to Wang and Ji(Reference Wang and Ji14) and exceeded the number in other similar research(Reference Wang, Brand-Miller and Mcveagh15). Because some mothers withdrew at the midway point, 246, 135, 85 and 48 breast milk samples were collected on days 2, 7, 30 and 90, respectively. Possible reasons for withdrawal: (1) not secreting enough milk to feed the baby, (2) tired of frequent interruptions, (3) returning to work, etc. This was a non-blinded observational study with a result of natural exposure.
Human milk samples (10 ml) were collected by manual expression or breast pump immediately before feeding at approximately the same time of the morning (09.00–12.00) on the 2nd (colostrum), 7th (transition milk), 30th (mature milk) and 90th (3 months) days after birth. The sample collection process was arranged in separate and quiet rooms and was performed by female operators. The key operations of the collection were conducted by caregivers from the Maternal and Child Care Service Centre, who have been in the maternal and child care profession for more than 5 years. The non-professional investigators have been trained systematically in theory and practice before taking up their jobs by doing auxiliary work. All samples were kept in sterile plastic lactation cups, brought back by investigators in coolers and stored at −20°C refrigerators.
Infant formulas
Infant formulas (n 24) suitable for full-term infants < 6 months of age were selected from the top-selling infant formulas in China for analysis. Most of the SA in infant formulas was bound to protein (70 %)(Reference Wang, Brand-Miller and Mcveagh15), the difference in SA content in milk-based formulas varies with the proportion of whey protein in the formula(Reference Neeser, Golliard and Vedovo16), so classified the infant formula by composition: 60 % whey formulas (formulas with a 60:40 whey: casein ratio, n 10), 40–50 % whey formulas (n 4), 40 % whey formulas (n 8) and soya milk formulas (n 2). All the formulas were reconstituted according to the manufacturer’s instructions using ultrapure water.
Analysis of sialic acids by HPLC-FLD
SA in human body fluids and infant formulas were determined using the HPLC-FLD method, which allows for the determination of Neu5Ac and Neu5Gc. Nue5Ac and Neu5Gc can react with 4,5-methylenedioxy-1,2-phenylenediamine dihydrochloride (DMB), a new fluorogenic reagent for α-keto acids, under acidic conditions to form corresponding derivatives(Reference Hara, Takemori and Yamaguchi17). These derivatives can produce strong fluorescence signals at specific wavelengths, allowing the chromatograph’s quantitative identification of Neu5Ac and Neu5Gc.
HPLC-FLD systems from Agilent (Santa Clara) were used: an Agilent-1200 HPLC system equipped with a fluorescence detector. Separation was performed on an X Terra MS-C18 analytical column (100 mm × 2·1 mm, 2·5 µm, Waters) using an X Terra MS-C18 Guard column (20 mm × 2·1 µm, Waters). The fluorescence signal was monitored at 373 nm (excitation wavelength) and 448 nm (emission wavelength). Samples were eluted isocratically using 2·5 % (v/v) methanol and 3·5 % (v/v) acetonitrile in water flowing at 0·3 ml/min. All injections were performed at 30°C.
Precisely 90 µl extracted sample or standard (Neu5Ac & Neu5Gc, Sigma) was mixed with 10 µl DMB derivative reagent (8 mmol/l DMB, 1·5 mol/l acetic acids, 0·25 mol/l sodium thiosulphate, 0·25 mol/l sodium hydrosulfite and 0·8 mmol/l 2-mercaptoethanol), and the mixture was derived 50°C for 150 min away from the light because the DMB reagent is light-sensitive(Reference Martín, Vázquez and Rueda18). The validation of the method showed that the average recovery of HPLC-FLD was 94·0 %, the precision RSD was 0·4 %, the stability RSD was 1·8 %, the reproducibility RSD was 0·8 % and the minimum detection limits were 0·02 μmol/l (Neu5Ac) and 0·03 μmol/l (Neu5Gc).
Sample preparation for the detection of free sialic acids
This section describes the direct detection of the concentration of free SA (C Free ). A 500 μl of milk sample, while reconstituted formula following the instructions on the packaging, was accurately aspirated into a centrifuge tube. An equal volume of trichloroacetic acid (10 %) was added to precipitate the protein. After mixing in a 10-minute ice bath, the sample was centrifuged at 4°C, 3000 r/min for 30 min at 4°C. The protein pellet in the precipitate was washed twice with cold 5 % trichloroacetic acid under the same conditions, and the supernatant of each wash was combined in a new centrifuge tube. Then, a proportion (500 µl) of the supernatant fluid was filtered directly through a 0·22 μm Microza (Chuding Analytical Instrument Limited Company) and left to derive.
Sample preparation for the detection of oligosaccharide-bound sialic acids
The remaining supernatant fluid was added to an equal volume of 0·1 mol/l trifluoroacetic acid and hydrolysed at 80°C for 30 min. After cooling to room temperature, the sample was filtered through a 0·22 μm microza and left to derive. The outcome of this section is the sum of the concentrations of free SA and oligosaccharide-bound SA (C FO ). The concentration of oligosaccharide-bound SA was determined by calculating (C Oligo = C FO – C Free ).
Sample preparation for the detection of protein-bound sialic acids
This section describes the direct detection of the concentration of protein-bound SA (C Pro ). The combined protein pellets were resuspended in 2 ml of 0·05 mol/l H2SO4 and heated for 120 min at 80°C. After cooling to room temperature, the sample was filtered through a 0·22 μm microza and left to derive. The final concentration of SA in each sample was expressed as mg/l of human milk or mg/g of infant formula.
Statistical analysis
Data were analysed using IBM SPSS Statistics v.26 (IBM Corporation) to compare the SA concentration of human milk with infant formulas and illustrate the dynamics of SA concentrations in human milk at different stages during the postpartum period. The Kolmogorov–Smirnov test was used to define normality of data. Preliminary tests showed SA concentrations in human milk were not following normal distributions. Thus, one-way ANOVA and Kruskal–Wallis H rank sum test (for non-normally distributed data) were used for comparisons of multiple samples. When there was a statistical difference (P < 0·05), multiple comparisons were performed using Bonferroni correction. Results are expressed as the mean with SD, median with IQR or box plots with medians and minimum-to-maximum whiskers. Differences were considered statistically significant at P < 0·05.
Results
Sialic acids concentration in human milk
The purpose and experimental design of this study were straightforward, so all analyses performed were pre-specified. We detected only SA of type Neu5Ac in the human milk collected in this study. Figure 1 shows the oligosaccharide-bound, protein-bound, free SA and total SA concentrations in human milk at different stages during the postpartum period. We presented all analysed data outcomes in the natural state and did not exclude any outliers. The total SA concentration in human term milk is the highest at the beginning of lactation and declined significantly over time. About 68·7–76·1 % of all SA in human milk is bound with oligosaccharides, 20·5–28·3 % with proteins and 0·9–5·6 % as free molecules.

Fig. 1. Concentration of each form of sialic acid, including oligosaccharide-bound (Oligo), protein-bound (Pro), free forms (Free) and total sialic acid (Total), in human milk during lactation. Human milk was collected on the 2nd (n 246), 7th (n 135), 30th (n 85) and 90th (n 48) day after birth. Box plots denote median (centre line), 25–75th percentile (limits), minimum and maximum values without outliers (whiskers) and outliers (dots). *, **: Significantly different from 90th day. *P < 0·05, **P < 0·001.
Trends in Sialic acids concentrations of human milk
We observed a significant downward trend in the total SA concentration in human milk at the four different time points (γ = 3, P < 0·005, Fig. 1). The amount of SA in colostrum was significantly higher than that in the other stages of lactation (γ = 510, P < 0·001); it was five times greater than that in mature milk expressed on the 90th day. Additionally, like the trend in total SA, the concentration of each form of SA, namely, oligosaccharide-bound, protein-bound and free SA, also showed the same time dependency. The concentrations of total SA in human milk on days 7th, 30th and 90th-day are 77·06 %, 33·87 % and 18·52 % on the 2nd day, respectively. Those values were estimated to be 74·51 % (7th/2nd), 33·84 % (30th/2nd) and 18·74 % (90th/2nd) for free SA, 76·89 %, 33·33 % and 18·01 % for oligosaccharide-bound SA and 77·49 %, 34·84 % and 19·80 % for protein-bound SA, respectively. All forms of SA exhibited an approximately parallel decrease to 18–20 % of the initial concentrations during the first three months of lactation.
Sialic acids content and distribution of infant formula
Two types of SA, Neu5Ac and Neu5Gc, were both detected in tested infant formulas, with Neu5Ac (1·63 ± 0·55 mg/g) accounting for approximately 96·5 % of the total amount of SA in the formula powders. In contrast, Neu5Gc (0·06 ± 0·04 mg/g) accounted for 3·46 % of it (Table 1). Approximately 63·0 % (95 % CI: 61·0–64·0 %) of all SA in infant formula is bound with proteins, while only 27·8 % (range: 26·4–29·2 %) with oligosaccharides and 9·2 % (range: 7·9–10·6 %) as free molecules. After reconstitution according to recommended guidelines, the mean Neu5Ac concentration in formula milk was 162·69 mg/l, with a maximum of 267·42 mg/l and a minimum of 63·13 mg/l.
Table 1. Sialic acid content and form in infant formulas (mg/g, mean ± sd)

* The same column values within the same group with different superscript letters are significantly different, P ≤ 0·005 (with Bonferroni’s adjustment for multiple comparisons).
Total SA content did not exhibit statistical differences among 60 % whey, 40–50 % whey, 40 % whey and soya milk formulas (P > 0·05, Table 1). Nevertheless, we observed numerical advantages in the SA content of the 60 % whey formula, which had a significantly higher protein-bound SA content than soya protein formulas (P = 0·027).
Estimation of sialic acids intake in infants
The SA concentrations in human milk were in all cases higher than those in infant formula tested. According to the Clinical Handbook of Paediatrics and Formula Powder Nutrition Facts, the average energy provided by Chinese mothers’ breast milk and infant formula milk is 670 and 520 kcal/l, respectively. Assuming an energy requirement of 110 kcal/kg·d for newborns, we estimated the average daily intake of SA concentration in human milk and infant formula for infants within the same energy intake range. Table 2 shows the estimated amount of SA ingested by breastfed infants within the first 3 months of life. The daily SA intake for breastfed infants did not change dynamically and was invariably higher than formula-fed infants. Even when estimated for infant formulas with the highest SA content, the mean daily intake (39·58 ± 9·80 mg/kg·d) was consistently lower than that of breastfed infants between 0 and 30th day (γ = 519, P < 0·05), which was only close to that of breast-feeding at 90th day (P = 1·0).
Table 2. Estimated daily intake of sialic acid of breastfed infants (median (IQR))

* Values in the same column with different superscript letters are significantly different, P ≤ 0·005 (with Bonferroni’s adjustment for multiple comparisons).
We analysed the proportions of the different forms of SA present, given the organism’s varying digestion, absorption and metabolism mechanisms (Fig. 2). Compared with formula-fed infants, the predominant amount of SA consumed by breastfed infants was oligosaccharide-bound (71·2 % v. 27·8 %) rather than protein-bound SA (25·6 % v. 63·0 %). In addition, formula-fed infants consume small amounts of Neu5Gc, which has not been detected in human milk.

Fig. 2. Differences in the distribution of sialic acid in human milk or infant formulas (90th day human milk v. infant formulas). The average proportion of sialic acid bound to oligosaccharides, protein or in free form in human milk and infant formula forms this stacked chart.
Discussion
The current study compared the concentrations of total SA in human milk and those of infant formulas and their distribution, including oligosaccharide-bound, protein-bound and free forms. In human milk, most SA (∼72·4 %) is bound to free oligosaccharides, and this proportion remains almost constant during lactation. Only a small proportion of SA (∼28·1 %) is bound to oligosaccharides in infant formula. Previous studies have documented similar distributions of SA in human milk and infant formulas as those found in the present study(Reference Wang, Brand-Miller and Mcveagh15,Reference Carlson19) . Since human milk is the only source of nutrition for the first stages of life, its composition is an essential criterion for the emulation of infant formulas. This research shows that during the 2–90 d of lactation, the total SA concentration in human milk, whether in colostrum or mature milk, was higher than in any of the infant formulas analysed. The body may not have an adequate capacity to synthesise SA to support its developmental requirements in early infancy. However, infancy is the most crucial stage in the structural and functional development of brain tissue, in which the modification of brain proteins by SA also occurs, making the supplementation of exogenous SA for infants essential. Studies have shown that intraperitoneal and oral administration of SA during the early postnatal period significantly increases the concentration of SA in brain gangliosides and glycoproteins(Reference Morgan and Myron20,Reference Wang21) . Supplementary SA is also associated with increased learning behaviour in the brains of young rats(Reference Bian, Wang and Huang22). In conjunction with the results of this study, the concentration of SA in human milk is more likely to meet the developmental demands of the organism in the early years of life.
The higher concentration of SA in human milk implies that the total amount of SA obtained by the infant through human milk will be higher than through infant formulas. The average volume of milk expressed by the mother on the 2nd, 7th, 30th and 90th postnatal days was approximately 100 ml, 500 ml, 800 ml and 800 ml(Reference Sun23), from which the total amount of SA secreted at each time point could be calculated to be 148·32 mg/d, 571·47 mg/d, 401·90 mg/d and 219·71 mg/d, respectively. Thus, the amount of SA infants obtained from human milk tends to decrease over time during the first 3 months of lactation. Even with the declining trend in human milk SA, feeding the infant formula that has the highest SA content would only offer about 68 % of the SA gained by a breastfed infant on the 90th day (the stage with the lowest observed intake). As an evolutionary adaptation, colostrum has a remarkably high level of SA with a relatively minimal secretion volume, accommodating the baby’s underdeveloped stomach (volume 25–50 ml only) and compensating for the infant’s partial capacity of the liver to synthesise endogenous SA. The liver’s synthesis capacity increases with age, followed by an adaptive decrease in the concentration of SA in human milk. The concentration of SA in human milk adaptively decreases with maturing liver function as age increases. Nevertheless, the SA content of infant formula is unlikely to be modified to suit the developmental demands of the infant in the same manner as human milk. Hence, SA in human milk can more scientifically compensate for the endogenous deficiency.
Estimates based on energy requirements showed that even for infant formulas with the highest SA content, the mean daily intake was consistently lower than that of breastfed infants between 0 and 30th day. Research has shown that the difference in SA content in milk-based formulas varies with the proportion of whey protein in the formula(Reference Neeser, Golliard and Vedovo16). There was also a tendency for SA content to increase with the proportion of whey protein, although the difference was not statistically significant. It is speculated that these formulas have a whey protein percentage that is more similar to that of human milk, which makes its SA content closer to the latter. Thus, the whey protein-rich formula may be a better source of SA than the formula high in casein.
Although SA digestion and absorption mechanisms have not yet been fully elucidated, studies on mammalian metabolism have shown that free SA is not fully utilised because of its short retention time in the body. In addition, compared with protein-bound SA, which has a long retention time but is resistant to conversion, oligosaccharide-bound or glycopeptide-bound SA has a suitable retention time and is readily absorbed and utilised(Reference Wang and Brand-Miller2). One study found that only 3′-sialyllactose appeared in the serum and urine of rats fed HMO, and barely any other HMOs appeared, suggesting the selective absorption of rat milk-specific oligosaccharides(Reference Jantscher-Krenn, Marx and Bode24). An in vitro experiment found that SA bound to oligosaccharides in human milk could not be digested in the small intestine of healthy infants(Reference Engfer, Stahl and Finke25), revealing the possibility that oligosaccharide-bound SA is fermented and used by microorganisms in the conjugate. Additionally, the glycans (including oligosaccharides, glycoproteins, glycolipids and mucins) that cover the intestinal mucosa are the first step in bacterial and viral infection, whereas sialylated oligosaccharides contain structural units similar to these glycans that may act as receptor analogs in the intestine, preventing infection by inhibiting pathogen adhesion(Reference Quinn, Joshi and Hickey4,Reference Newburg26) .
These high proportions of oligosaccharide-bound SA may also be important contributors to the appropriate intestinal flora distribution in infants. Most HMO reach the distal small intestine and colon intact and at high concentrations, as these oligosaccharides are not only a form of dietary fibre required by infants but have also been observed to act as ‘bifidogenic factors’(Reference Lars27). Research has consistently demonstrated the essential role of HMO in promoting the growth of probiotics (Bifidobacterium, Lactobacillus, Bacteroides, etc.) and in inhibiting the growth of other parthenogenic anaerobic bacteria in the intestine(Reference Rudloff, Pohlentz and Borsch28). Furthermore, studies have reported that HMO can indirectly improve immune function in infants, presumably because sialylated HMO can affect lymphocyte maturation, leading to a shift in the T-cell response towards a more balanced and stable immune state(Reference Eiwegger, Stahl and Schmitt6,Reference Rudloff, Pohlentz and Borsch28) . Restrictions on the source of raw materials and ethical issues have made it difficult for HMO to be naturally added to infant formulas, together with challenges in the full range of chemical simulations. Thus, as long as the oligosaccharide component of infant formula has not been optimised, breast-feeding remains a safer choice.
Unlike human milk, infant formulas contain approximately 3·46 % Neu5Gc in addition to Neu5Ac. Neu5Gc is a hydroxylated product of Neu5Ac, in which the CMAH gene encoding the hydroxylase CMP-Neu5Ac has been mutated during evolution in healthy human tissues, leaving them unable to synthesise Neu5Gc(Reference Varki29). Hence, Neu5Gc has not been detected in healthy human tissues or body fluids yet(Reference Muchmore, Diaz and Varki30). However, consuming foods containing Neu5Gc, such as dairy products, red meat and some aquatic products, can accumulate Neu5Gc in the body(Reference Vamecq, Mestdagh and Henichart31). The accumulation of Neu5Gc cross-links with Neu5Ac-containing sugar chains in the tissue, resulting in ‘heterologous autoantibody’ called anti-Neu5Gc production. Studies have shown that the higher the intake of Neu5Gc in humans, the more anti-Neu5Gc is generated and the higher the risk of cancer. Epidemiological links exist between Neu5Gc accumulation and cancer development(Reference Varki32). This link suggests that breast-feeding is a safer option to avoid the additional intake of Neu5Gc, which might be detrimental to human health.
The limitations of our study should be considered. It was not possible to know the SA content of human milk for more than 90 d and the difference between human milk and infant formula. We do not know whether factors such as age and education level are factors that influence exclusive breast-feeding by mothers. Samples were collected at the same time of day (09.00–12.00), and it was impossible to know if there was a circadian rhythm of SA content in breast milk.
In this study, we did not tend to encourage parents to adopt any certain infant feeding style. All experimental outcomes are recommendations that formula producers could take into consideration. Parents only need to choose the most appropriate plan on an informed basis.
Overall, our findings reinforce the dynamic nature of SA production in human milk and demonstrate that it is a preferable and safer source of SA than infant formulas in terms of total SA content, dynamics, distribution and type. Such knowledge could facilitate the development and clinical application of personalised infant formulas. Further experiments are definitely required to make an in-depth exploration, not only stalling on simple comparisons but also on the factors influencing mammary gland secretion of SA concentration, such as dietary or ethnic factors, or rational addition of supplementary SA in infant formula, which all can contribute to dairy products beneficial effects.
Key messages
-
(a) Human milk is rich in SA, with the highest concentrations in colostrum, which gradually decreases as breast-feeding progresses.
-
(b) Most of the SA in human milk are bound to oligosaccharides, whereas in infant formula are mostly bound to proteins.
-
(c) Small amounts of non-human SA, namely Neu5Gc, were detected in the infant formulas.
-
(d) During the 2–90 d of lactation, the total SA concentration in human milk was higher than in any of the infant formulas analysed.
Acknowledgements
The authors gratefully acknowledge the active participation of all mothers.
This research was funded by the Zhongshan Hospital, Fudan University (Xiamen Branch) (grant number 2020ZSXMYS24).
Y. L., X. W. and H. L. contributed to the planning and the design of the study. Y. L. and X. W. act as the project administrator. J. H.,W. Z., M. Z., K. D., Y. Z. and L. Z. collected the data. J. H. and W. Z. contributed significantly to the data analysis and manuscript preparation. Y. L., X. W. wrote the manuscript. H. L. helped perform the analysis with constructive discussions. All authors read and approved the final manuscript.
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses or interpretation of data; in the writing of the manuscript or in the decision to publish the results.
The data that support the findings of this study are available on request from the corresponding author.
Xiamen Maternal and Child Care Service Centre ethics committee approved the study, and all mothers provided written informed consent before inclusion in the study. The study in accordance with the Helsinki Declaration of 1975, as revised in 2008.